Cell-Based Modeling of Tissue Developing in the Scaffold Pores of Varying Cross-Sections
Abstract
:1. Introduction
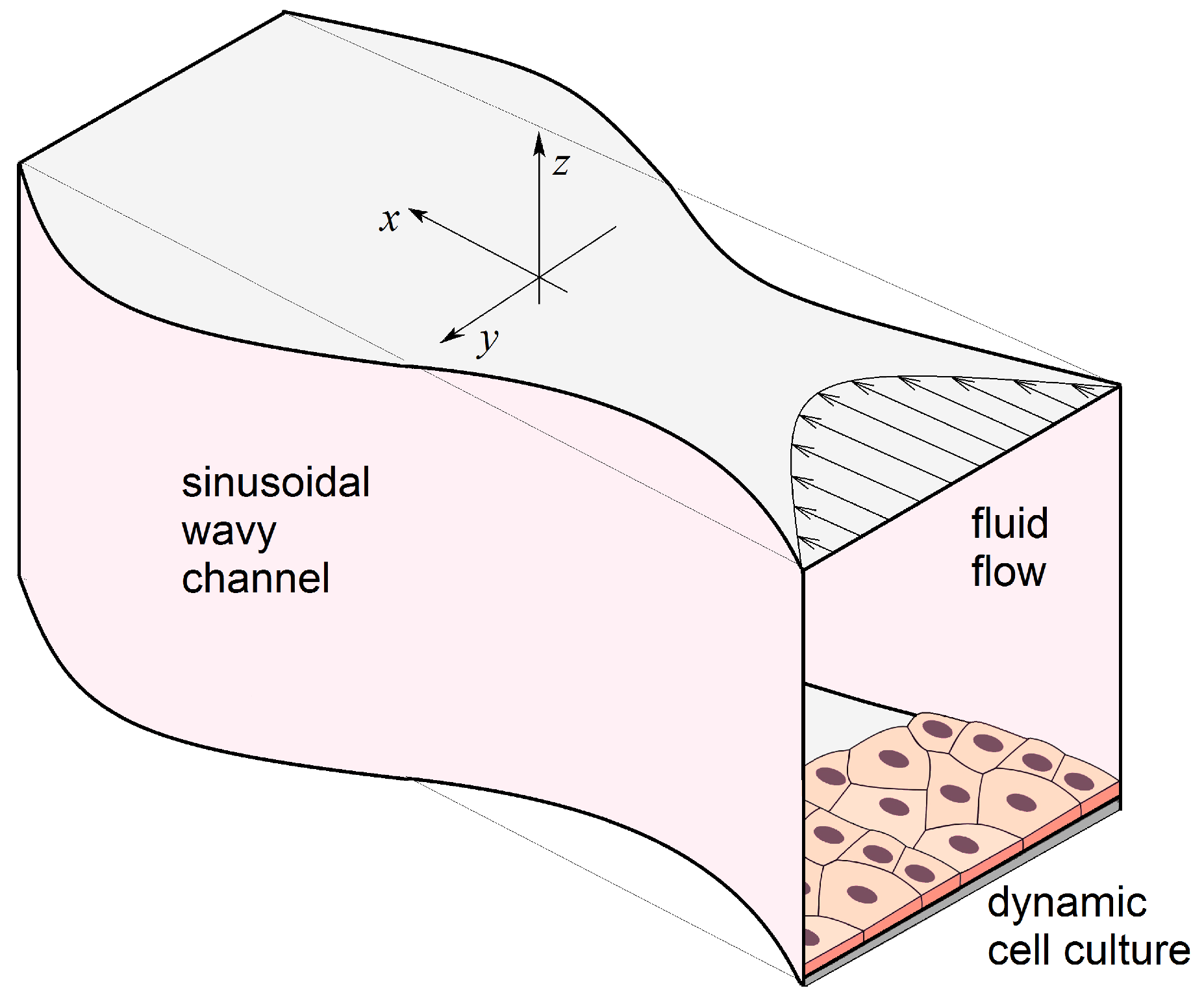
2. Mathematical Formulation
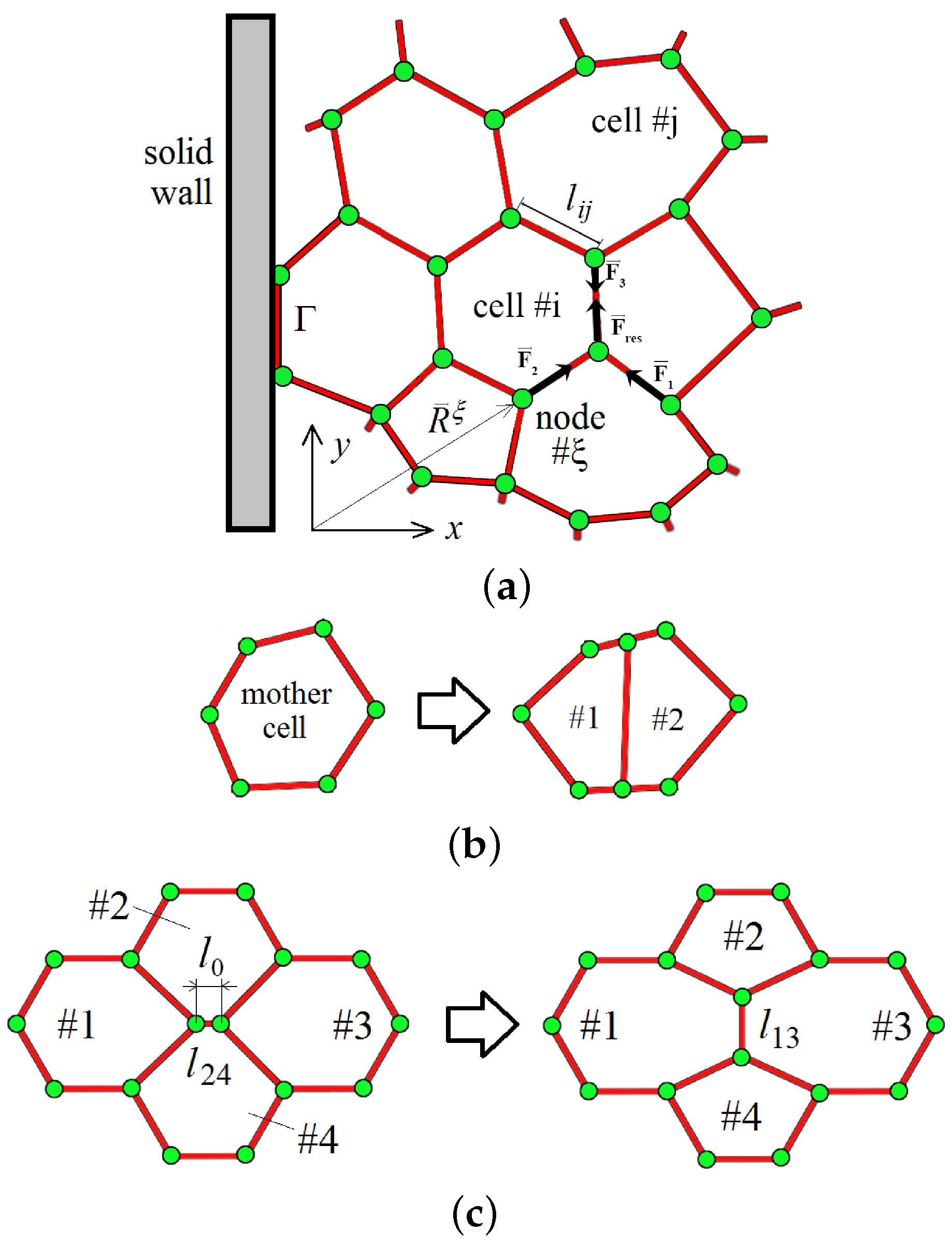
2.1. Two-Dimensional Vertex Model for Simulating Tissue Growth
- Determine the longest side of the mother cell.
- If the number of sides is even, then
- (a)
- Connect the middle of the longest side with the middle of the opposite side;
- (b)
- Create two new nodes;
- (c)
- Remove the mother cell from the list;
- (d)
- Add two new cells to the list.
- If the number of sides is odd, then
- (a)
- Connect the middle of the longest side with the middle of the longest opposite side (choose one of two);
- (b)
- Create two new nodes;
- (c)
- Remove the mother cell from the list;
- (d)
- Add two new cells to the list.
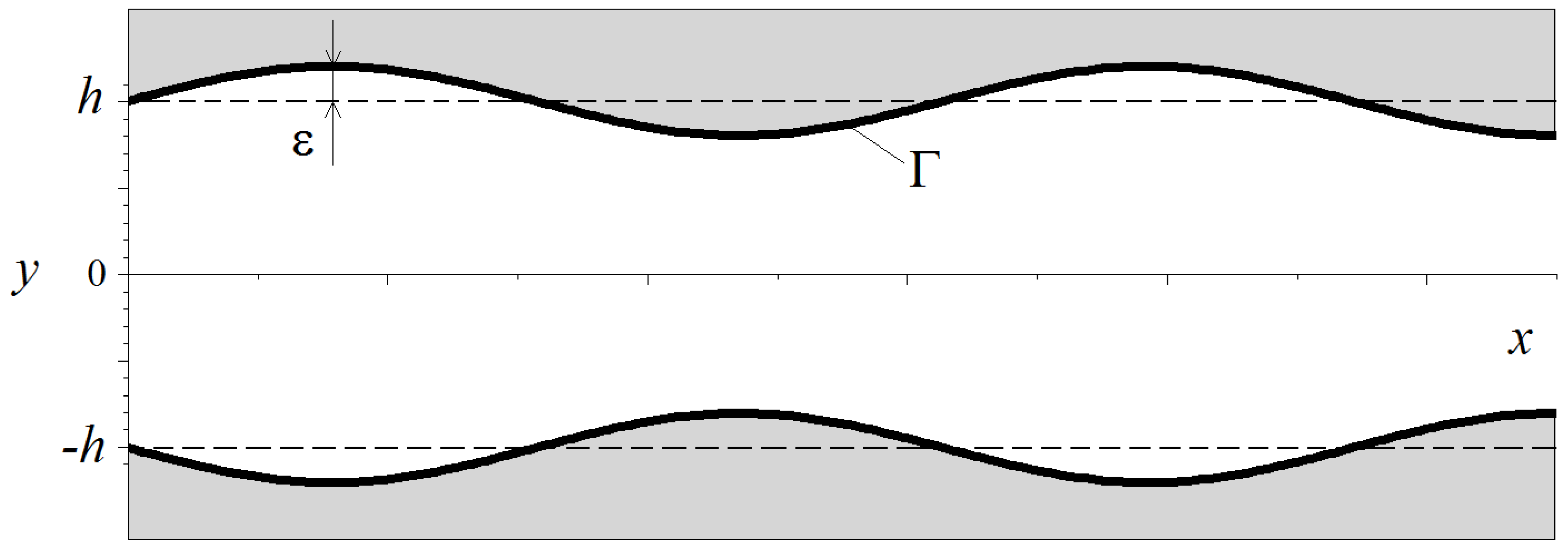
2.2. Hydrodynamic Model for Shear Stresses
2.3. Numerical Method and Quantitative Measurements
3. Numerical Results
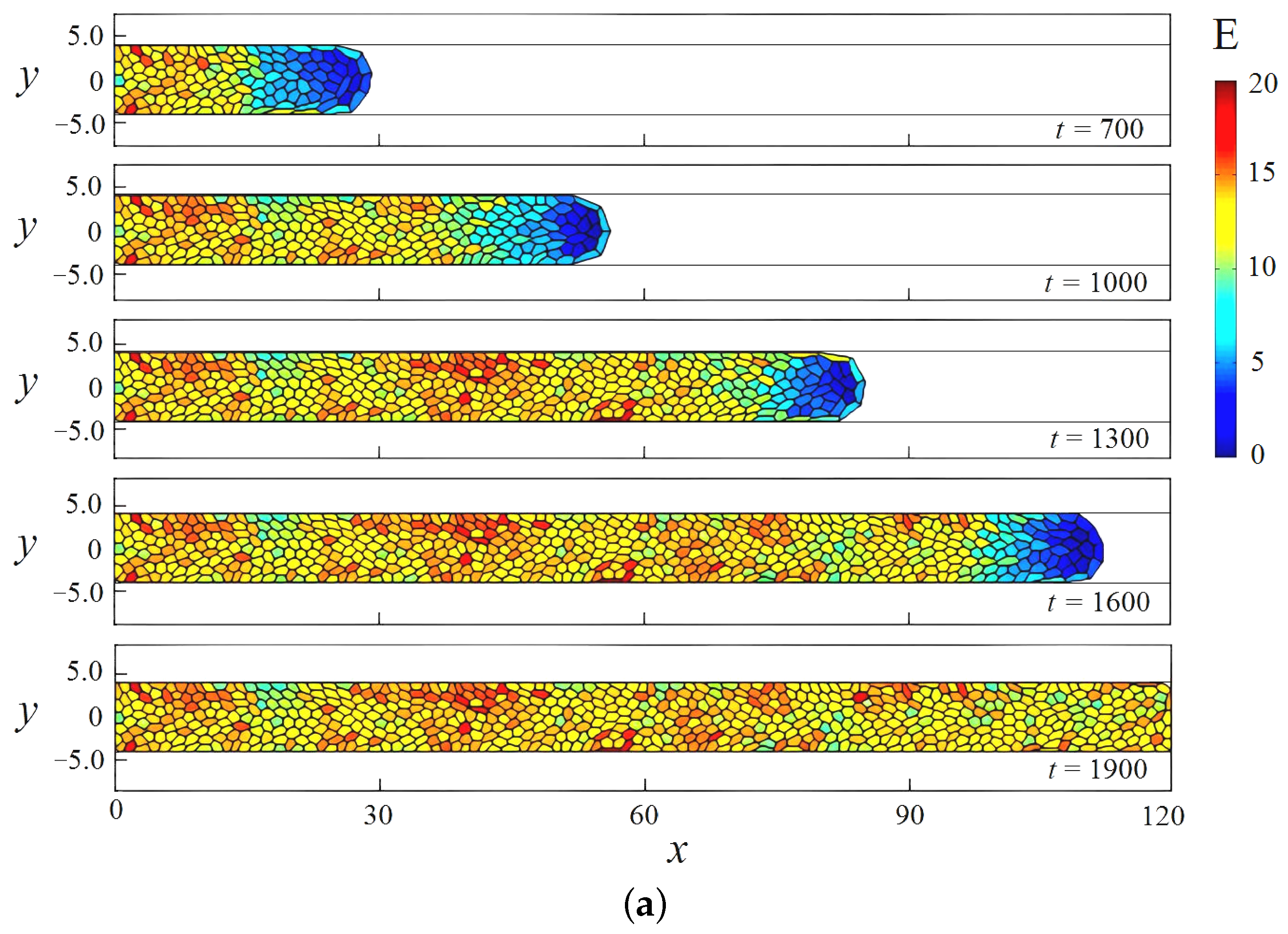
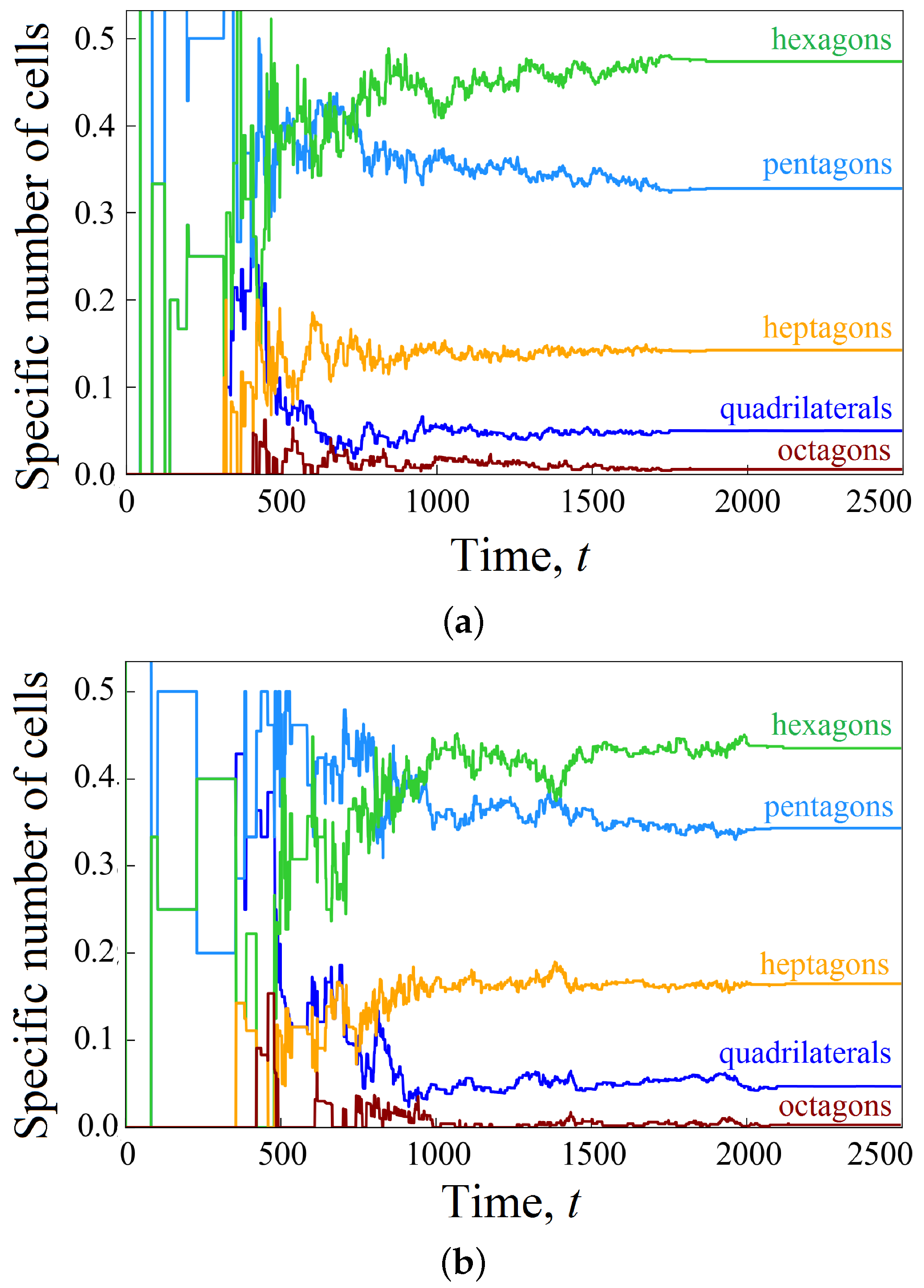
3.1. Uniform Cell Growth in a Scaffold Pore
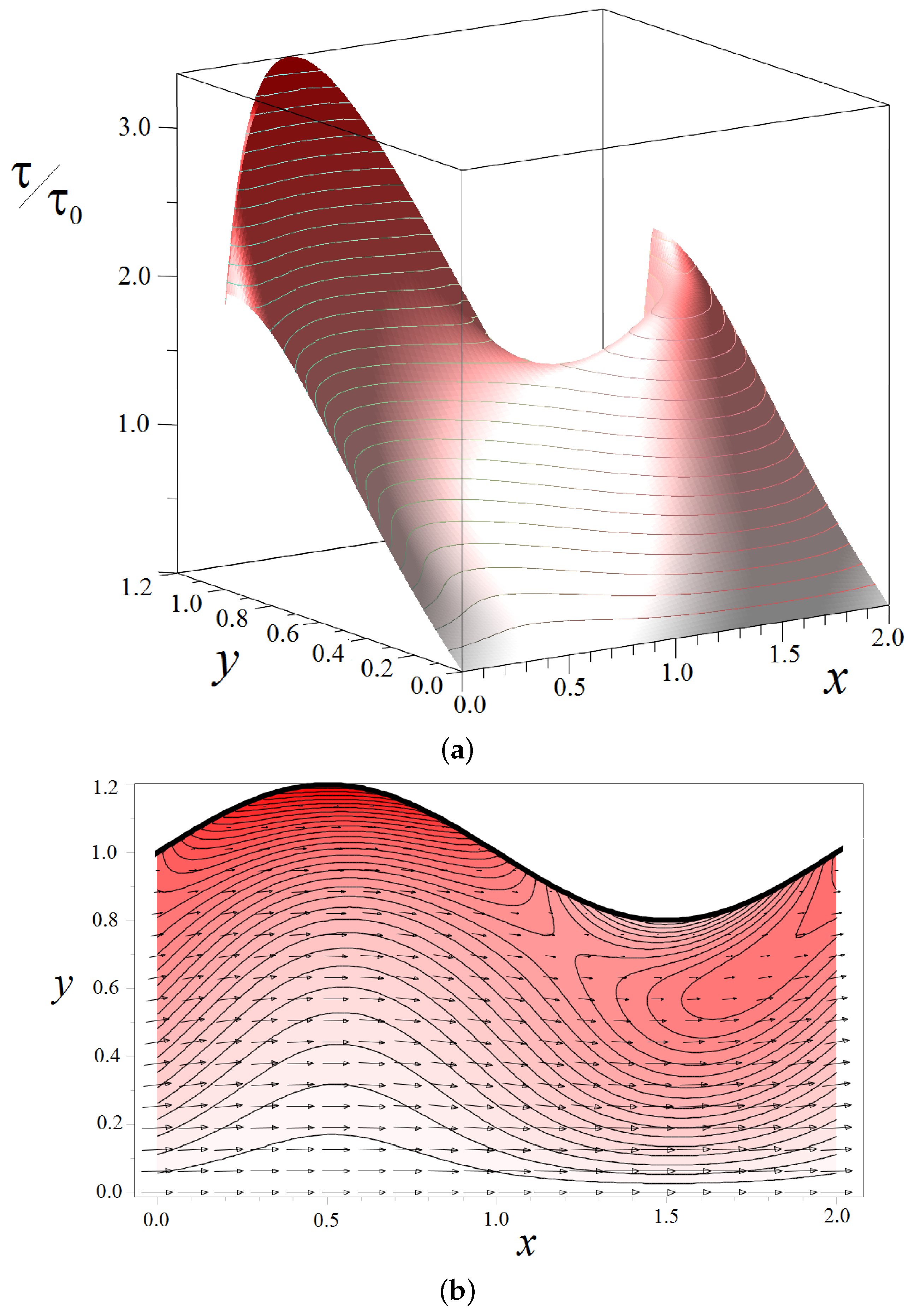
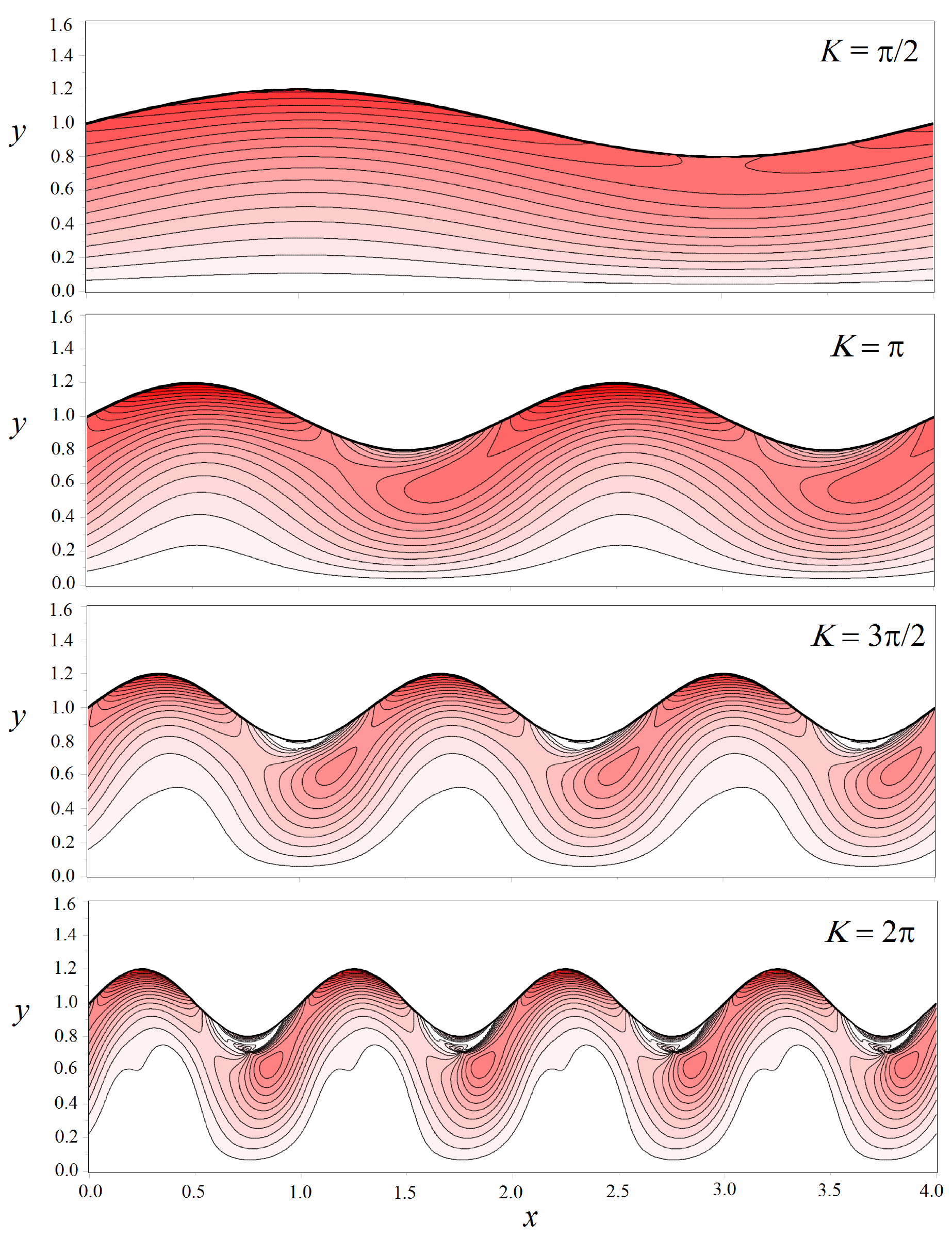
3.2. Shear Stresses in the Fluid Flow through a Wavy Channel
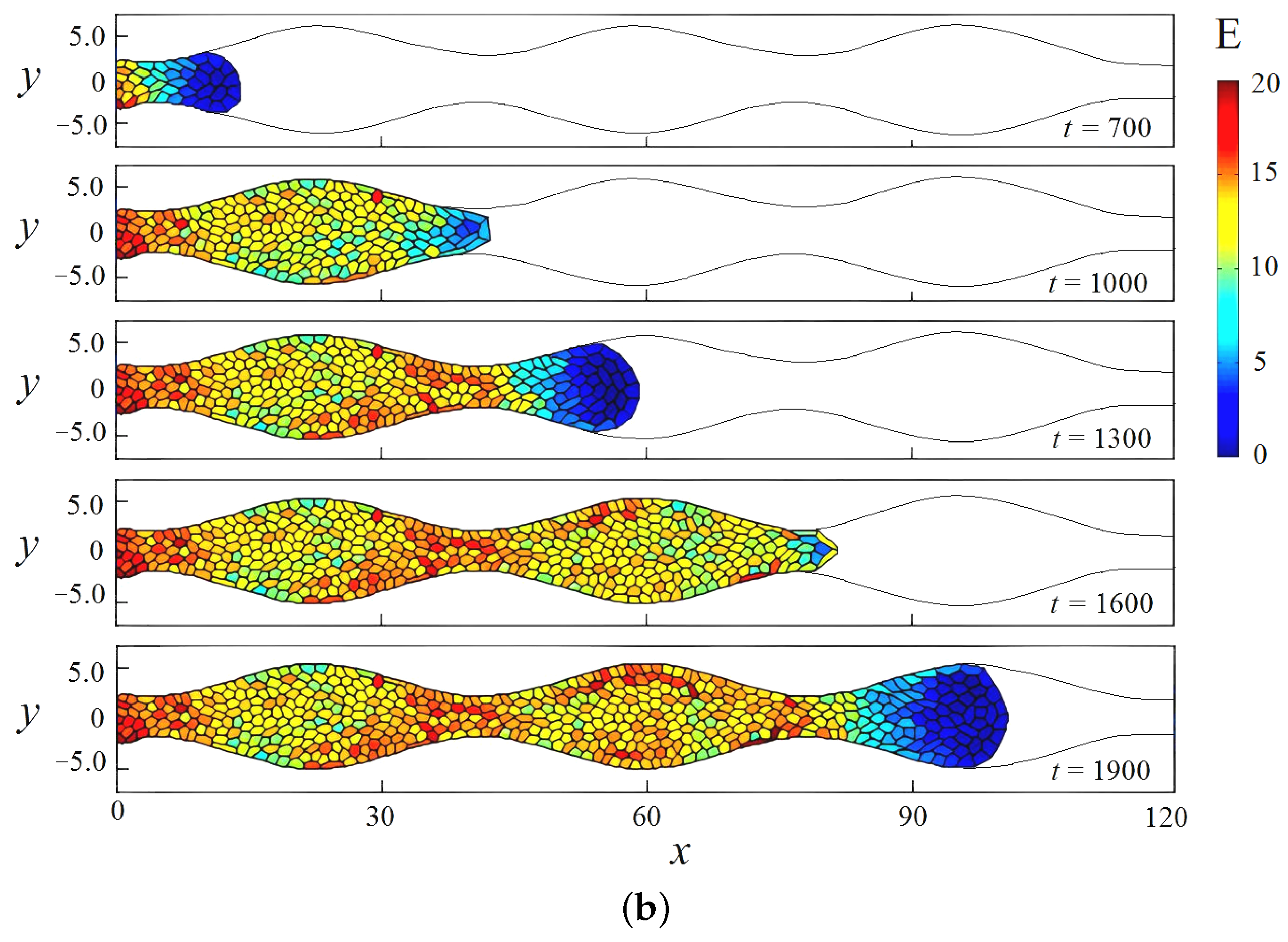
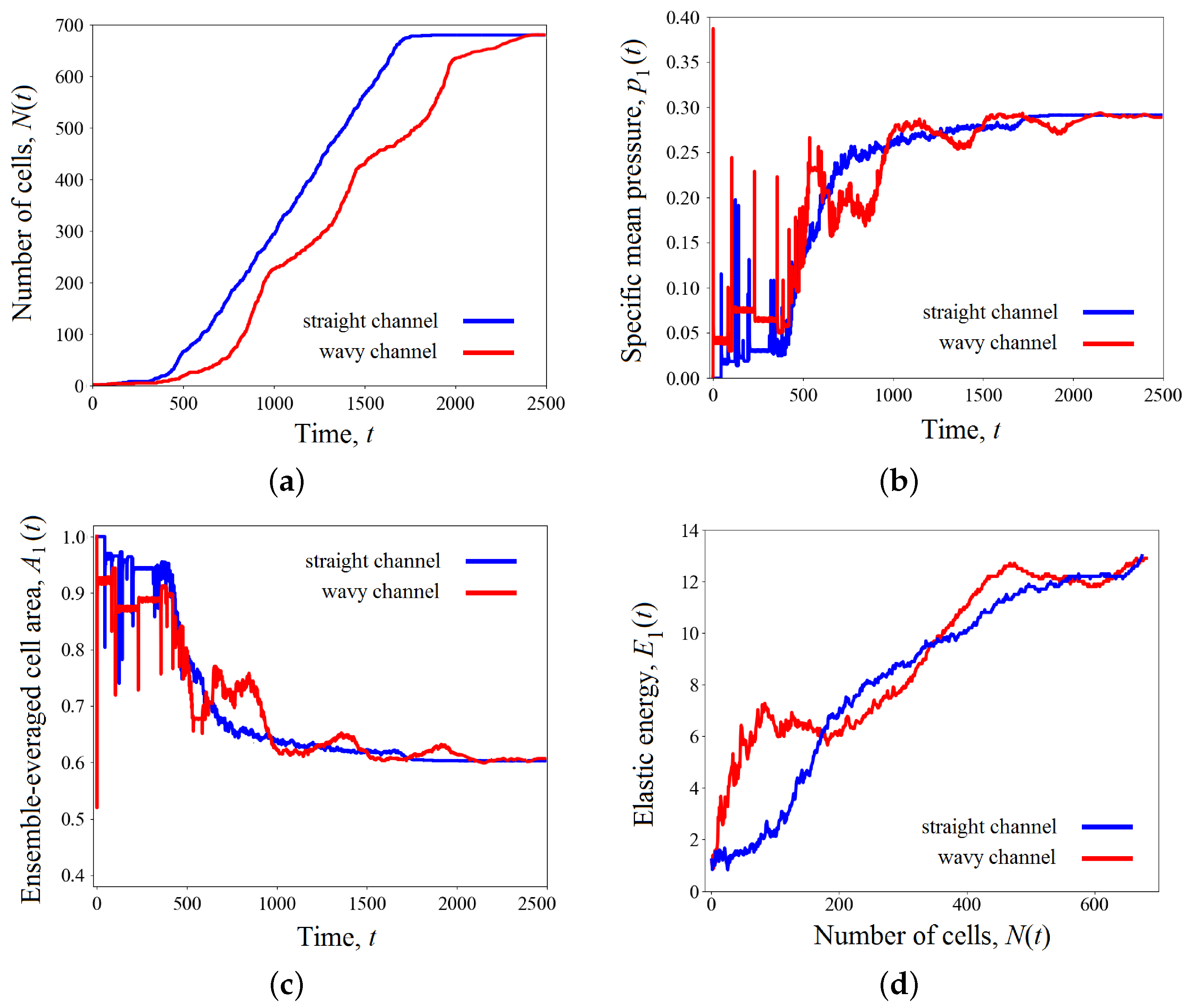
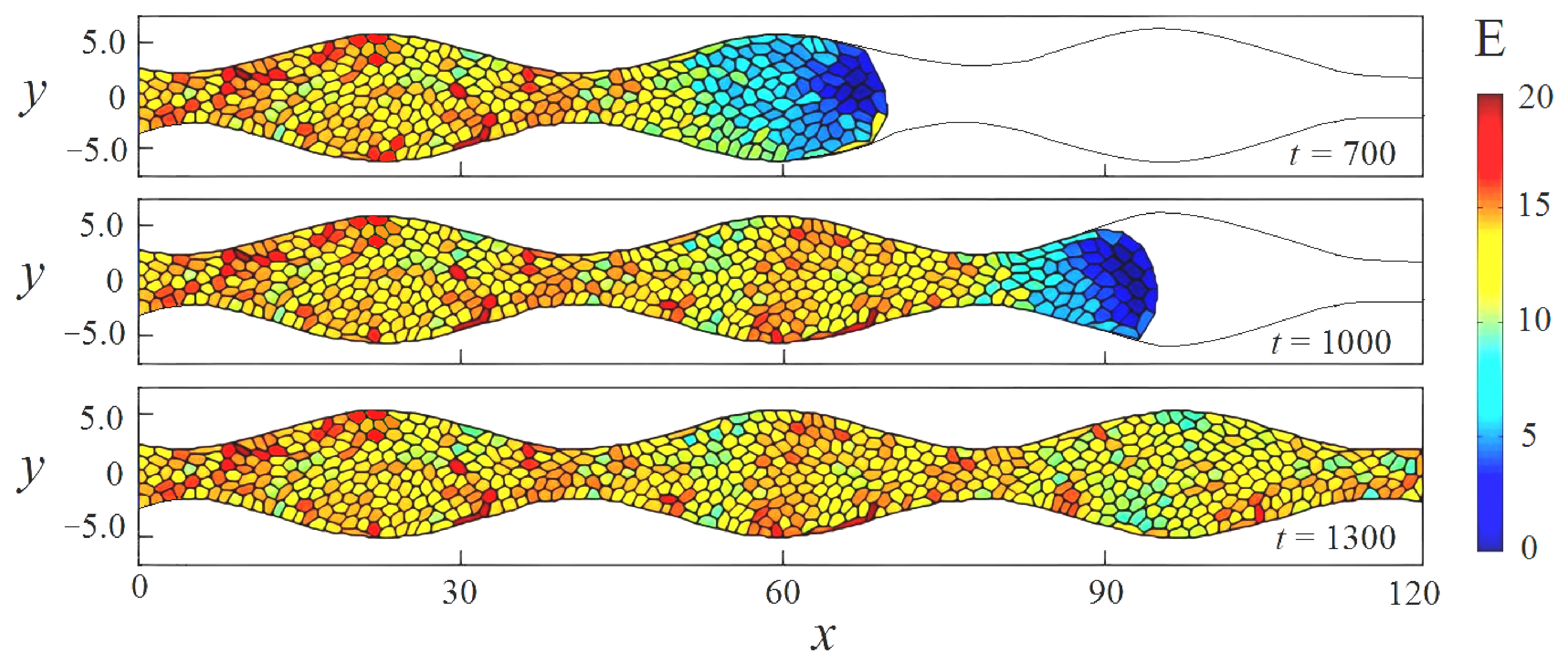
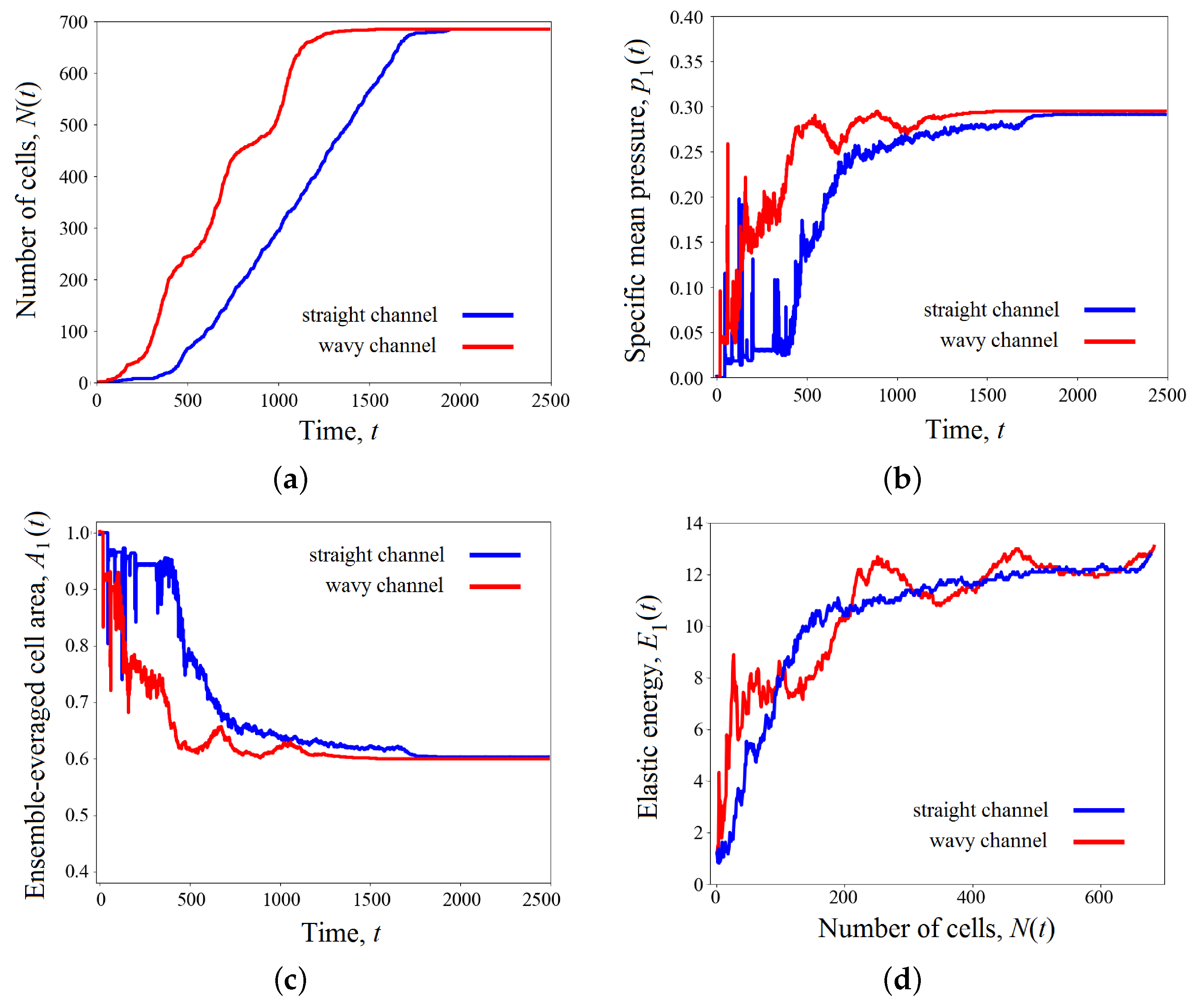
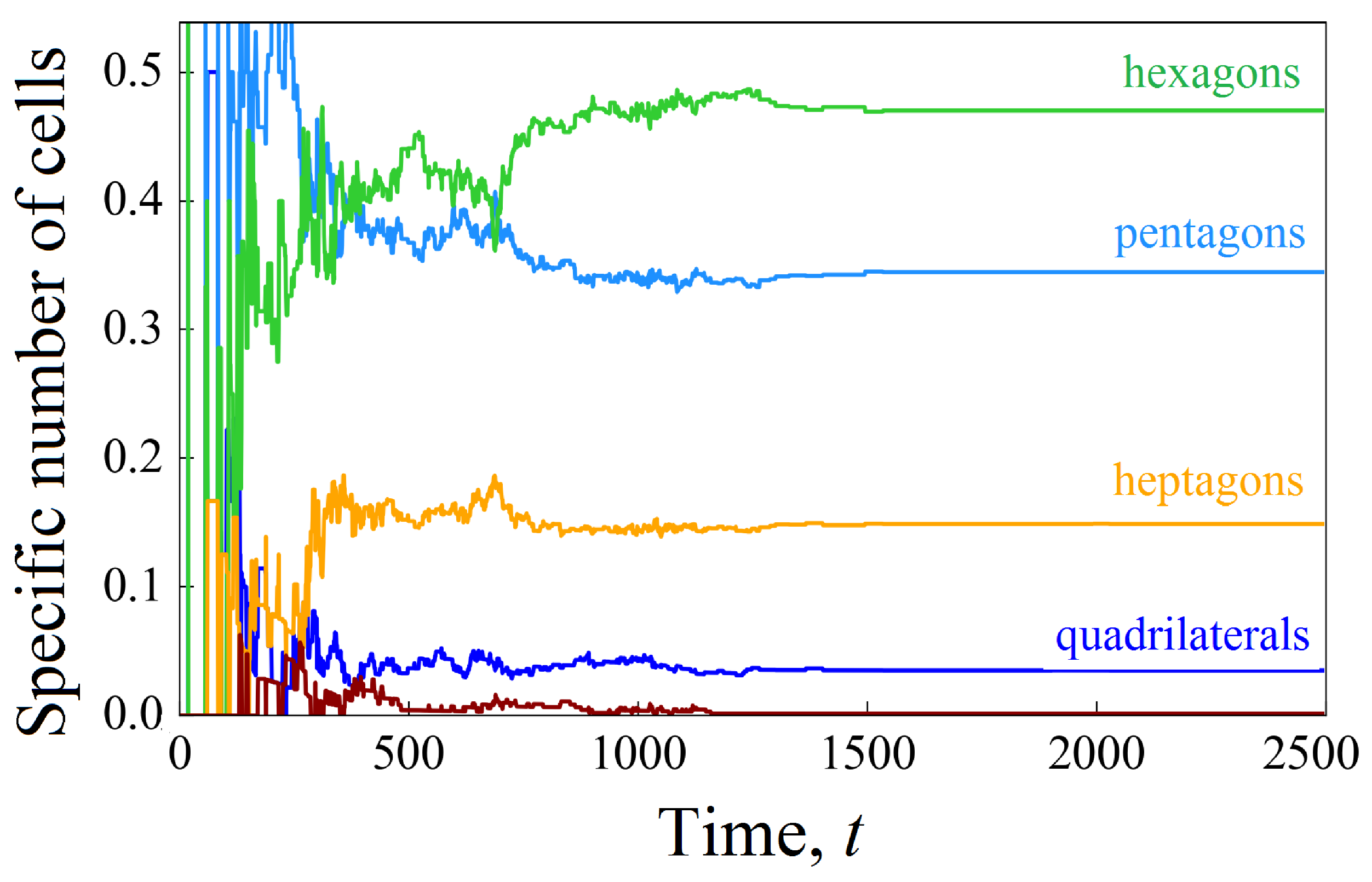
3.3. Cell Growth in a Scaffold Pore Affected by Shear Stresses
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Pallua, N.; Suscheck, C.V. Tissue Engineering; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Slack, J.M.W. Essential Developmental Biology; Wiley-Blackwell: Oxford, UK, 2013. [Google Scholar]
- Kesharwani, R.K.; Keservani, R.K.; Sharma, A.K. Tissue Engineering: Applications and Advancements; Apple Academic Press: Palm Bay, FL, USA, 2022. [Google Scholar]
- Mertsching, H.; Schanz, J.; Steger, V.; Schandar, M.; Schenk, M.; Hansmann, J.; Dally, I.; Friedel, G.; Walles, T. Generation and transplantation of an autologous vascularized bioartificial human tissue. Transplantation 2009, 88, 203–210. [Google Scholar] [CrossRef]
- Mokhtari-Jafari, F.; Amoabediny, G.; Haghighipour, N.; Zarghami, R.; Saatchi, A.; Akbari, J.; Salehi-Nik, N. Mathematical modeling of cell growth in a 3D scaffold and validation of static and dynamic cultures. Eng. Life Sci. 2016, 16, 290–298. [Google Scholar] [CrossRef]
- Porter, B.; Zauel, R.; Stockman, H.; Guldberg, R. 3-D computational modeling of media flow through scaffolds in a perfusion bioreactor. J. Biomech. 2005, 38, 543–549. [Google Scholar] [CrossRef]
- Zhao, F.; Vaughan, T.; McNamara, L. Quantification of Fluid Shear Stress in Bone Tissue Engineering Scaffolds with Spherical and Cubical Pore Architectures. Biomech. Model. Mechanobiol. 2016, 15, 561–577. [Google Scholar] [CrossRef]
- Blecha, L.D.; Rakotomanana, L.; Razafimahery, F.; Terrier, A. Mechanical interaction between cells and fluid for bone tissue engineering scaffold modulation of the interfacial shear stress. J. Biomech. 2010, 43, 933–937. [Google Scholar] [CrossRef]
- Yeatts, A.; Fisher, J. Bone tissue engineering bioreactors: Dynamic culture and the influence of shear stress. Bone 2011, 48, 171–181. [Google Scholar] [CrossRef]
- Jiang, G.; White, C.; Stevens, H.; Frangos, J. Temporal gradients in shear stimulate osteoblastic proliferation via erk1/2 and retinoblastoma protein. Am. J. Physiol. Endocrinol. 2002, 283, E383–E389. [Google Scholar] [CrossRef]
- Cartmell, S.; Porter, B.; Garcia, A.; Guldberg, R. Effects of medium perfusion rate on cell-seeded three-dimensional bone constructs in vitro. Tissue Eng. 2003, 9, 1197–1203. [Google Scholar] [CrossRef]
- Nokhbatolfoghahaei, H.; Bohlouli, M.; Adavi, K.; Paknejad, Z.; Rezai Rad, M.; Khani, M.M.; Salehi-Nik, N.; Khojasteh, A. Computational modeling of media flow through perfusion-based bioreactors for bone tissue engineering. Proc. Inst. Mech. Eng. H 2020, 234, 1397–1408. [Google Scholar] [CrossRef]
- Zhao, F.; Xiong, Y.; Ito, K.; van Rietbergen, B.; Hofmann, S. Porous Geometry Guided Micro-mechanical Environment Within Scaffolds for Cell Mechanobiology Study in Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 736489. [Google Scholar] [CrossRef]
- Freyman, T.M.; Yannas, I.V.; Pek, Y.S.; Yokoo, R.; Gibson, L. Micromechanics of fibroblast contraction of a collagen-GAG matrix. Exp. Cell Res. 2001, 269, 140–153. [Google Scholar] [CrossRef]
- Jungreuthmayer, C.; Jaasma, M.J.; Al-Munajjed, A.; Zanghellini, J.; Kelly, D.; O’Brien, F. Deformation simulation of cells seeded on a collagen-GAG scaffold in a flow perfusion bioreactor using a sequential 3D CFD-elastostatics model. Med. Eng. Phys. 2009, 31, 420–427. [Google Scholar] [CrossRef]
- Mccoy, R.J.; Jungreuthmayer, C.; O’Brien, F.J. Influence of Flow Rate and Scaffold Pore Size on Cell Behavior During Mechanical Stimulation in a Flow Perfusion Bioreactor. Biotechnol. Bioeng. 2012, 109, 1583–1594. [Google Scholar] [CrossRef]
- Guo, R.; Lu, S.; Page, J.M.; Merkel, A.; Basu, S.; Sterling, J. Fabrication of 3D Scaffolds with Precisely Controlled Substrate Modulus and Pore Size by Templated-Fused Deposition Modeling to Direct Osteogenic Differentiation. Adv. Healthc. Mater. 2015, 4, 1826–1832. [Google Scholar] [CrossRef]
- Yamashita, T.; Kollmannsberger, P.; Mawatari, K.; Kitamori, T.; Vogel, V. Cell Sheet Mechanics: How Geometrical Constraints Induce the Detachment of Cell Sheets from Concave Surfaces. Acta Biomater. 2016, 45, 85–97. [Google Scholar] [CrossRef]
- Wohlgemuth, M.; Yufa, N.; Hoffman, J.; Thomas, E.L. Triply periodic bicontinuous cubic microdomain morphologies by symmetries. Macromolecules 2001, 34, 6083–6089. [Google Scholar] [CrossRef]
- Liu, F.; Mao, Z.; Zhang, P.; Zhang, D.Z.; Jiang, J.; Ma, Z. Functionally graded porous scaffolds in multiple patterns: New design method, physical and mechanical properties. Mater. Des. 2018, 160, 849–860. [Google Scholar] [CrossRef]
- Zhao, F.; Lacroix, D.; Ito, K.; van Rietbergen, B.; Hofmann, S. Changes in Scaffold Porosity during Bone Tissue Engineering in Perfusion Bioreactors Considerably Affect Cellular Mechanical Stimulation for Mineralization. Bone Rep. 2020, 12, 100265. [Google Scholar] [CrossRef]
- Elenskaya, N.; Tashkinov, M. Numerical simulation of deformation behavior of additively manufactured polymer lattice structures with a porosity gradient. Procedia Struct. Integr. 2022, 37, 692–697. [Google Scholar] [CrossRef]
- Yuan, L.; Ding, S.; Wen, C. Additive manufacturing technology for porous metal implant applications and triple minimal surface structures: A review. Bioact. Mater. 2019, 4, 56–70. [Google Scholar] [CrossRef]
- Abdel-Wahab, A.; Alam, K.; Silberschmidt, V. Analysis of anisotropic viscoelastoplastic properties of cortical bone tissues. J. Mech. Behav. Biomed. Mater. 2011, 4, 807–820. [Google Scholar] [CrossRef]
- Li, S.; Abdel-Wahab, A.; Silberschmidt, V. Analysis of fracture processes in cortical bone tissue. Eng. Fract. Mech. 2013, 110, 448–458. [Google Scholar] [CrossRef]
- Elsayed, Y.; Lekakou, C.; Tomlins, P. Modeling, simulations, and optimization of smooth muscle cell tissue engineering for the production of vascular grafts. Biotechnol. Bioeng. 2019, 116, 1509–1522. [Google Scholar] [CrossRef]
- Egan, P.; Shea, K.; Ferguson, S. Simulated Tissue Growth for 3D Printed Scaffolds. Biomech. Model. Mechanobiol. 2018, 17, 1481–1495. [Google Scholar] [CrossRef]
- Nava, M.; Raimondi, M.; Pietrabissa, R. A multiphysics 3D model of tissue growth under interstitial perfusion in a tissue-engineering bioreactor. Biomech. Model. Mechanobiol. 2013, 12, 1169–1179. [Google Scholar] [CrossRef]
- Ait Mahiout, L.; Kazmierczak, B.; Volpert, V. Viral Infection Spreading and Mutation in Cell Culture. Mathematics 2022, 10, 256. [Google Scholar] [CrossRef]
- Wilson, D.; King, J.; Byrne, H. Modelling Scaffold Occupation by a Growing, Nutrient-Rich Tissue. Math. Model. Methods Appl. Sci. 2007, 17, 1721–1750. [Google Scholar] [CrossRef]
- Vedula, S.; Leong, M.; Lai, T.; Hersen, P.; Kabla, A.; Lim, C.; Ladoux, B. Emerging modes of collective cell migration induced by geometrical constraints. Proc. Natl. Acad. Sci. USA 2012, 109, 12974–12979. [Google Scholar] [CrossRef]
- Shakeel, M. 2-D coupled computational model of biological cell proliferation and nutrient delivery in a perfusion bioreactor. Math. Biosci. 2013, 242, 86–94. [Google Scholar] [CrossRef]
- Liu, R.; Song, K.; Hu, Z.; Cao, W.; Shuai, J.; Chen, S.; Nan, H.; Zheng, Y.; Jiang, X.; Zhang, H.; et al. Diversity of collective migration patterns of invasive breast cancer cells emerging during microtrack invasion. Phys. Rev. E 2019, 99, 062403. [Google Scholar] [CrossRef]
- Bouchnita, A.; Bocharov, G.; Meyerhans, A.; Volpert, V. Hybrid approach to model the spatial regulation of T cell responses. BMC Immunol. 2017, 18, 29. [Google Scholar] [CrossRef]
- Neumann, J.v. Theory of Self-Reproducing Automata; University of Illinois: Chicago, IL, USA, 1966. [Google Scholar]
- Simpson, M.; Landman, K.; Hughes, B. Distinguishing between directed and undirected cell motility within an invading cell population. Bull. Math. Biol. 2009, 71, 781–799. [Google Scholar] [CrossRef]
- Ruben, I.; Reinaldo, R.R.; Fernando, V.R.; Ariel, R.T.; Ribeiro, C.; Canci, A. Tumor growth modelling by cellular automata. Math. Mech. Complex Syst. 2017, 5, 239–259. [Google Scholar] [CrossRef]
- Bodenstein, L. A dynamic simulation model of tissue growth and cell patterning. Cell Differ. 1986, 19, 19–33. [Google Scholar] [CrossRef]
- Basan, M.; Prost, J.; Joanny, J.F.; Elgeti, J. Dissipative particle dynamics simulations for biological tissues: Rheology and competition. Phys. Biol. 2011, 8, 026014. [Google Scholar] [CrossRef]
- Bessonova, N.; Pujo-Menjouet, L.; Volpert, V. Cell Modelling of Hematopoiesis. Math. Model. Nat. Phenom. 2008, 2, 81–103. [Google Scholar] [CrossRef]
- Alt, S.; Ganguly, P.; Salbreux, G. Vertex models: From cell mechanics to tissue morphogenesis. Phil. Trans. R. Soc. B 2016, 372, 20150520. [Google Scholar] [CrossRef]
- Nagai, T.; Honda, H. A dynamic cell model for the formation of epithelial tissues. Philos. Mag. B 2001, 81, 699–719. [Google Scholar] [CrossRef]
- Salbreux, G.; Barthel, L.; Raymond, P.; Lubensky, D. Coupling mechanical deformations and planar cell polarity to create regular patterns in the zebrafish retina. PLoS Comput Biol. 2012, 8, e1002618. [Google Scholar] [CrossRef]
- Bratsun, D.; Krasnyakov, I.; Pismen, L. Biomechanical modeling of invasive breast carcinoma under a dynamic change in cell phenotype: Collective migration of large groups of cells. Biomech. Model. Mechanobiol. 2020, 19, 723–743. [Google Scholar] [CrossRef]
- Misra, M.; Audoly, B.; Kevrekidis, I.; Shvartsman, S. Shape Transformations of Epithelial Shells. Biophys. J. 2016, 110, 1670–1678. [Google Scholar] [CrossRef]
- Salm, M.; Pismen, L. Chemical and mechanical signaling in epithelial spreading. Phys. Biol. 2012, 9, 026009–026023. [Google Scholar] [CrossRef]
- Bratsun, D.; Merkuriev, D.; Zakharov, A.; Pismen, L. Multiscale modeling of tumor growth induced by circadian rhythm disruption in epithelial tissue. J. Biol. Phys. 2016, 42, 107–132. [Google Scholar] [CrossRef]
- Bratsun, D. Protein pattern formation induced by the joint effect of noise and delay in a multicellular system. Math. Model. Nat. Phenom. 2022, 17, 16. [Google Scholar] [CrossRef]
- Krasnyakov, I.; Bratsun, D.; Pismen, L. Mathematical modelling of epithelial tissue growth. Russ. J. Biomech. 2020, 24, 375–388. [Google Scholar]
- Bratsun, D.; Krasnyakov, I. Modeling the cellular microenvironment near a tissue-liquid interface during cell growth in a porous scaffold. Interfacial Phenom. Heat Transf. 2022, 10, 25–44. [Google Scholar] [CrossRef]
- Ji, S.; Guvendiren, M. 3D Printed Wavy Scaffolds Enhance Mesenchymal Stem Cell Osteogenesis. Micromachines 2019, 11, 31. [Google Scholar] [CrossRef]
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef]
- Chavey, D. Tilings by regular polygons—II: A catalog of tilings. Comput Math Appl. 1989, 17, 147–165. [Google Scholar] [CrossRef]
- Miklius, M.P.; Hilgenfeldt, S. Epithelial tissue statistics: Eliminating bias reveals morphological and morphogenetic features. Eur. Phys. J. E 2011, 34, 50. [Google Scholar] [CrossRef]
- Chan, K.; Yan, C.C.; Roan, H. Skin cells undergo asymmetric fission to expand body surfaces in zebrafish. Nature 2022, 605, 119–125. [Google Scholar] [CrossRef]
- Tsangaris, S.; Leiter, E. On laminar steady flow in sinusoidal channels. J. Eng. Math. 1984, 18, 89–103. [Google Scholar] [CrossRef]
- Nikolic, D.L.; Boettiger, A.N.; Bar-Sagi, D.; Carbeck, J.D.; Shvartsman, S.Y. Role of boundary conditions in an experimental model of epithelial wound healing. Am. J. Physiol. Cell Physiol. 2006, 291, 68–75. [Google Scholar] [CrossRef]


| k | a | q | m | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.0 | 4.0 | 0.5 | 0.1 | 2 | 6 | 1.4 | 0.7 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krasnyakov, I.; Bratsun, D. Cell-Based Modeling of Tissue Developing in the Scaffold Pores of Varying Cross-Sections. Biomimetics 2023, 8, 562. https://doi.org/10.3390/biomimetics8080562
Krasnyakov I, Bratsun D. Cell-Based Modeling of Tissue Developing in the Scaffold Pores of Varying Cross-Sections. Biomimetics. 2023; 8(8):562. https://doi.org/10.3390/biomimetics8080562
Chicago/Turabian StyleKrasnyakov, Ivan, and Dmitry Bratsun. 2023. "Cell-Based Modeling of Tissue Developing in the Scaffold Pores of Varying Cross-Sections" Biomimetics 8, no. 8: 562. https://doi.org/10.3390/biomimetics8080562






