The Influence of Physiological Blood Clot on Osteoblastic Cell Response to a Chitosan-Based 3D Scaffold—A Pilot Investigation
Abstract
1. Introduction
2. Materials And Methods
2.1. Ethics Aspects
2.2. 3D Chitosan/-TCP Scaffolds
2.3. Osteoblastic Line
2.4. “Physiological” Blood Clot Formation
2.5. Ex Vivo Assay
2.5.1. Flow Citometry
2.5.2. Cell Culture on Substrate (SCA + PhC)
2.5.3. Cell Viability
2.5.4. Scanning Electron Microscopy
2.5.5. Detection of Calcium Accumulations (Mineralized Matrix Formation)
2.5.6. Cell Morphology and Three-Dimensional Analysis of Substrate by Confocal Microscopy
2.6. Statistical Analysis
3. Results
3.1. Higher Prevalence of Platelets in PhC
3.2. Cells Viability Was More Evident When Phc Was Incorporated into the Sca
3.3. PhC Showed a Rich Fibrin Mesh
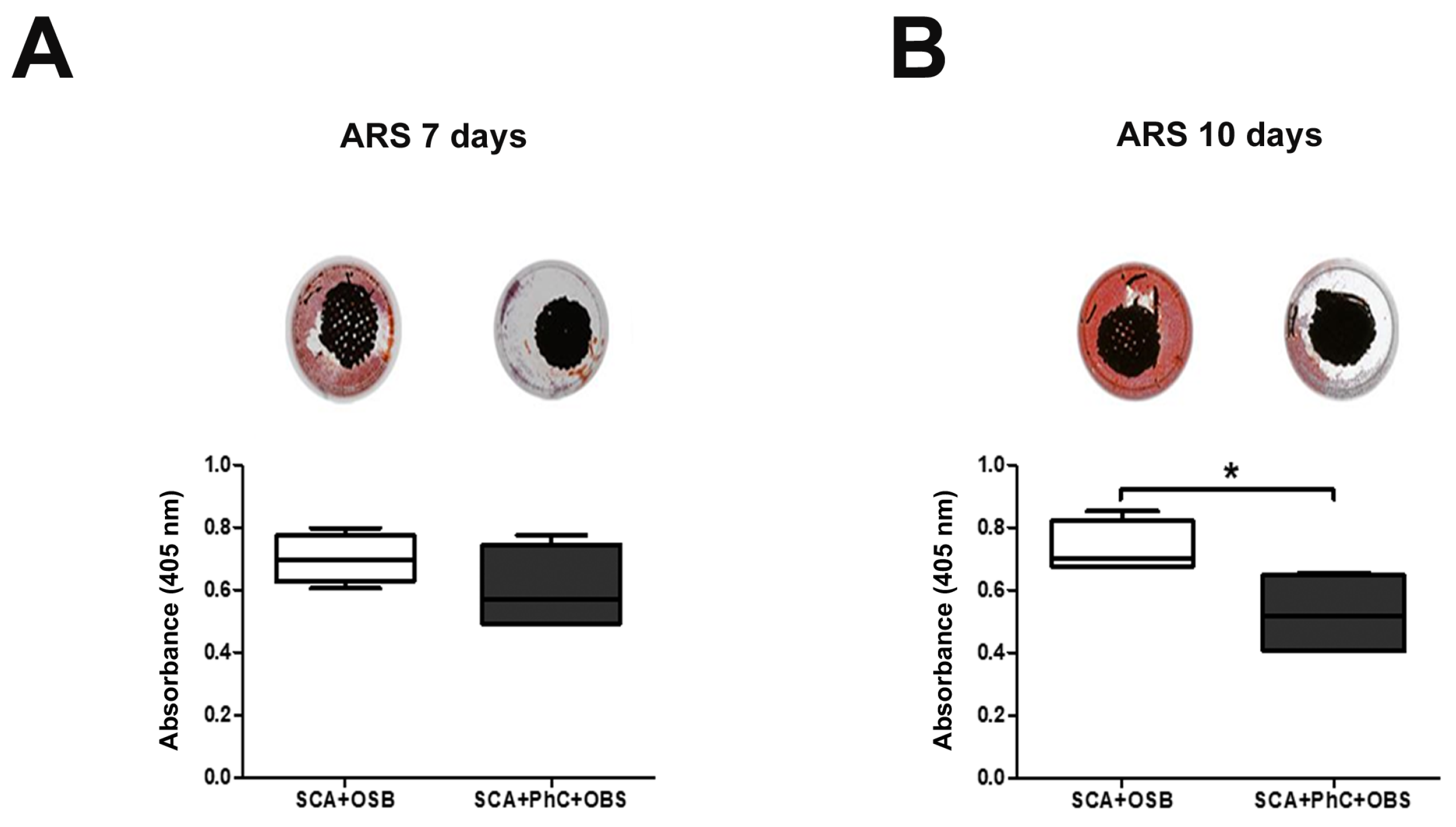
3.4. Ex Vivo Models May Delay the Mineralization Process
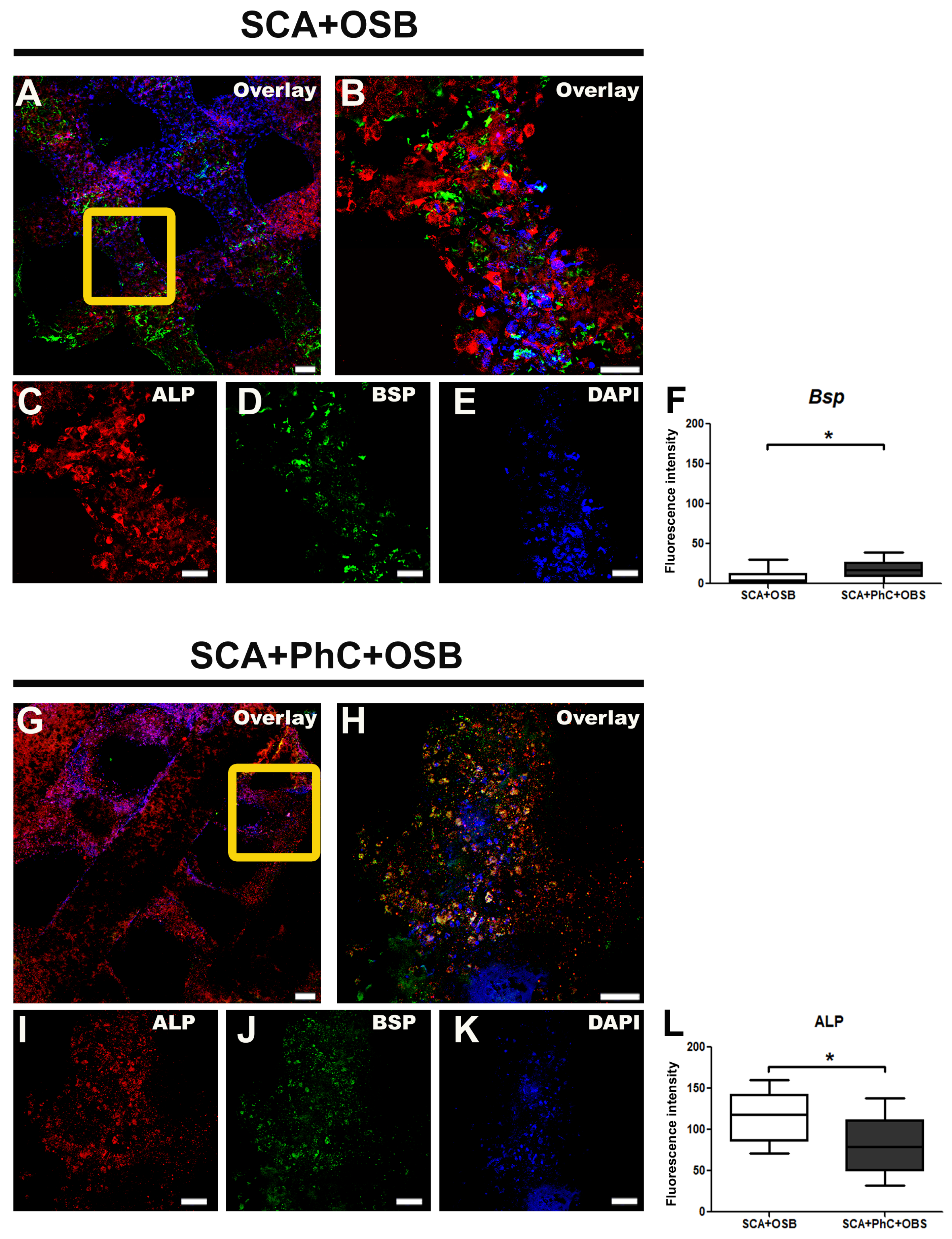
3.5. ALP and BSP Immunodetection Was Different in Each Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.; Shrestha, N.; Préat, V.; Beloqui, A. An overview of in vitro, ex vivo and in vivo models for studying the transport of drugs across intestinal barriers. Adv. Drug Deliv. Rev. 2021, 175, 113795. [Google Scholar] [CrossRef] [PubMed]
- Ud-Din, S.; Bayat, A. Non-animal models of wound healing in cutaneous repair: In silico, in vitro, ex vivo, and in vivo models of wounds and scars in human skin. Wound Repair Regen. 2017, 25, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Lebonvallet, N.; Jeanmaire, C.; Danoux, L.; Sibille, P.; Pauly, G.; Misery, L. The evolution and use of skin explants: Potential and limitations for dermatological research. Eur. J. Dermatol. 2010, 20, 671–684. [Google Scholar]
- Morozova, D.S.; Martyanov, A.A.; Obydennyi, S.I.; Korobkin, J.J.D.; Sokolov, A.V.; Shamova, E.V.; Gorudko, I.V.; Khoreva, A.L.; Shcherbina, A.; Panteleev, M.A.; et al. Ex vivo observation of granulocyte activity during thrombus formation. Bmc Biol. 2022, 20, 32. [Google Scholar] [CrossRef] [PubMed]
- Jung, O.; Barbeck, M.; Fan, L.; Korte, F.; Zhao, C.; Krastev, R.; Pantermehl, S.; Xiong, X. Republication: In Vitro and Ex Vivo Analysis of Collagen Foams for Soft and Hard Tissue Regeneration. In Vivo 2023, 37, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.P.; Miller-Dorey, S.; Jenne, C.N. Platelets and coagulation in infection. Clin. Transl. Immunol. 2016, 5, e89. [Google Scholar] [CrossRef] [PubMed]
- Nurden, A.T. Platelet membrane glycoproteins: A look back into the past and a view to the future. Thromb. Haemost. 2007, 98, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Lopes, V.R.; Birgersson, U.; Manivel, V.A.; Hulsart-Billström, G.; Gallinetti, S.; Aparicio, C.; Hong, J. Human whole blood interactions with craniomaxillofacial reconstruction materials: Exploring In vitro the role of blood cascades and leukocytes in early healing events. J. Funct. Biomater. 2023, 14, 361. [Google Scholar] [CrossRef]
- Shiu, H.T.; Goss, B.; Lutton, C.; Crawford, R.; Xiao, Y. Formation of blood clot on biomaterial implants influences bone healing. Tissue Eng. Part B Rev. 2014, 20, 697–712. [Google Scholar] [CrossRef]
- Tran, P.A. Blood clots and tissue regeneration of 3D printed dual scale porous polymeric scaffolds. Mater. Lett. 2021, 285, 129184. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. 2013, 19. [Google Scholar] [CrossRef] [PubMed]
- Cámara-Torres, M.; Sinha, R.; Mota, C.; Moroni, L. Improving cell distribution on 3D additive manufactured scaffolds through engineered seeding media density and viscosity. Acta Biomater. 2020, 101, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, Z.; Irani, S.; Ardeshirylajimi, A.; Seyedjafari, E. Enhanced osteogenic differentiation of stem cells by 3D printed PCL scaffolds coated with collagen and hydroxyapatite. Sci. Rep. 2022, 12, 12359. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, G.; Jeon, Y.C.; Koh, Y.; Kim, W. 3D polycaprolactone scaffolds with controlled pore structure using a rapid prototyping system. J. Mater. Sci. Mater. Med. 2009, 20, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Bertassoni, L.E.; Cecconi, M.; Manoharan, V.; Nikkhah, M.; Hjortnaes, J.; Cristino, A.L.; Barabaschi, G.; Demarchi, D.; Dokmeci, M.R.; Yang, Y.; et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip 2014, 14, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- Wei, S.; Ma, J.X.; Xu, L.; Gu, X.S.; Ma, X.L. Biodegradable materials for bone defect repair. Mil. Med. Res. 2020, 7, 1–25. [Google Scholar] [CrossRef]
- Parisi, L.; Galli, C.; Bianchera, A.; Lagonegro, P.; Elviri, L.; Smerieri, A.; Lumetti, S.; Manfredi, E.; Bettini, R.; Macaluso, G. Anti-fibronectin aptamers improve the colonization of chitosan films modified with D-(+) Raffinose by murine osteoblastic cells. J. Mater. Sci. Mater. Med. 2017, 28, 1–12. [Google Scholar] [CrossRef]
- Qasim, S.B.; Najeeb, S.; Delaine-Smith, R.M.; Rawlinson, A.; Rehman, I.U. Potential of electrospun chitosan fibers as a surface layer in functionally graded GTR membrane for periodontal regeneration. Dent. Mater. 2017, 33, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.; Silva, S.; Pina, C.; Tavaria, F.; Pintado, M. Evaluation and insights into chitosan antimicrobial activity against anaerobic oral pathogens. Anaerobe 2012, 18, 305–309. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. J. Pharmacol. Pharmacother. 2010, 1, 94–99. [Google Scholar] [CrossRef]
- Clements, P.J.; Bolon, B.; McInnes, E.; Mukaratirwa, S.; Scudamore, C. Chapter 17-Animal Models in Toxicologic Research: Rodents. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology, 4th ed.; Haschek, W.M., Rousseaux, C.G., Wallig, M.A., Bolon, B., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 653–694. [Google Scholar] [CrossRef]
- Zuardi, L.R.; Silva, C.L.A.; Rego, E.M.; Carneiro, G.V.; Spriano, S.; Nanci, A.; de Oliveira, P.T. Influence of a Physiologically Formed Blood Clot on Pre-Osteoblastic Cells Grown on a BMP-7-Coated Nanoporous Titanium Surface. Biomimetics 2023, 8, 123. [Google Scholar] [CrossRef]
- Elviri, L.; Foresti, R.; Bergonzi, C.; Zimetti, F.; Marchi, C.; Bianchera, A.; Bernini, F.; Silvestri, M.; Bettini, R. Highly defined 3D printed chitosan scaffolds featuring improved cell growth. Biomed. Mater. 2017, 12, 045009. [Google Scholar] [CrossRef]
- Da Costa, N.M.M.; Parisi, L.; Ghezzi, B.; Elviri, L.; de Souza, S.L.S.; Novaes, A.B.; De Oliveira, P.T.; Macaluso, G.M.; Palioto, D.B. Anti-Fibronectin Aptamer Modifies Blood Clot Pattern and Stimulates Osteogenesis: An Ex Vivo Study. Biomimetics 2023, 8, 582. [Google Scholar] [CrossRef] [PubMed]
- Monroe, D.M.; Hoffman, M. The clotting system–a major player in wound healing. Haemophilia 2012, 18, 11–16. [Google Scholar] [CrossRef]
- Ma, L.; Chang, Q.; Pei, F.; Liu, M.; Zhang, W.; Hong, Y.K.; Chai, Y.; Chen, J.F. Skull progenitor cell-driven meningeal lymphatic restoration improves neurocognitive functions in craniosynostosis. Cell Stem Cell 2023, 30, 1472–1485.e7. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Boccafoschi, F.; Rasponi, M.; Ramella, M.; Ferreira, A.M.; Vesentini, S.; Cannas, M. Short-term effects of microstructured surfaces: Role in cell differentiation toward a contractile phenotype. J. Appl. Biomater. Funct. Mater. 2015, 13, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, P.T.; Nanci, A. Nanotexturing of titanium-based surfaces upregulates expression of bone sialoprotein and osteopontin by cultured osteogenic cells. Biomaterials 2004, 25, 403–413. [Google Scholar] [CrossRef]
- Yang, Y.; Xiao, Y. Biomaterials regulating bone hematoma for osteogenesis. Adv. Healthc. Mater. 2020, 9, 2000726. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.; Schäfer, C.; Hess, S.; Folz-Donahue, K.; Nelles, M.; Minassian, A.; Schwarz, M.K.; Kukat, C.; Ehrlich, M.; Zaehres, H.; et al. The in vivo timeline of differentiation of engrafted human neural progenitor cells. Stem Cell Res. 2019, 37, 101429. [Google Scholar] [CrossRef] [PubMed]
- Strobel, L.; Johswich, K.O. Anticoagulants impact on innate immune responses and bacterial survival in whole blood models of Neisseria meningitidis infection. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- McGovern, J.A.; Griffin, M.; Hutmacher, D.W. Animal models for bone tissue engineering and modelling disease. Dis. Model. Mech. 2018, 11, dmm033084. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.; Fernandes, M. Rodent models in bone-related research: The relevance of calvarial defects in the assessment of bone regeneration strategies. Lab. Anim. 2011, 45, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Couly, G.F.; Coltey, P.M.; Douarin, N.M.L. The triple origin of skull in higher vertebrates: A study in quail-chick chimeras. Development 1993, 117, 409–429. [Google Scholar] [CrossRef] [PubMed]
- Olsen, B.R.; Reginato, A.M.; Wang, W. Bone development. Annu. Rev. Cell Dev. Biol. 2000, 16, 191–220. [Google Scholar] [CrossRef] [PubMed]
- Quarto, N.; Wan, D.C.; Kwan, M.D.; Panetta, N.J.; Li, S.; Longaker, M.T. Origin matters: Differences in embryonic tissue origin and Wnt signaling determine the osteogenic potential and healing capacity of frontal and parietal calvarial bones. J. Bone Miner. Res. 2010, 25, 1680–1694. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.; Gohlke, J.; Friis, T.; Quent, V.; Hutmacher, D. Mesodermal and neural crest derived ovine tibial and mandibular osteoblasts display distinct molecular differences. Gene 2013, 525, 99–106. [Google Scholar] [CrossRef]
- Bennett, J.S.; Berger, B.; Billings, P. The structure and function of platelet integrins. J. Thromb. Haemost. 2009, 7, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Ivanciu, L.; Stalker, T. Spatiotemporal regulation of coagulation and platelet activation during the hemostatic response in vivo. J. Thromb. Haemost. 2015, 13, 1949–1959. [Google Scholar] [CrossRef] [PubMed]
- Rendu, F.; Brohard-Bohn, B. The platelet release reaction: Granules’ constituents, secretion and functions. Platelets 2001, 12, 261–273. [Google Scholar] [CrossRef]
- Hankenson, K.; Gagne, K.; Shaughnessy, M. Extracellular signaling molecules to promote fracture healing and bone regeneration. Adv. Drug Deliv. Rev. 2015, 94, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, M.; Du, W.; Zhao, J.; Ling, G.; Zhang, P. Chitosan-based high-strength supramolecular hydrogels for 3D bioprinting. Int. J. Biol. Macromol. 2022, 219, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, J.; Jin, G.; Li, L.; Li, Z.; Li, C. Research of osteoblastic induced rat bone marrow mesenchymal stem cells cultured on β-TCP/PLLA porous scaffold. Int. J. Clin. Exp. Med. 2015, 8, 3202. [Google Scholar]
- Hatt, L.P.; van der Heide, D.; Armiento, A.R.; Stoddart, M.J. β-TCP from 3D-printed composite scaffolds acts as an effective phosphate source during osteogenic differentiation of human mesenchymal stromal cells. Front. Cell Dev. Biol. 2023, 11, 1258161. [Google Scholar] [CrossRef] [PubMed]
- Vimalraj, S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene 2020, 754, 144855. [Google Scholar] [CrossRef]
- Golub, E.E.; Boesze-Battaglia, K. The role of alkaline phosphatase in mineralization. Curr. Opin. Orthop. 2007, 18, 444–448. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Costa, N.M.M.; Caetano, H.I.P.; Aguiar, L.M.; Parisi, L.; Ghezzi, B.; Elviri, L.; Zuardi, L.R.; de Oliveira, P.T.; Palioto, D.B. The Influence of Physiological Blood Clot on Osteoblastic Cell Response to a Chitosan-Based 3D Scaffold—A Pilot Investigation. Biomimetics 2024, 9, 782. https://doi.org/10.3390/biomimetics9120782
da Costa NMM, Caetano HIP, Aguiar LM, Parisi L, Ghezzi B, Elviri L, Zuardi LR, de Oliveira PT, Palioto DB. The Influence of Physiological Blood Clot on Osteoblastic Cell Response to a Chitosan-Based 3D Scaffold—A Pilot Investigation. Biomimetics. 2024; 9(12):782. https://doi.org/10.3390/biomimetics9120782
Chicago/Turabian Styleda Costa, Natacha Malu Miranda, Hilary Ignes Palma Caetano, Larissa Miranda Aguiar, Ludovica Parisi, Benedetta Ghezzi, Lisa Elviri, Leonardo Raphael Zuardi, Paulo Tambasco de Oliveira, and Daniela Bazan Palioto. 2024. "The Influence of Physiological Blood Clot on Osteoblastic Cell Response to a Chitosan-Based 3D Scaffold—A Pilot Investigation" Biomimetics 9, no. 12: 782. https://doi.org/10.3390/biomimetics9120782
APA Styleda Costa, N. M. M., Caetano, H. I. P., Aguiar, L. M., Parisi, L., Ghezzi, B., Elviri, L., Zuardi, L. R., de Oliveira, P. T., & Palioto, D. B. (2024). The Influence of Physiological Blood Clot on Osteoblastic Cell Response to a Chitosan-Based 3D Scaffold—A Pilot Investigation. Biomimetics, 9(12), 782. https://doi.org/10.3390/biomimetics9120782






