Simulated Dopamine Modulation of a Neurorobotic Model of the Basal Ganglia
Abstract
1. Introduction
2. A Robot Model of Action Selection by the Basal Ganglia
2.1. Requirements for Effective Selection
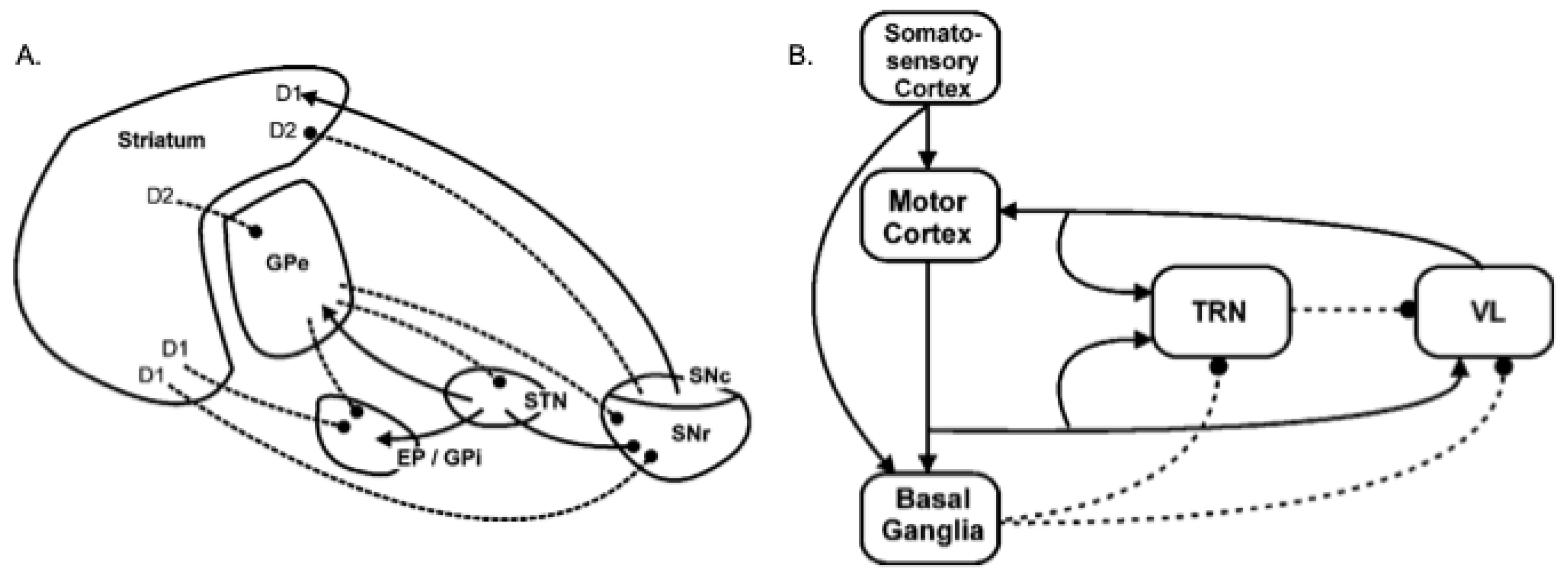
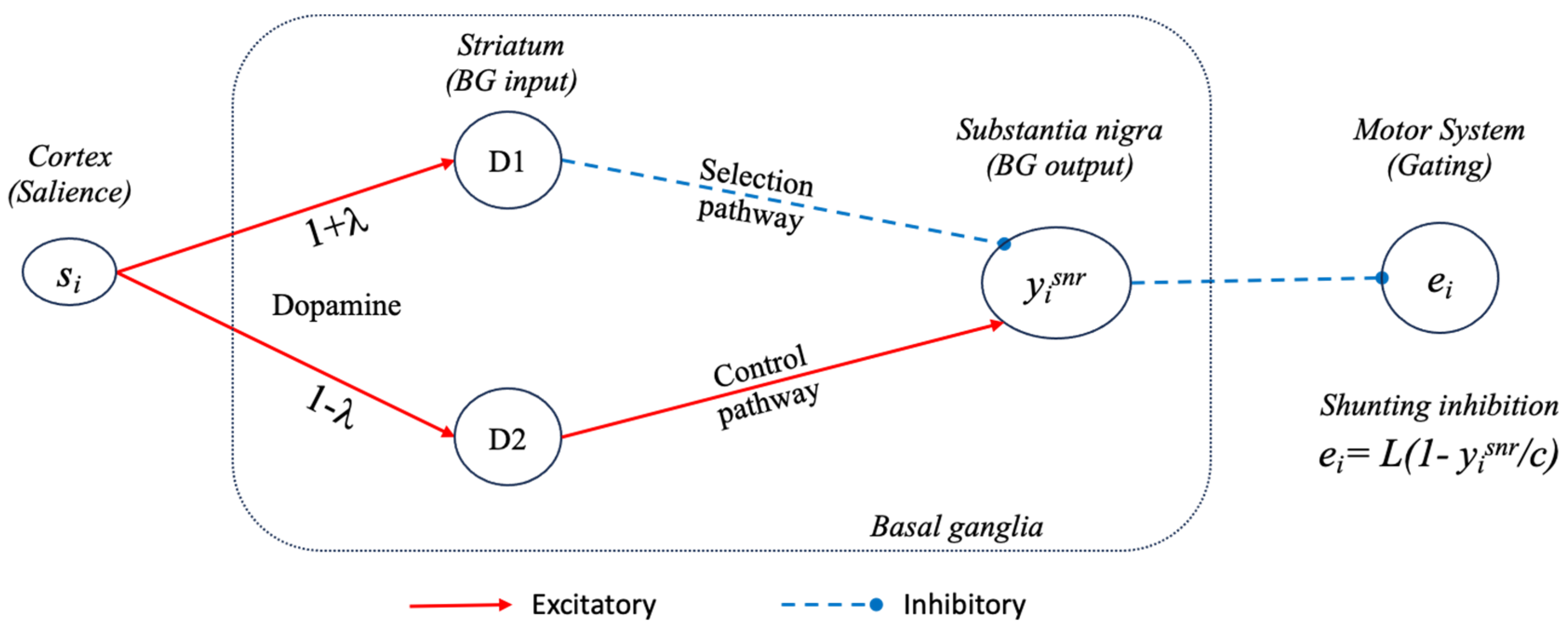
2.2. A Model of Basal Ganglia Intrinsic Circuitry
2.3. A Model of the Extended Basal Ganglia
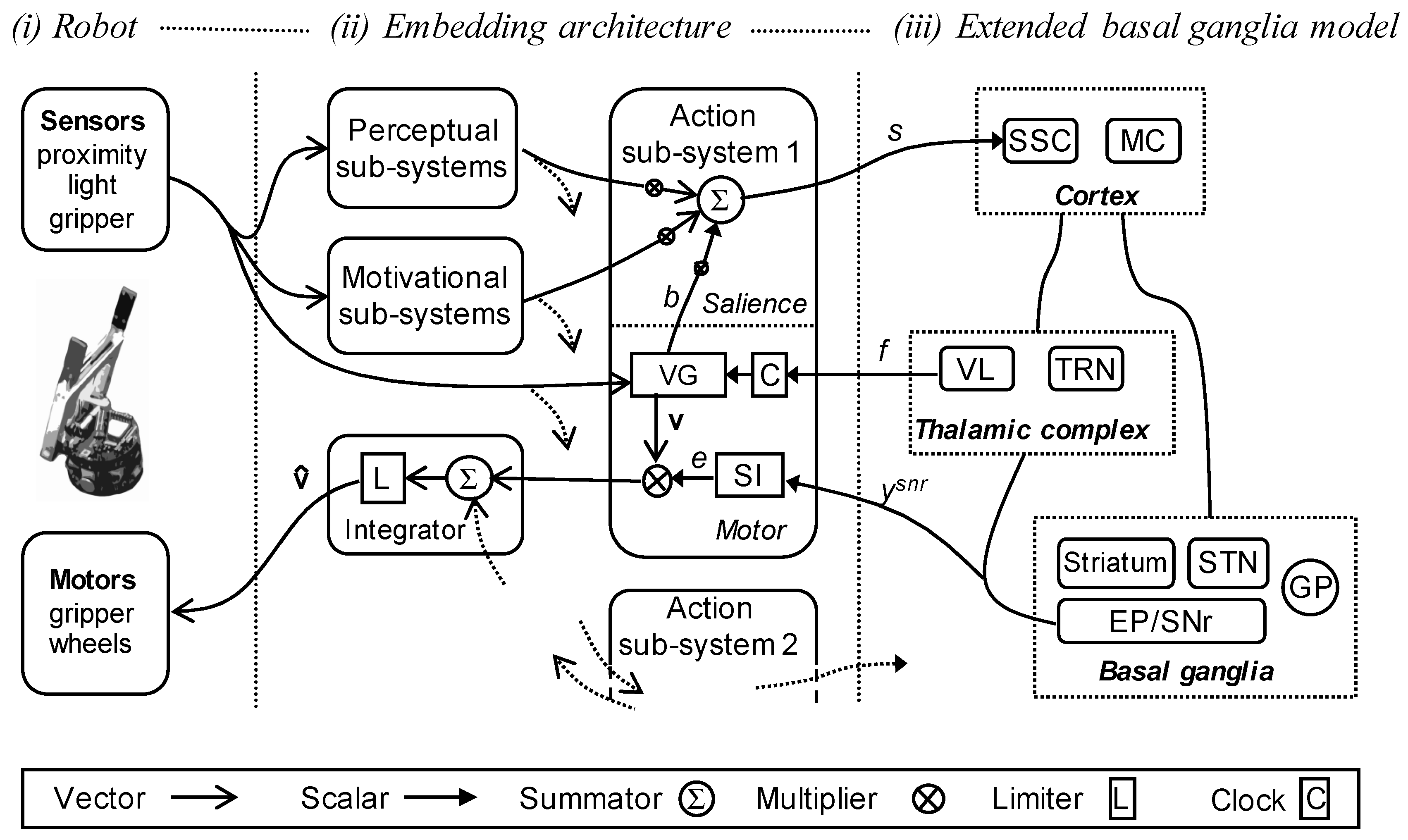
2.4. Robot Embedding of the Extended Basal Ganglia Model
3. Study 1: Tonic Dopamine Modulation in the Extended Basal Ganglia Model
3.1. Methods
3.1.1. Tonic Dopamine Modulation of the Model Basal Ganglia
3.1.2. Using Basal Ganglia Outputs as Selection Signals
3.1.3. Metrics for Measuring Effective Selection
3.1.4. Procedure
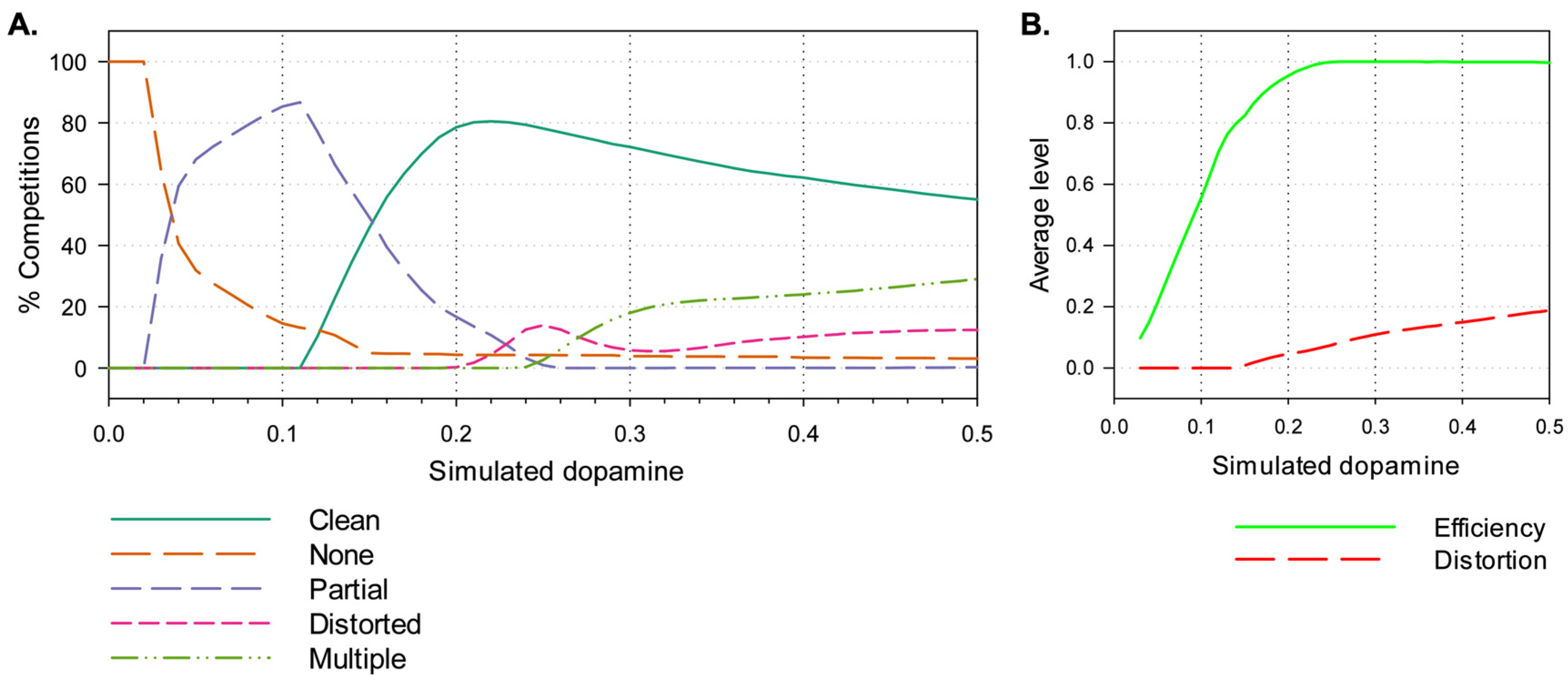
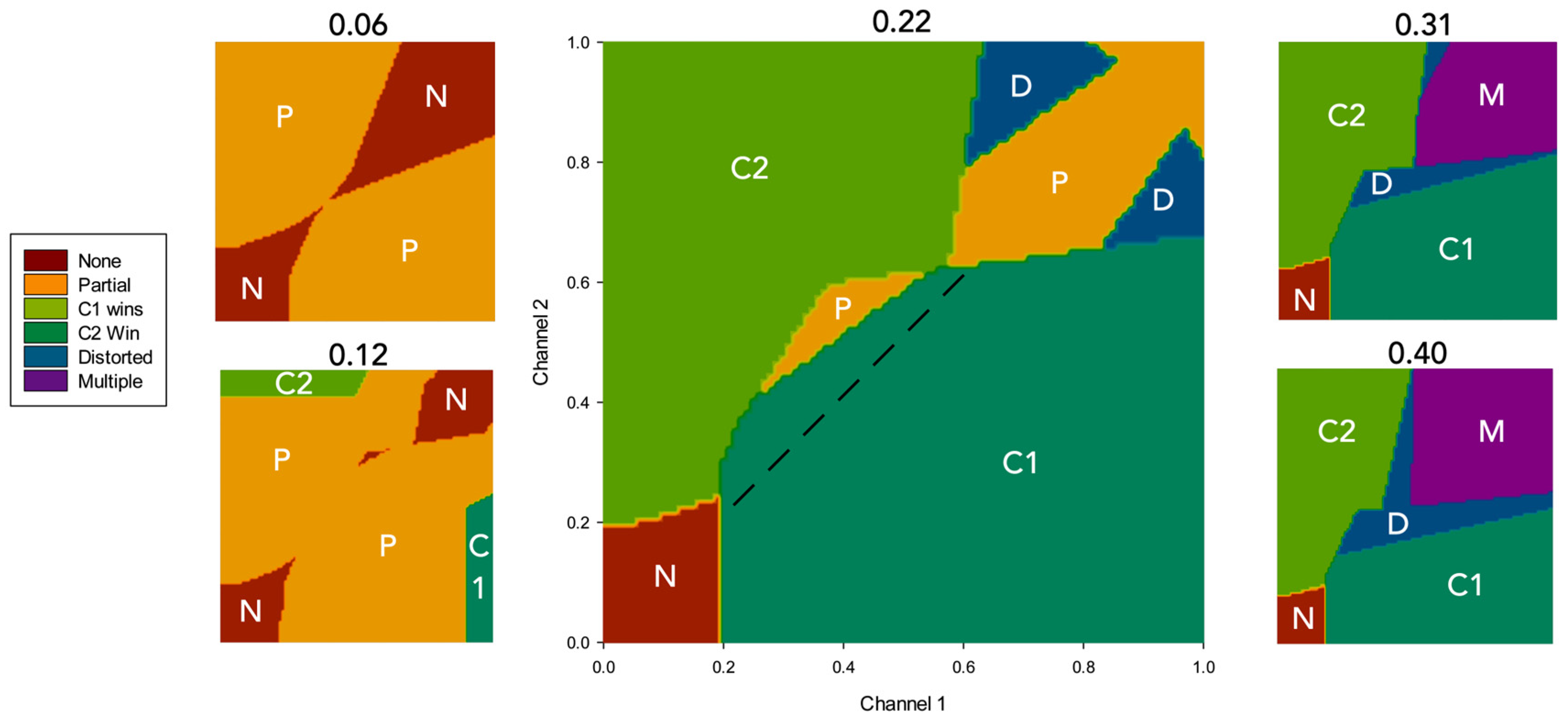
3.2. Results
4. Study 2: Selection in the Neurorobotic Basal Ganglia Model
4.1. Methods
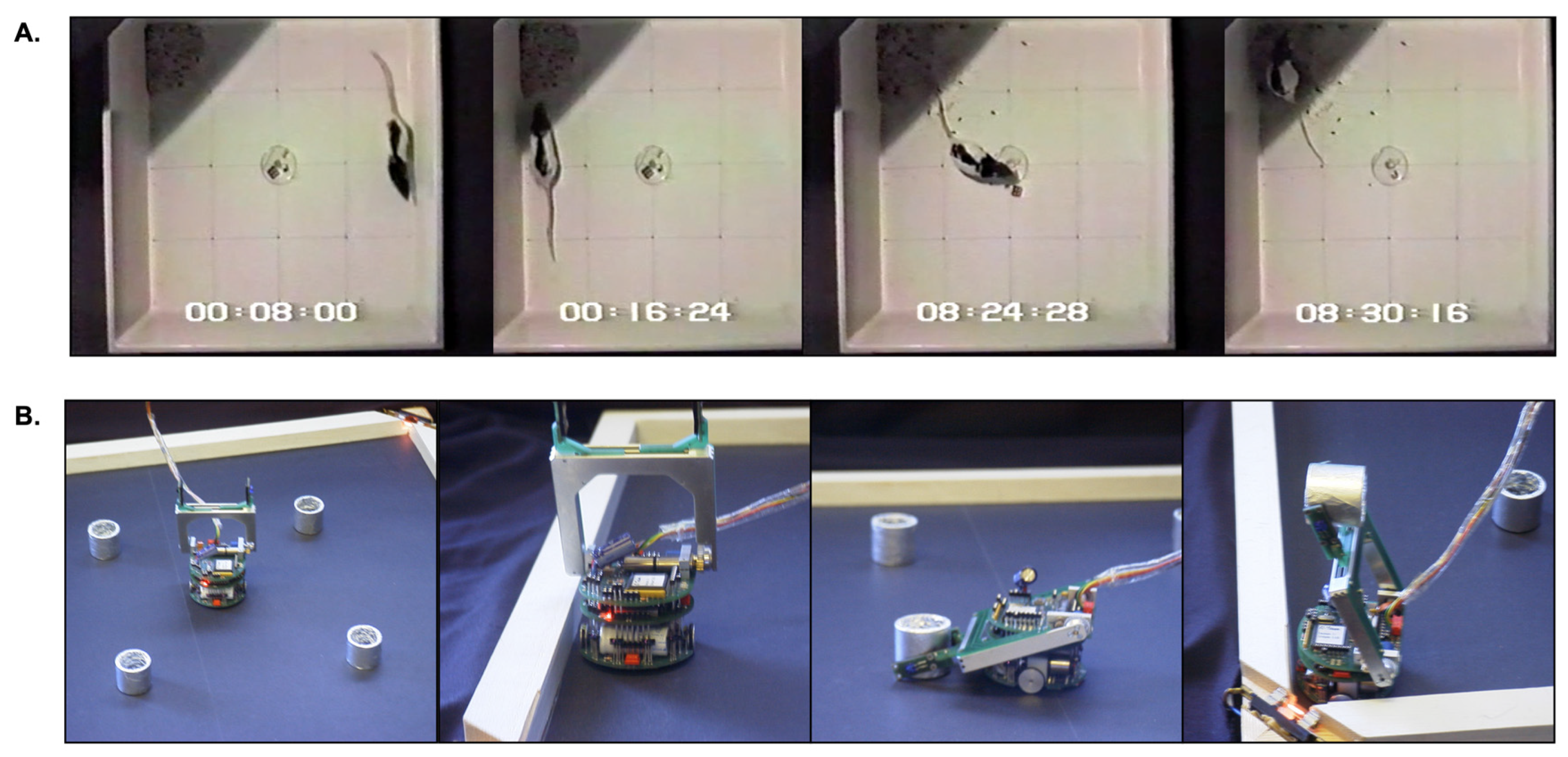
4.1.1. Measuring Effective Action Selection in the Robot Model
- (i)
- Successful avoidance is activity resulting in the discovery of a wall (ignoring any cylinders encountered en route) followed by movement covering some distance along the wall’s length.
- (ii)
- Successful foraging is activity resulting in the deposition of a cylinder in a ‘nest’ area.
4.1.2. Procedure
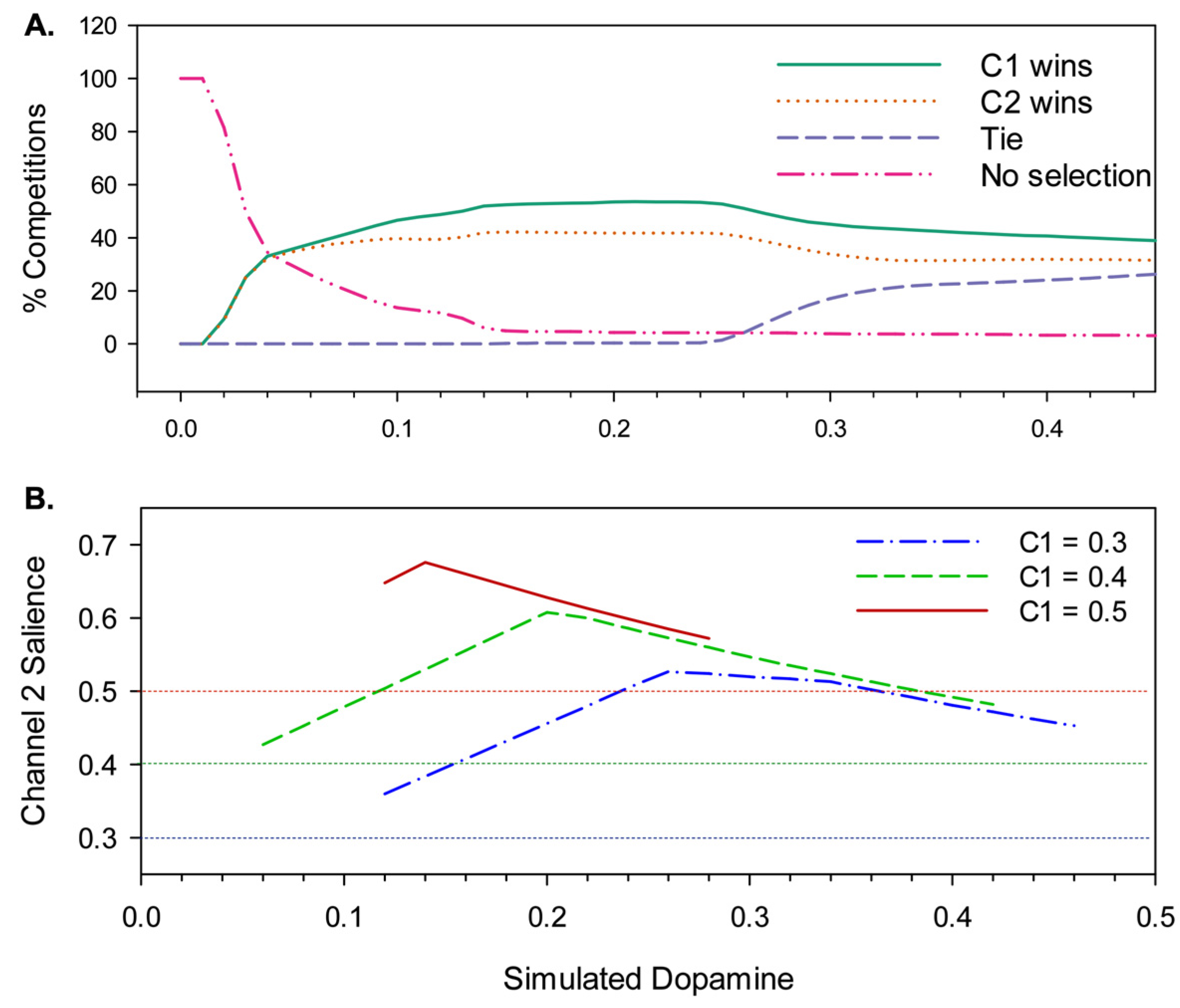
4.2. Results
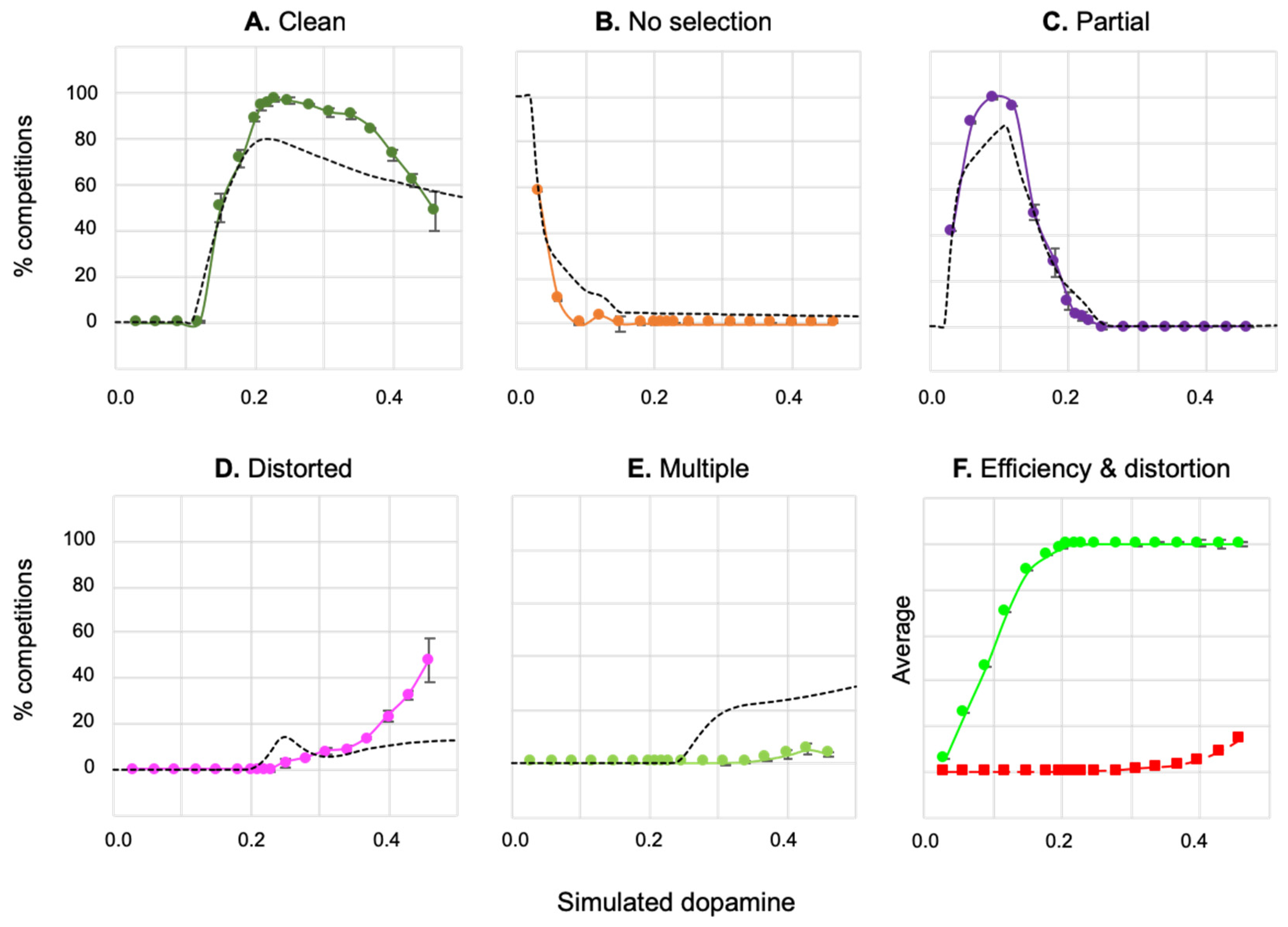
4.2.1. Effects of Simulated Dopamine Modulation on Behavioural Outcome
4.2.2. Behavioural Consequences of Low Simulated Tonic Dopamine (λ < 0.2)
4.2.3. Behavioural Consequences of High Simulated Tonic Dopamine (λ > 0.3)
4.2.4. Effects of Distortion on Behavioural Persistence
5. Discussion
5.1. Effects of Simulated Dopamine Modulation on Robot Behaviour
5.2. The Role of Dopamine in Basal Ganglia Dysfunction in Animals and Humans
5.3. Dopamine-Depleting Interventions and Neurological Conditions Associated with Reduced Striatal Dopamine
5.4. Dopamine-Increasing Interventions, and Neurological Conditions Involving Increased Striatal Dopamine
5.5. Limitations and Related Work
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Detailed Commentary on Robot Behaviour in Figure 11
References
- Grillner, S.; Hellgren, J.; Menard, A.; Saitoh, K.; Wikstrom, M. Mechanisms for selection of basic motor programs—Roles for the striatum and pallidum. Trends Neurosci. 2005, 28, 364–370. [Google Scholar] [CrossRef]
- Mink, J.W. The basal ganglia: Focused selection and inhibition of competing motor programs. Prog. Neurobiol. 1996, 50, 381–425. [Google Scholar] [CrossRef] [PubMed]
- Redgrave, P.; Prescott, T.J.; Gurney, K. The basal ganglia: A vertebrate solution to the selection problem? Neuroscience 1999, 89, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Prescott, T.J.; Redgrave, P.; Gurney, K. Layered control architectures in robots and vertebrates. Adapt. Behav. 1999, 7, 99–127. [Google Scholar] [CrossRef]
- Balleine, B.W.; Delgado, M.R.; Hikosaka, O. The Role of the Dorsal Striatum in Reward and Decision-Making. J. Neurosci. 2007, 27, 8161. [Google Scholar] [CrossRef] [PubMed]
- Grillner, S.; Robertson, B. The basal ganglia downstream control of brainstem motor centres—An evolutionarily conserved strategy. Curr. Opin. Neurobiol. 2015, 33, 47–52. [Google Scholar] [CrossRef]
- Mink, J.W. The basal ganglia and involuntary movements: Impaired inhibition of competing motor patterns. Arch. Neurol. 2003, 60, 1365–1368. [Google Scholar] [CrossRef]
- Schultz, W. Multiple Dopamine Functions at Different Time Courses. Annu. Rev. Neurosci. 2007, 30, 259–288. [Google Scholar] [CrossRef]
- Arber, S.; Costa, R.M. Networking brainstem and basal ganglia circuits for movement. Nat. Rev. Neurosci. 2022, 23, 342–360. [Google Scholar] [CrossRef]
- Salamone, J.D. Dopaminergic involvement in activational aspects of motivation—Effects of haloperidol on schedule-induced activity, feeding, and foraging in rats. Psychobiology 1988, 16, 196–206. [Google Scholar] [CrossRef]
- Salamone, J.D.; Zigmond, M.J.; Stricker, E.M. Characterization of the impaired feeding-behavior in rats given haloperidol or dopamine-depleting brain-lesions. Neuroscience 1990, 39, 17–24. [Google Scholar] [CrossRef]
- Salamone, J.D. Behavioral pharmacology of dopamine systems: A new synthesis. In The Mesolimbic Dopamine System: From Motivation to Action; Willner, P., Scheel-Kruger, J., Eds.; Wiley and Sons: Hoboken, NJ, USA, 1991. [Google Scholar]
- Bakshi, V.P.; Kelley, A.E. Dopaminergic regulation of feeding-behavior.1. differential-effects of haloperidol microinfusion into 3 striatal subregions. Psychobiology 1991, 19, 223–232. [Google Scholar] [CrossRef]
- Salamone, J.D.; Mahan, K.; Rogers, S. Ventrolateral striatal dopamine depletions impair feeding and food handling in rats. Pharmacol. Biochem. Behav. 1993, 44, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Bury, D.; Schmidt, W.J. Effects of systemically and intrastriatally injected haloperidol and apomorphine on grooming, feeding and locomotion in the rat. Behav. Process. 1987, 15, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Salamone, J.D.; Correa, M. The Mysterious Motivational Functions of Mesolimbic Dopamine. Neuron 2012, 76, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Gaddy, J.R.; Neill, D.B. Differential behavioral changes following intrastriatal application of 6-hydroxydopamine. Brain Res. 1977, 119, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Cousins, M.S.; Salamone, J.D. Involvement of ventrolateral striatal dopamine in movement initiation and execution—A microdialysis and behavioral investigation. Neuroscience 1996, 70, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Cousins, M.S.; Salamone, J.D. Skilled motor deficits in rats induced by ventrolateral striatal dopamine depletions—Behavioral and pharmacological characterization. Brain Res. 1996, 732, 186–194. [Google Scholar] [CrossRef]
- Koob, G.F.; Riley, S.J.; Smith, S.C.; Robbins, T.W. Effects of 6-Hydroxydopamine lesions of the nucleus accumbens septi and olfactory tubercle on feeding, locomotor activity, and amphetamine anorexia in the rat. J. Comp. Physiol. Psychol. 1978, 92, 917–927. [Google Scholar] [CrossRef]
- Cools, A.R. Role of the neostriatal dopaminergic activity in sequencing and selecting behavioural strategies: Facilitation of processes involved in selecting the best strategy in a stressful situation. Behav. Brain Res. 1980, 1, 361–378. [Google Scholar] [CrossRef]
- Gelissen, M.; Cools, A. Effect of intracaudate haloperidol and apomorphine on switching motor patterns upon current behavior of cats. Behav. Brain Res. 1988, 29, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Marin, C.; Engber, T.M.; Bonastre, M.; Chase, T.N.; Tolosa, E. Effect of long-term haloperidol treatment on striatal neuropeptides—Relation to stereotyped behavior. Brain Res. 1996, 731, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.F.; Levitan, D.; Stricker, E.M. Activation-induced restoration of sensorimotor functions in rats with dopamine-depleting brain lesions. J. Comp. Physiol. Psychol. 1976, 90, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, P.; Schallert, T.; Whishaw, I.Q. Sources of spontaneity in motivated behaviour. In Handbook of Behavioural Neurobiology; Teitelbaum, P., Satinoff, E., Eds.; Plenum Press: New York, NY, USA, 1983; pp. 23–66. [Google Scholar]
- Oades, R.D. The role of noradrenaline in tuning and dopamine in switching between signals in the cns. Neurosci. Biobehav. Rev. 1985, 9, 261–282. [Google Scholar] [CrossRef][Green Version]
- Iversen, S.D. Striatal function and stereotyped behaviour. In Psychobiology of the Striatum; Cools, A.R., Ed.; Elsevier: Amsterdam, The Netherlands, 1977; pp. 99–117. [Google Scholar]
- Kelley, A.E.; Iversen, S.D. Substance P infusion into substantia nigra of the rat: Behavioural analysis and involvement of striatal dopamine. Eur. J. Pharmacol. 1979, 60, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, V.P.; Kelley, A.E. Dopaminergic regulation of feeding-behavior: 2. differential-effects of amphetamine microinfusion into 3 striatal subregions. Psychobiology 1991, 19, 233–242. [Google Scholar] [CrossRef]
- Langen, M.; Kas, M.J.; Staal, W.G.; van Engeland, H.; Durston, S. The neurobiology of repetitive behavior: … and men. Neurosci. Biobehav. Rev. 2011, 35, 345–355. [Google Scholar] [CrossRef]
- Jankovic, J.J.; Tolosa, E. (Eds.) Parkinson’s Disease and Movement Disorders, 4th ed.; Lipincott, Williams, & Wilkins: Philadelphia, PA, USA, 2002. [Google Scholar]
- Moore, H.; West, A.R.; Grace, A.A. The regulation of forebrain dopamine transmission: Relevance to the pathophysiology and psychopathology of schizophrenia. Biol. Psychiatry 1999, 46, 40–55. [Google Scholar] [CrossRef]
- Brisch, R.; Saniotis, A.; Wolf, R.; Bielau, H.; Bernstein, H.G.; Steiner, J.; Bogerts, B.; Braun, K.; Jankowski, Z.; Kumaratilake, J.; et al. The Role of Dopamine in Schizophrenia from a Neurobiological and Evolutionary Perspective: Old Fashioned, but Still in Vogue. Front. Psychiatry 2014, 5, 47. [Google Scholar]
- Joel, D. Current animal models of obsessive compulsive disorder: A critical review. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2006, 30, 374–388. [Google Scholar] [CrossRef]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Prescott, T.J.; González, F.M.M.; Gurney, K.; Humphries, M.D.; Redgrave, P. A robot model of the basal ganglia: Behaviour and intrinsic processing. Neural Netw. 2006, 19, 31–61. [Google Scholar] [CrossRef] [PubMed]
- Montague, P.R.; Dolan, R.J.; Friston, K.J.; Dayan, P. Computational psychiatry. Trends Cogn. Sci. 2012, 16, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Tolu, S.; Strohmer, B.; Zahra, O. Perspective on investigation of neurodegenerative diseases with neurorobotics approaches. Neuromorphic Comput. Eng. 2023, 3, 013001. [Google Scholar] [CrossRef]
- Gurney, K.; Prescott, T.J.; Redgrave, P. A computational model of action selection in the basal ganglia: I. A new functional anatomy. Biol. Cybern. 2001, 84, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Gurney, K.; Prescott, T.J.; Redgrave, P. A computational model of action selection in the basal ganglia: II. Analysis and simulation of behaviour. Biol. Cybern. 2001, 84, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Humphries, M.D.; Gurney, K. The role of intra-thalamic and thalamocortical circuits in action selection. Netw. Comput. Neural Syst. 2002, 13, 131–156. [Google Scholar] [CrossRef]
- McFarland, D.; Bosser, T. Intelligent Behaviour in Animals and Robots; MIT Press: Cambridge, MA, USA, 1993. [Google Scholar]
- Snaith, S.; Holland, O. An investigation of two mediation strategies suitable for behavioural control in animals and animats. In From Animals to Animats: Proceedings of the First International Conference Simulation of Adaptive Behaviour; Meyer, J.-A., Wilson, S., Eds.; MIT Press: Cambridge, MA, USA, 1990; pp. 255–262. [Google Scholar]
- Prescott, T.J. Action Selection. Scholarpedia 2008, 3, 2705. [Google Scholar] [CrossRef]
- Prescott, T.J. Forced moves or good tricks in design space? Landmarks in the evolution of neural mechanisms for action selection. Adapt. Behav. 2007, 15, 9–31. [Google Scholar] [CrossRef]
- Ludlow, A.R. Applications of Computer Modelling to Behavioural Coordination. Ph.D. Thesis, University of London, London, UK, 1983. [Google Scholar]
- McFarland, D. Problems of Animal Behaviour; Longman: Harlow, UK, 1989. [Google Scholar]
- McFarland, D.J.; Sibly, R.M. The behavioural final common path. Philos. Trans. R. Soc. B 1975, 270, 34–293. [Google Scholar]
- Gillies, A.; Willshaw, D. Models of the subthalamic nucleus: The importance of intranuclear connectivity. Med. Eng. Phys. 2004, 26, 723–732. [Google Scholar] [CrossRef]
- Parent, A.; Hazrati, L.N. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res. Brain Res. Rev. 1995, 20, 128–154. [Google Scholar] [CrossRef]
- Suryanarayana, S.M.; Kotaleski, J.H.; Grillner, S.; Gurney, K.N. Roles for globus pallidus externa revealed in a computational model of action selection in the basal ganglia. Neural Netw. 2019, 109, 113–136. [Google Scholar] [CrossRef]
- Gerfen, C.R.; Surmeier, D.J. Modulation of Striatal Projection Systems by Dopamine. Annu. Rev. Neurosci. 2011, 34, 441–466. [Google Scholar] [CrossRef]
- Akkal, D.; Burbaud, P.; Audin, J.; Bioulac, B. Responses of substantia nigra pars reticulata neurons to intrastriatal D1 and D2 dopaminergic agonist injections in the rat. Neurosci. Lett. 1996, 213, 66–70. [Google Scholar] [CrossRef]
- Cui, G.; Jun, S.B.; Jin, X.; Pham, M.D.; Vogel, S.S.; Lovinger, D.M.; Costa, R.M. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature 2013, 494, 238–242. [Google Scholar] [CrossRef]
- Tecuapetla, F.; Jin, X.; Lima, S.Q.; Costa, R.M. Complementary Contributions of Striatal Projection Pathways to Action Initiation and Execution. Cell 2016, 166, 703–715. [Google Scholar] [CrossRef]
- González, F.M.; Prescott, T.J.; Gurney, K.; Humphries, M.; Redgrave, P. An embodied model of action selection mechanisms in the vertebrate brain. In From Animals to Animats 6: Proceedings of the 6th International Conference on the Simulation of Adaptive Behavior; Meyer, J.A., Ed.; MIT Press: Cambridge, MA, USA, 2000; pp. 157–166. [Google Scholar]
- Hinde, R.A. Animal Behaviour: A Synthesis of Ethology and Comparative Psychology; McGraw-Hill: London, UK, 1966. [Google Scholar]
- Lorenz, K. Der Kumpan in der Umwelt des Vogels. J. Ornithol. 1935, 83, 137–213, 289–413. [Google Scholar] [CrossRef]
- Schultz, W.; Dayan, P.; Montague, P.R. A neural substrate for prediction and reward. Science 1997, 275, 1593–1599. [Google Scholar] [CrossRef]
- Blomfield, S. Arithmetical operations performed by nerve cells. Brain Res. 1974, 69, 115–124. [Google Scholar] [CrossRef]
- Koch, C.; Poggio, T.; Torre, V. Nonlinear interactions in a dendritic tree: Localization, timing, and role in information processing. Proc. Natl. Acad. Sci. USA 1983, 80, 2799–2802. [Google Scholar] [CrossRef]
- Tsumori, T.; Yasui, Y. Organization of the nigro-tecto-bulbar pathway to the parvicellular reticular formation: A light- and electron-microscopic study in the rat. Exp. Brain Res. 1997, 116, 341–350. [Google Scholar] [CrossRef] [PubMed]
- A Rossi, M.; E Li, H.; Lu, D.; Kim, I.H.; A Bartholomew, R.; Gaidis, E.; Barter, J.W.; Kim, N.; Cai, M.T.; Soderling, S.H.; et al. A GABAergic nigrotectal pathway for coordination of drinking behavior. Nat. Neurosci. 2016, 19, 742–748. [Google Scholar] [CrossRef]
- Lehner, P.N. Handbook of Ethological Methods, 2nd ed.; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Prescott, T.J.; Wilson, S.P. Understanding brain functional architecture through robotics. Sci. Robot. 2023, 8, eadg6014. [Google Scholar] [CrossRef] [PubMed]
- Hallam, J.C.; Malcolm, C.A. Behaviour: Perception, action and intelligence—The view from situated robotics. Philos. Trans. R. Soc. London. Ser. A Phys. Eng. Sci. 1994, 349, 29–42. [Google Scholar]
- Brooks, R.A. Coherent behaviour from many adaptive processes. In From Animals to Animats 3: Proceedings of the Third International Conference on the Simulation of Adaptive Behaviour; Cliff, D., Husbands, P., Meyer, J.-A., Wilson, S.W., Eds.; MIT Press: Cambridge, MA, USA, 1994; pp. 22–29. [Google Scholar]
- Prescott, T.J.; Ayers, J.O.S.E.P.H.; Grasso, F.W.; Verschure, P.F. Embodied models and neurorobotics. In From Neuron to Cognition via Computational Neuroscience; Arbib, M.A., Bonaiuto, J.J., Eds.; MIT Press: Cambridge, MA, USA, 2016; pp. 483–512. [Google Scholar]
- Verschure, P.F.M.J.; Voegtlin, T.; Douglas, R.J. Environmentally mediated synergy between perception and behaviour in mobile robots. Nature 2003, 425, 620–624. [Google Scholar] [CrossRef]
- Obeso, J.A.; Marin, C.; Rodriguez-Oroz, C.; Blesa, J.; Benitez-Temiño, B.; Mena-Segovia, J.; Rodríguez, M.; Olanow, C.W. The basal ganglia in Parkinson’s disease: Current concepts and unexplained observations. Ann. Neurol. 2008, 64, S30–S46. [Google Scholar] [CrossRef]
- Frank, M.J. Dynamic dopamine modulation in the basal ganglia: A neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism. J. Cogn. Neurosci. 2005, 17, 51–72. [Google Scholar] [CrossRef]
- Guthrie, M.; Myers, C.E.; Gluck, M.A. A neurocomputational model of tonic and phasic dopamine in action selection: A comparison with cognitive deficits in Parkinson’s disease. Behav. Brain Res. 2009, 200, 48–59. [Google Scholar] [CrossRef]
- Frank, M.J.; Santamaria, A.; O’Reilly, R.C.; Willcutt, E. Testing Computational Models of Dopamine and Noradrenaline Dysfunction in Attention Deficit/Hyperactivity Disorder. Neuropsychopharmacology 2007, 32, 1583–1599. [Google Scholar] [CrossRef]
- Sonnenschein, S.F.; Gomes, F.V.; Grace, A.A. Dysregulation of Midbrain Dopamine System and the Pathophysiology of Schizophrenia. Front Psychiatry 2020, 11, 613. [Google Scholar] [CrossRef]
- Maia, T.V.; Conceicao, V.A. The Roles of Phasic and Tonic Dopamine in Tic Learning and Expression. Biol. Psychiatry 2017, 82, 401–412. [Google Scholar] [CrossRef]
- Singer, H.S.; Szymanski, S.; Giuliano, J.; Yokoi, F.; Dogan, A.S.; Brasic, J.R.; Zhou, Y.; Grace, A.A.; Wong, D.F. Elevated Intrasynaptic Dopamine Release in Tourette’s Syndrome Measured by PET. Am. J. Psychiatry 2002, 159, 1329–1336. [Google Scholar] [CrossRef]
- Xue, J.; Qian, D.; Zhang, B.; Yang, J.; Li, W.; Bao, Y.; Qiu, S.; Fu, Y.; Wang, S.; Yuan, T.-F.; et al. Midbrain dopamine neurons arbiter OCD-like behavior. Proc. Natl. Acad. Sci. USA 2022, 119, e2207545119. [Google Scholar] [CrossRef]
- Jones, C.A.; Watson, D.J.G.; Fone, K.C.F. Animal models of schizophrenia. Br. J. Pharmacol. 2011, 164, 1162–1194. [Google Scholar] [CrossRef]
- Betarbet, R.; Sherer, T.B.; Greenamyre, J.T. Animal models of Parkinson’s disease. BioEssays 2002, 24, 308–318. [Google Scholar] [CrossRef]
- Blesa, J.; Przedborski, S. Parkinson’s disease: Animal models and dopaminergic cell vulnerability. Front. Neuroanat. 2014, 8, 155. [Google Scholar] [CrossRef]
- Dawson, T.M.; Ko, H.S.; Dawson, V.L. Genetic Animal Models of Parkinson’s Disease. Neuron 2010, 66, 646–661. [Google Scholar] [CrossRef]
- Schober, A. Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res. 2004, 318, 215–224. [Google Scholar] [CrossRef]
- Wise, R.A. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004, 5, 483–494. [Google Scholar] [CrossRef]
- Ikemoto, S.; Panksepp, J. The role of nucleus accumbens dopamine in motivated behavior: A unifying interpretation with special reference to reward-seeking. Brain Res. Rev. 1999, 31, 6–41. [Google Scholar] [CrossRef]
- Salamone, J.D.; Correa, M.; Yang, J.-H.; Rotolo, R.; Presby, R. Dopamine, Effort-Based Choice, and Behavioral Economics: Basic and Translational Research. Front. Behav. Neurosci. 2018, 12, 52. [Google Scholar] [CrossRef]
- Berridge, K.C. From prediction error to incentive salience: Mesolimbic computation of reward motivation. Eur. J. Neurosci. 2012, 35, 1124–1143. [Google Scholar] [CrossRef]
- Berardelli, A.; Rothwell, J.C.; Thompson, P.D.; Hallett, M. Pathophysiology of bradykinesia in Parkinson’s disease. Brain 2001, 124, 2131–2146. [Google Scholar] [CrossRef]
- Blackburn, J.R.; Phillips, A.G.; Fibiger, H.C. Dopamine and preparatory behavior: 1. effects of pimozide. Behav. Neurosci. 1987, 101, 352–360. [Google Scholar] [CrossRef]
- Kelley, A.E.; Stinus, L. Dissapearance of hoarding behavior after 6-hydroxydopamine lesions of the mesolimbic dopamine neurons and its reinstatement with l-dopa. Behav. Neurosci. 1985, 99, 531–545. [Google Scholar] [CrossRef]
- Keefe, K.A.; Salamone, J.D.; Zigmond, M.J.; Stricker, E.M. Paradoxical kinesia in parkinsonism is not caused by dopamine release. Studies in an animal model. Arch. Neurol. 1989, 46, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- McDowell, S.-A.; Harris, J. Irrelevant peripheral visual stimuli impair manual reaction times in Parkinson’s disease. Vis. Res. 1997, 37, 3549–3558. [Google Scholar] [CrossRef] [PubMed]
- Schwab, R.S. Akinesia paradoxica. Electroencephalogr. Clin. Neurophysiol. 1972, 31, 87–92. [Google Scholar]
- Benecke, R.; Rothwell, J.C.; Dick, J.P.R.; Day, B.L.; Marsden, C.D. Performance of simultaneous movements in patients with parkinson’s disease. Brain 1986, 109, 739–757. [Google Scholar] [CrossRef]
- Shukla, A.W.; Ounpraseuth, S.; Okun, M.S.; Gray, V.; Schwankhaus, J.; Metzer, W.S. Micrographia and related deficits in Parkinson’s disease: A cross-sectional study. BMJ Open 2012, 2, e000628. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.M.; Prescott, T.J. Response times for visually guided saccades in persons with Parkinson’s disease: A meta-analytic review. Neuropsychologia 2010, 48, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Rebec, G.V.; Bashore, T.R. Critical issues in assessing the behavioral effects of amphetamine. Neurosci. Biobehav. Rev. 1984, 8, 153–159. [Google Scholar] [CrossRef]
- Seiden, L.S.; Sabol, K.E.; Ricaurte, G.A. Amphetamine: Effects on Catecholamine Systems and Behavior. Annu. Rev. Pharmacol. Toxicol. 1993, 33, 639–676. [Google Scholar] [CrossRef] [PubMed]
- Kelley, A.E.; Winnock, M.; Stinus, L. Amphetamine, apomorphine and investigatory behavior in the rat: Analysis of the structure and pattern of responses. Psychopharmacology 1986, 88, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Eilam, D. From an animal model to human patients: An example of a translational study on obsessive compulsive disorder (OCD). Neurosci. Biobehav. Rev. 2017, 76 Pt A, 67–76. [Google Scholar] [CrossRef]
- Zhuang, X.; Oosting, R.S.; Jones, S.R.; Gainetdinov, R.R.; Miller, G.W.; Caron, M.G.; Hen, R. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc. Natl. Acad. Sci. USA 2001, 98, 1982–1987. [Google Scholar] [CrossRef]
- Cinque, S.; Zoratto, F.; Poleggi, A.; Leo, D.; Cerniglia, L.; Cimino, S.; Tambelli, R.; Alleva, E.; Gainetdinov, R.R.; Laviola, G.; et al. Behavioral Phenotyping of Dopamine Transporter Knockout Rats: Compulsive Traits, Motor Stereotypies, and Anhedonia. Front Psychiatry 2018, 9, 43. [Google Scholar] [CrossRef]
- Allport, A. Selection for action: Some behavioial and neurophysiological considerations of attention and action. In Perspectives on Perception and Action; Heuer, H., Sanders, A.F., Eds.; Erlbaum: Hillsdale, NJ, USA, 1987; pp. 395–420. [Google Scholar]
- Humphries, M.D.; Gurney, K. Making decisions in the dark basement of the brain: A look back at the GPR model of action selection and the basal ganglia. Biol. Cybern. 2021, 115, 323–329. [Google Scholar] [CrossRef]
- Humphries, M.D.; Lepora, N.; Wood, R.; Gurney, K. Capturing dopaminergic modulation and bimodal membrane behaviour of striatal medium spiny neurons in accurate, reduced models. Front. Comput. Neurosci. 2009, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Bahuguna, J.; Weidel, P.; Morrison, A. Exploring the role of striatal D1 and D2 medium spiny neurons in action selection using a virtual robotic framework. Eur. J. Neurosci. 2019, 49, 737–753. [Google Scholar] [CrossRef]
- Humphries, M.D.; Prescott, T.J. The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog. Neurobiol. 2010, 90, 385–417. [Google Scholar] [CrossRef]
- Cope, A.J.; Chambers, J.M.; Prescott, T.J.; Gurney, K.N. Basal Ganglia Control of Reflexive Saccades: A Computational Model Integrating Physiology Anatomy and Behaviour. bioRxiv 2017. [Google Scholar] [CrossRef]
- Prescott, T.J.; Mitchinson, B.; Lepora, N.F.; Wilson, S.P.; Anderson, S.R.; Porrill, J.; Dean, P.; Fox, C.W.; Pearson, M.J.; Sullivan, J.C.; et al. The robot vibrissal system: Understanding mammalian sensorimotor co-ordination through biomimetics. In Sensorimotor Integration in the Whisker System; Krieger, P., Groh, A., Eds.; Springer: New York, NY, USA, 2015; pp. 213–240. [Google Scholar]
- Sarvestani, I.K.; Kozlov, A.; Harischandra, N.; Grillner, S.; Ekeberg, Ö. A computational model of visually guided locomotion in lamprey. Biol. Cybern. 2013, 107, 497–512. [Google Scholar] [CrossRef]
- Verschure, P.F.M.J.; Pennartz, C.M.A.; Pezzulo, G. The why, what, where, when and how of goal-directed choice: Neuronal and computational principles. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130483. [Google Scholar] [CrossRef] [PubMed]
- Girard, B.; Tabareau, N.; Pham, Q.; Berthoz, A.; Slotine, J.-J. Where neuroscience and dynamic system theory meet autonomous robotics: A contracting basal ganglia model for action selection. Neural Netw. 2008, 21, 628–641. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jimenez-Rodriguez, A.; Prescott, T.J. Motivational Modulation of Consummatory Behaviour and Learning in a Robot Model of Spatial Navigation. In Biomimetic and Biohybrid Systems; Springer Nature: Cham, Switzerland, 2023. [Google Scholar]
- Marinelli, M.; McCutcheon, J.E. Heterogeneity of dopamine neuron activity across traits and states. Neuroscience 2014, 282, 176–197. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.E.; Patel, J.C.; Cragg, S.J. Dopamine release in the basal ganglia. Neuroscience 2011, 198, 112–137. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Otani, S.; Grace, A.A. The Yin and Yang of dopamine release: A new perspective. Neuropharmacology 2007, 53, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Krichmar, J.L. The neuromodulatory system: A framework for survival and adaptive behavior in a challenging world. Adapt. Behav. 2008, 16, 385–399. [Google Scholar] [CrossRef]
- Krichmar, J. A neurorobotic platform to test the influence of neuromodulatory signaling on anxious and curious behavior. Front. Neurorobotics 2013, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jusup, M.; Shi, L.; Lee, J.-H.; Iwasa, Y.; Boccaletti, S. Exploiting a cognitive bias promotes cooperation in social dilemma experiments. Nat. Commun. 2018, 9, 2954. [Google Scholar] [CrossRef] [PubMed]
- Hommel, B.; Chapman, C.S.; Cisek, P.; Neyedli, H.F.; Song, J.-H.; Welsh, T.N. No one knows what attention is. Atten. Percept. Psychophys. 2019, 81, 2288–2303. [Google Scholar] [CrossRef] [PubMed]
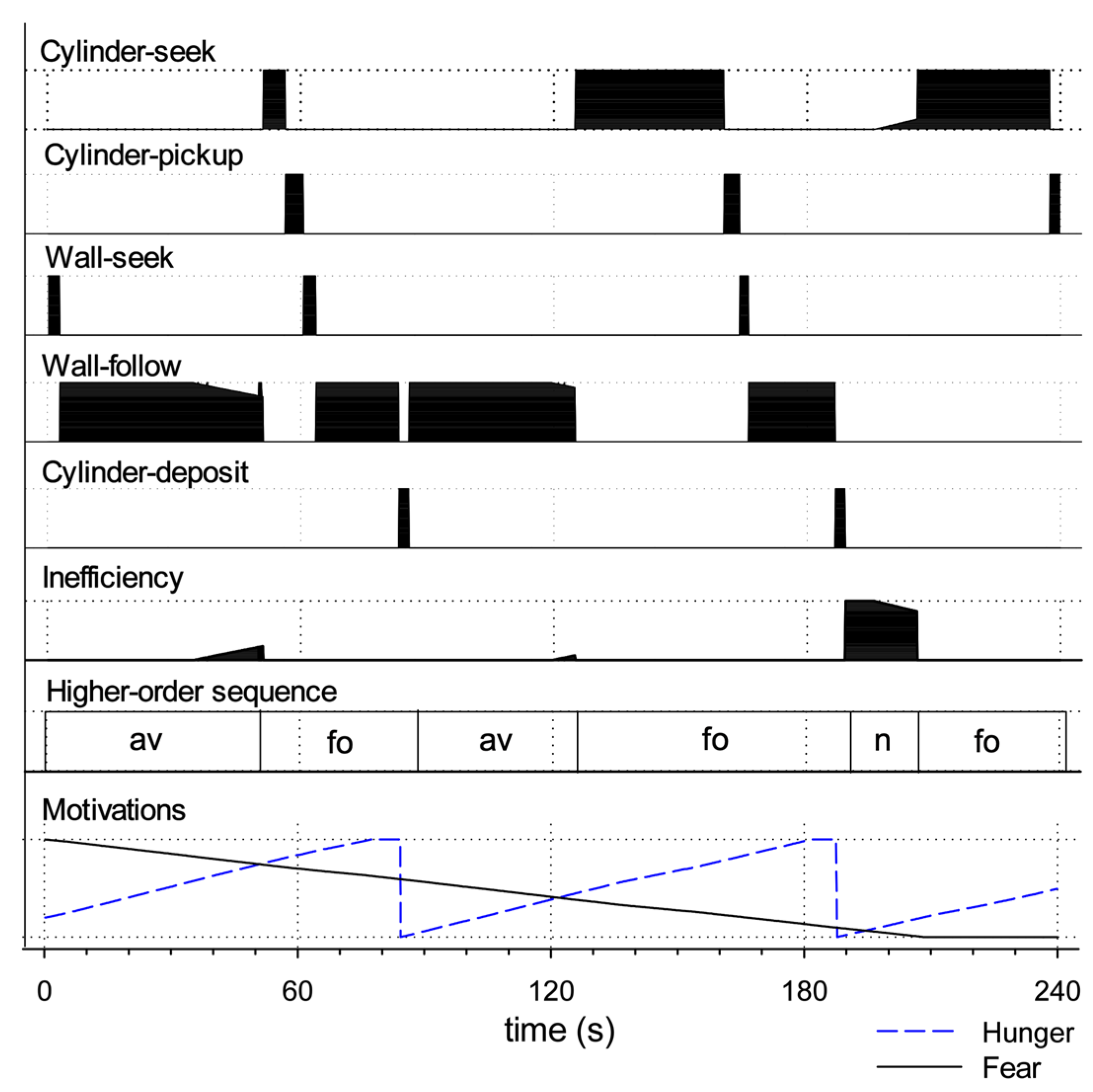
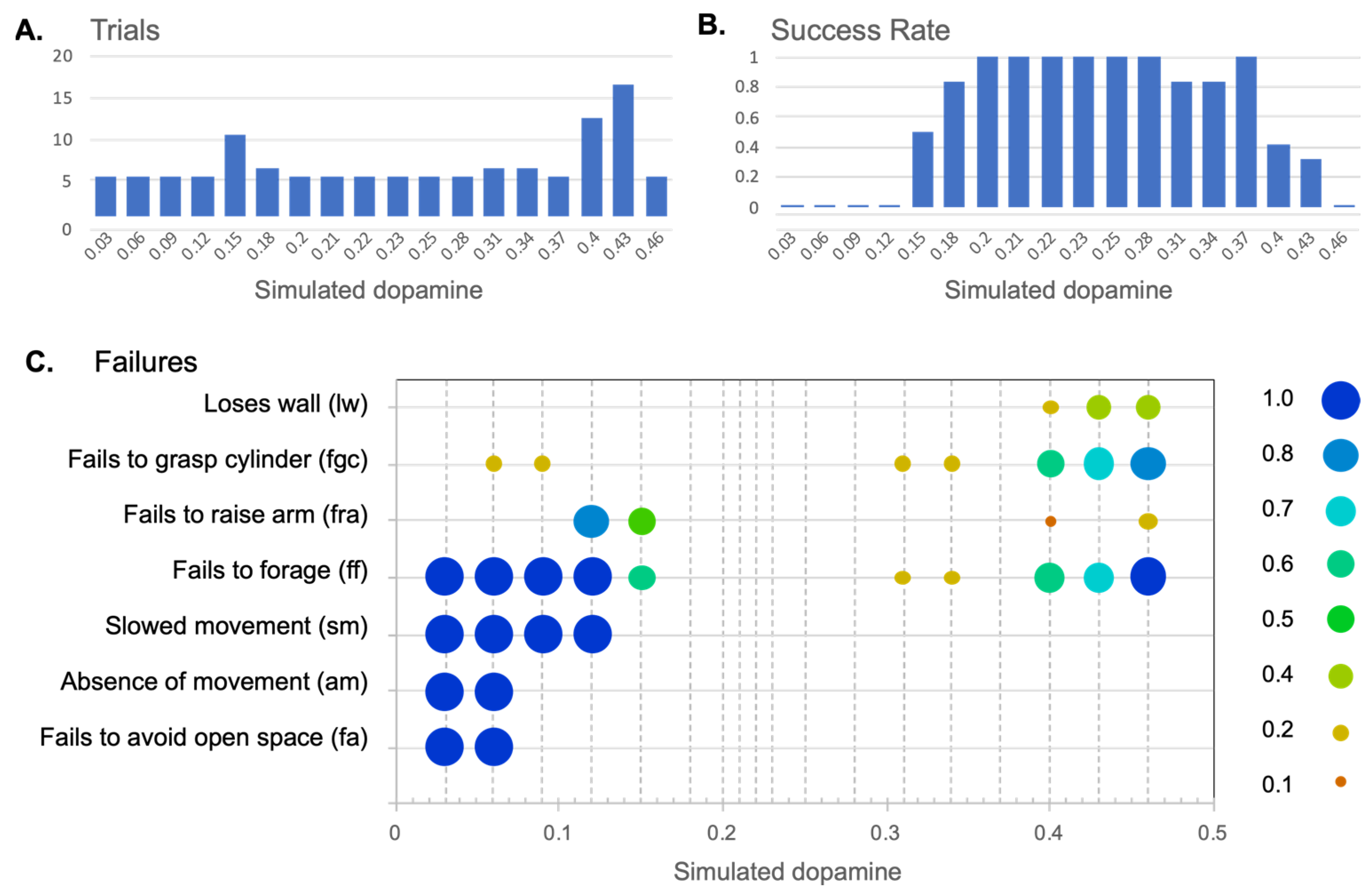


| Failure to meet success criterion | |
| Fails to avoid open space (fa) | Failure with respect to criterion (i) above. |
| Fails to forage (ff) | Failure with respect to criterion (ii) above. |
| Behaviours typically leading to fa or ff | |
| Absence of movement (am) | Failure to express movement despite being motivated. Typically leads to fa as the robot fails to leave open space. |
| Fails to raise arm (fra) | Fails to lift the arm after grasping a cylinder. Typically leads to ff as the lowered arm blocks the infrared sensor’s ability to detect the environment. |
| Fails to grasp cylinder (fgc) | Fails to lower the arm sufficiently to grasp a cylinder (therefore grasping at air). This can lead to ff, as, when the robot fails to grasp the cylinder, it then immediately looks for another cylinder. This generally leads to repeated cycles of cylinder-seek followed by (unsuccessful) cylinder-pickup. |
| Forms of behavioural disintegration typically not leading to fa or ff | |
| Slowed movement (sm) | Scored when behaviour, such as wheeled movement, is slowed to 75% or less of the usual speed (as measured by the output motor signal). |
| Loses wall (lw) | Losing contact with the wall while expressing wall-follow behaviour. Determined to occur if contact has been lost a minimum of four times in sequence (since occasional losses can occur due to sensor noise). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prescott, T.J.; Montes González, F.M.; Gurney, K.; Humphries, M.D.; Redgrave, P. Simulated Dopamine Modulation of a Neurorobotic Model of the Basal Ganglia. Biomimetics 2024, 9, 139. https://doi.org/10.3390/biomimetics9030139
Prescott TJ, Montes González FM, Gurney K, Humphries MD, Redgrave P. Simulated Dopamine Modulation of a Neurorobotic Model of the Basal Ganglia. Biomimetics. 2024; 9(3):139. https://doi.org/10.3390/biomimetics9030139
Chicago/Turabian StylePrescott, Tony J., Fernando M. Montes González, Kevin Gurney, Mark D. Humphries, and Peter Redgrave. 2024. "Simulated Dopamine Modulation of a Neurorobotic Model of the Basal Ganglia" Biomimetics 9, no. 3: 139. https://doi.org/10.3390/biomimetics9030139
APA StylePrescott, T. J., Montes González, F. M., Gurney, K., Humphries, M. D., & Redgrave, P. (2024). Simulated Dopamine Modulation of a Neurorobotic Model of the Basal Ganglia. Biomimetics, 9(3), 139. https://doi.org/10.3390/biomimetics9030139








