Automatic Assist Level Adjustment Function of a Gait Exercise Rehabilitation Robot with Functional Electrical Stimulation for Spinal Cord Injury: Insights from Clinical Trials
Abstract
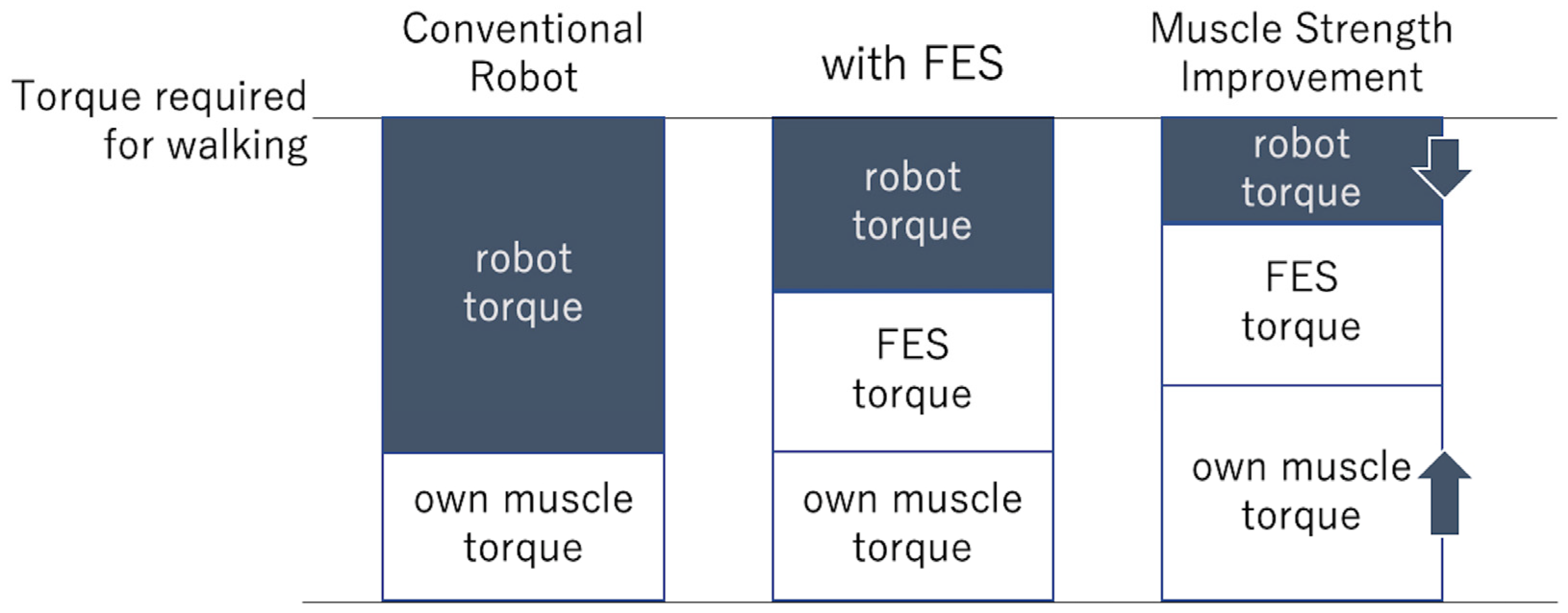
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Spinal Cord Injury. Available online: https://www.who.int/news-room/fact-sheets/detail/spinal-cord-injury (accessed on 5 September 2024).
- Miyakoshi, N.; Suda, K.; Kudo, D.; Sakai, H.; Nakagawa, Y.; Mikami, Y.; Suzuki, S.; Tokioka, T.; Tokuhiro, A.; Takei, H.; et al. A nationwide survey on the incidence and characteristics of traumatic spinal cord injury in Japan in 2018. Spinal Cord 2021, 59, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Hubli, M.; Dietz, V. The physiological basis of neurorehabilitation—Locomotor training after spinal cord injury. J. Neuroeng. Rehabil. 2013, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Veerbeek, J.M.; van Wegen, E.; van Peppen, R.; van der Wees, P.J.; Hendriks, E.; Rietberg, M.; Kwakkel, G. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS ONE 2014, 9, e87987. [Google Scholar] [CrossRef]
- Holsheimer, J. Concepts and methods in neuromodulation and functional electrical stimulation: An introduction. Neuromodulation 1998, 1, 57–61. [Google Scholar] [CrossRef]
- Illis, L.S. Central nervous system regeneration does not occur. Spinal Cord 2012, 50, 259–263. [Google Scholar] [CrossRef]
- Kralj, A.; Bajd, T.; Turk, R. Enhancement of gait restoration in spinal injured patients by functional electrical stimulation. Clin. Orthop. Relat. Res. 1988, 233, 34–43. [Google Scholar] [CrossRef]
- Andrews, B.J.; Baxendale, R.H.; Barnett, R. Hybrid FES orthosis incorporating closed loop control and sensory feedback. J. Biomed. Eng. 1988, 10, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Solomonow, M.; Baratta, R.; Hirokawa, S.; Rightor, N.; Walker, W.; Beaudette, P.; Shoji, H.; D’Ambrosia, R. The RGO generation II: Muscle stimulation powered orthosis as a practical walking system for thoracic paraplegics. Orthopedics 1989, 12, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.H.; Ko, Y.J.; Chang, W.H.; Lee, J.H.; Lee, K.B.; Park, Y.J.; Ha, H.G.; Kim, Y.H. Effects of Robot-assisted Gait Training Combined with Functional Electrical Stimulation on Recovery of Locomotor Mobility in Chronic Stroke Patients: A Randomized Controlled Trial. J. Phys. Ther. Sci. 2014, 26, 1949–1953. [Google Scholar] [CrossRef]
- Schicketmueller, A.; Rose, G.; Hofmann, M. Feasibility of a Sensor-Based Gait Event Detection Algorithm for Triggering Functional Electrical Stimulation during Robot-Assisted Gait Training. Sensors 2019, 19, 4804. [Google Scholar] [CrossRef]
- Bao, X.; Molazadeh, V.; Dodson, A.; Dicianno, B.E.; Sharma, N. Using person-specific muscle fatigue characteristics to optimally allocate control in a hybrid exoskeleton—Preliminary results. IEEE Trans. Med. Robot. Bionics 2020, 2, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Patathong, T.; Klaewkasikum, K.; Woratanarat, P.; Rattanasiri, S.; Anothaisintawee, T.; Woratanarat, T.; Thakkinstian, A. The efficacy of gait rehabilitations for the treatment of incomplete spinal cord injury: A systematic review and network meta-analysis. J. Orthop. Surg. Res. 2023, 18, 60. [Google Scholar] [CrossRef]
- Kirsch, N.A.; Bao, X.; Alibeji, N.A.; Dicianno, B.E.; Sharma, N. Model-based dynamic control allocation in a hybrid neuroprosthesis. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ren, Y.; Gui, K.; Jia, J.; Xu, W. Cooperative control for a hybrid rehabilitation system combining functional electrical stimulation and robotic exoskeleton. Front. Neurosci. 2017, 11, 725. [Google Scholar] [CrossRef]
- del-Ama, A.J.; Gil-Agudo, Á.; Pons, J.L.; Moreno, J.C. Hybrid FES–robot cooperative control of ambulatory gait rehabilitation exoskeleton. J. Neuroeng. Rehabil. 2014, 11, 27. [Google Scholar] [CrossRef]
- Bersch, I.; Alberty, M.; Fridén, J. Robot-assisted training with functional electrical stimulation enhances lower extremity function after spinal cord injury. Artif. Organs 2022, 46, 2009–2014. [Google Scholar] [CrossRef] [PubMed]
- Kimura, R.; Matsunaga, T.; Iwami, T.; Kudo, D.; Saitoh, K.; Hatakeyama, K.; Watanabe, M.; Takahashi, Y.; Miyakoshi, N.; Shimada, Y. Development of a Rehabilitation Robot Combined with Functional Electrical Stimulation Controlled by Non-disabled Lower Extremity in Hemiplegic Gait. Prog. Rehabil. Med. 2018, 3, 20180005. [Google Scholar] [CrossRef]
- Inoue, J.; Kimura, R.; Shimada, Y.; Saito, K.; Kudo, D.; Hatakeyama, K.; Watanabe, M.; Maeda, K.; Iwami, T.; Matsunaga, T.; et al. Development of a gait rehabilitation robot using an exoskeleton and functional electrical stimulation: Validation in a pseudo-paraplegic model. Prog. Rehabil. Med. 2022, 7, 20220001. [Google Scholar] [CrossRef]
- Maeda, K.; Iwami, T.; Kimura, R.; Shimada, Y. Development of an automatic adjustment system for the amount of assist using reinforcement leaning in gait rehabilitation robot for hemiplegic patients. Jpn. J. Inst. Ind. Appl. Eng. 2022, 10, 28–37. (In Japanese) [Google Scholar]
- Sutton, R.S.; Barto, A.G. Reinforcement Learning: An Introduction, 2nd ed.; MIT Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Marquez-Chin, C.; Popovic, M.R. Functional electrical stimulation therapy for restoration of motor function after spinal cord injury and stroke: A review. Biomed. Eng. Online 2020, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, N.; Masani, K.; Craven, C.; Giangregorio, L.M.; Hitzig, S.L.; Richards, K.; Popovic, M.R. A randomized trial of functional electrical stimulation for walking in incomplete spinal cord injury: Effects on walking competency. J. Spinal Cord. Med. 2014, 37, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Pizzolato, C.; Saxby, D.J.; Palipana, D.; Diamond, L.E.; Barrett, R.S.; Teng, Y.D.; Lloyd, D.G. Neuromusculoskeletal modeling-based prostheses for recovery after spinal cord injury. Front. Neurorobot 2019, 13, 97. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Androwis, G.; Adamovich, S.; Nunez, E.; Su, H.; Zhou, X. Robust walking control of a lower limb rehabilitation exoskeleton coupled with a musculoskeletal model via deep reinforcement learning. J. Neuroeng. Rehabil. 2023, 20, 34. [Google Scholar] [CrossRef]
- Xu, J.; Xu, L.; Cheng, G.; Shi, J.; Liu, J.; Liang, X.; Fan, S. A robotic system with reinforcement learning for lower extremity hemiparesis rehabilitation. Ind. Robot. Int. J. Robot. Res. Appl. 2021, 48, 388–400. [Google Scholar] [CrossRef]
- Spiess, M.R.; Steenbrink, F.; Esquenazi, A. Getting the best out of advanced rehabilitation technology for the lower limbs: Minding motor learning principles. PM&R 2018, 10, S165–S173. [Google Scholar] [CrossRef]
- Gonzalez-Vazquez, A.; Garcia, L.; Kilby, J.; McNair, P. Soft wearable rehabilitation robots with artificial muscles based on smart materials: A review. Adv. Intell. Syst. 2023, 5, 2200159. [Google Scholar] [CrossRef]

| (Nm) | Case 1 | Case 2 | ||||
|---|---|---|---|---|---|---|
| FES (−) | FES (+) | p | FES (−) | FES (+) | p | |
| Hip Right | 18.6 ± 1.5 | 16.6 ± 1.7 | 0.0237 | 17.9 ± 1.2 | 8.7 ± 1.2 | <0.001 |
| Hip Left | 18.1 ± 1.4 | 16 ± 1.6 | 0.0181 | 14.7 ± 1.3 | 11.9 ± 1.7 | <0.001 |
| Knee Right | 20.3 ± 1.8 | 13.1 ± 1.5 | <0.001 | 15.4 ± 1.2 | 13.4 ± 1.6 | 0.0047 |
| Knee Left | 19.2 ± 1.4 | 17.1 ± 1.9 | 0.0226 | 18.4 ± 1.5 | 12.2 ± 1.3 | <0.001 |
| (Nm) | Case 1 | Case 2 | ||||
|---|---|---|---|---|---|---|
| FES (−) | FES (+) | p | FES (−) | FES (+) | p | |
| Hip Right | 28.5 ± 0.5 | 26.5 ± 0.5 | <0.001 | 42.9 ± 0.3 | 30.9 ± 0.7 | <0.001 |
| Hip Left | 24.1 ± 0.6 | 23.4 ± 0.7 | 0.0445 | 38.9 ± 0.3 | 30.9 ± 0.3 | <0.001 |
| Knee Right | 30.8 ± 0.4 | 23.9 ± 0.3 | <0.001 | 25.4 ± 0.5 | 20.6 ± 0.5 | <0.001 |
| Knee Left | 29.5 ± 0.5 | 17.5 ± 0.5 | <0.001 | 33.9 ± 0.3 | 21.4 ± 0.5 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimura, R.; Sato, T.; Kasukawa, Y.; Kudo, D.; Iwami, T.; Miyakoshi, N. Automatic Assist Level Adjustment Function of a Gait Exercise Rehabilitation Robot with Functional Electrical Stimulation for Spinal Cord Injury: Insights from Clinical Trials. Biomimetics 2024, 9, 621. https://doi.org/10.3390/biomimetics9100621
Kimura R, Sato T, Kasukawa Y, Kudo D, Iwami T, Miyakoshi N. Automatic Assist Level Adjustment Function of a Gait Exercise Rehabilitation Robot with Functional Electrical Stimulation for Spinal Cord Injury: Insights from Clinical Trials. Biomimetics. 2024; 9(10):621. https://doi.org/10.3390/biomimetics9100621
Chicago/Turabian StyleKimura, Ryota, Takahiro Sato, Yuji Kasukawa, Daisuke Kudo, Takehiro Iwami, and Naohisa Miyakoshi. 2024. "Automatic Assist Level Adjustment Function of a Gait Exercise Rehabilitation Robot with Functional Electrical Stimulation for Spinal Cord Injury: Insights from Clinical Trials" Biomimetics 9, no. 10: 621. https://doi.org/10.3390/biomimetics9100621
APA StyleKimura, R., Sato, T., Kasukawa, Y., Kudo, D., Iwami, T., & Miyakoshi, N. (2024). Automatic Assist Level Adjustment Function of a Gait Exercise Rehabilitation Robot with Functional Electrical Stimulation for Spinal Cord Injury: Insights from Clinical Trials. Biomimetics, 9(10), 621. https://doi.org/10.3390/biomimetics9100621





