Recent Advances in Scaffolds for Guided Bone Regeneration
Abstract
1. Introduction
2. Methodology
3. Guided Bone Regeneration Technique and Its Role in Alveolar Bone Defects
3.1. Biomaterials for Bone Regeneration
- Biocompatibility, a critical property that prevents an inflammatory response;
- Controlled biodegradability;
- Adequate pore size, minimum requirement is 100 μm, but larger than 300 μm is the optimal for vascularization and bone formation;
- Interconnected porosity, which allows the diffusion bone cells, nutrients and waste products;
- Appropriate surface, that allows cell attachment, migration and proliferation while promoting vascular ingrowth;
- Tolerable elasticity and mechanical compressive strength, supporting the adjacent tissue load.
3.2. Membranes (Resorbable/Non-Resorbable)
- Biocompatibility, to integrate with host’s tissues without initiating an inflammatory response;
- Biodegradability, with an appropriate degradation profile according to the host’s tissue;
- Biological activity;
- Competent physical and mechanical properties;
- Porosity and occlusive properties;
- Tolerable strength to withstand the forces of adjacent tissues, preventing membranes collapse;
- Exposure tolerance.
4. Scaffolds in BTE/RM
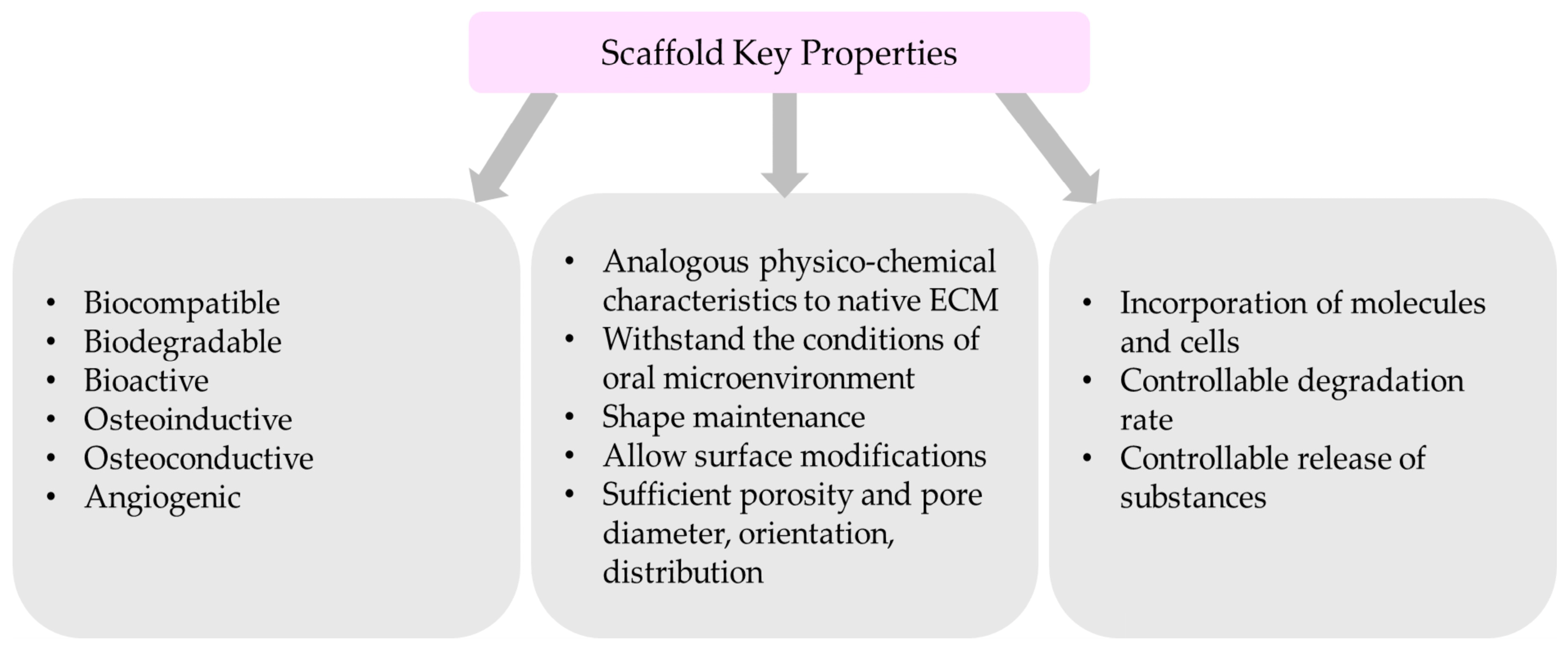
4.1. The Critical Properties of Scaffolds
- Biocompatible nanomaterial with non-toxic degradation;
- Bioactivity, which will promote the interaction of a material’s surface and the adjacent cells;
- Analogous physicochemical characteristics similar to extracellular matrix (ECM) of the targeted bone native state;
- Ability to withstand the conditions of oral microenvironment (pH, temperature)
- Shape maintenance after implantation;
- Sufficient porosity and adequate pore diameter, orientation, and distribution
- Allow the incorporation of molecules and cells;
- Allow surface modifications;
- Degradable;
- Controllable degradation and release of substances. Scaffold’s degradation should be similar to the tissue regenerated;
- Osteoinductive and osteoconductive properties to promote cell infiltration;
- Angiogenic;
4.2. Scaffold Architecture
4.3. Scaffold Fabrication Methods
4.3.1. Electrospinning
- High voltage power supply;
- Syringe pump;
- Metallic needle;
- Stationary or rotating metallic collector for fiber collection.
4.3.2. Additive Manufacturing
4.3.3. Bioprinting
4.3.4. Freeze Drying
4.3.5. Solvent-Casting and Particulate Leaching
4.3.6. Gas-Foaming Process
4.3.7. Decellularization
4.4. Biodegradable and Nonbiodegradable Scaffolds
4.5. Additional Scaffold Categories
4.5.1. Monophasic Scaffolds
4.5.2. Multiphasic Scaffolds
4.5.3. Hybrid Scaffolds
4.5.4. Smart Scaffolds
- Biomimetic and bionic. Mittal et al. (2010) developed a porous biomimetic scaffold containing PLGA microspheres and peptides. This system was able to replicate the structure and composition of natural tissues [201].
- Immune sensitives. Zeng et al. (2017) coated a mesoporous bioactive glass scaffold with amino functional groups and reported its osteoimmunomodulatory efficacy on MSCs, macrophages, and bone marrow [202].
- Shape memory. Liu et al. (2014) loaded a shape-memory nanoporous scaffold with growth factors (BMP-2), attempting to repair a mandibular bone defect. The authors stated that the nanosystem could be applied in bone-regenerative medicine due to its potential [203].
- Electromechanical stimulus. Damaraju et al. (2017) developed flexible 3D fibrous scaffolds that are able to initiate the differentiation of MSCs and tissue formation [204]. A similar scaffold (Piezoelectric poly(vinylidene fluoride-trifluoroethylene)) incorporating zinc oxide nanoparticle enhanced the adhesion and proliferation of hMSCs while also improving blood vessel formation [205].
4.5.5. Personalized Scaffolds CAD/CAM
4.6. Scaffolds as Drug Delivery Systems
4.6.1. Antimicrobial Effect
4.6.2. Anti-Inflammatory Effect
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, D.; Yang, F.; Zheng, Y.; Liu, Y.; Zhou, Y. Research Status of Biodegradable Metals Designed for Oral and Maxillofacial Applications: A Review. Bioact. Mater. 2021, 6, 4186–4208. [Google Scholar] [CrossRef]
- Wang, B.; Feng, C.; Liu, Y.; Mi, F.; Dong, J. Recent Advances in Biofunctional Guided Bone Regeneration Materials for Repairing Defective Alveolar and Maxillofacial Bone: A Review. Jpn. Dent. Sci. Rev. 2022, 58, 233–248. [Google Scholar] [CrossRef]
- Armiento, A.R.; Hatt, L.P.; Sanchez Rosenberg, G.; Thompson, K.; Stoddart, M.J. Functional Biomaterials for Bone Regeneration: A Lesson in Complex Biology. Adv. Funct. Mater. 2020, 30, 1909874. [Google Scholar] [CrossRef]
- Lagopati, N.; Agathopoulos, S. Hydroxyapatite Scaffolds Produced from Cuttlefish Bone via Hydrothermal Transformation for Application in Tissue Engineering and Drug Delivery Systems. In Marine-Derived Biomaterials for Tissue Engineering Applications; Choi, A., Ben-Nissan, B., Eds.; Springer Series in Biomaterials Science and Engineering; Springer: Singapore, 2019; Volume 14. [Google Scholar] [CrossRef]
- Sailer, I.; Karasan, D.; Todorovic, A.; Ligoutsikou, M.; Pjetursson, B.E. Prosthetic failures in dental implant therapy. Periodontol. 2000 2022, 88, 130–144. [Google Scholar] [CrossRef]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Nyman, S.; Lindhe, J.; Karring, T.; Rylander, H. New attachment following surgical treatment of human periodontal disease. J. Clin. Periodontol. 1982, 9, 290–296. [Google Scholar] [CrossRef]
- Gottlow, J.; Nyman, S.; Karring, T.; Lindhe, J. New attachment formation as the result of controlled tissue regeneration. J. Clin. Periodontol. 1984, 11, 494–503. [Google Scholar] [CrossRef]
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological principle and therapeutic applications. Clin. Oral Implants Res. 2010, 21, 567–576. [Google Scholar] [CrossRef]
- Dahlin, C.; Linde, A.; Gottlow, J.; Nyman, S. Healing of bone defects by guided tissue regeneration. Plast. Reconst. Surg. 1988, 81, 672–676. [Google Scholar] [CrossRef]
- Dahlin, C.; Sennerby, L.; Lekholm, U.; Linde, A.; Nyman, S. Generation of new bone around titanium implants using a membrane technique: An experimental study in rabbits. Int. J. Oral Maxillofac. Implants 1989, 4, 19–25. [Google Scholar]
- Donos, N.; Akcali, A.; Padhye, N.; Sculean, A.; Calciolari, E. Bone regeneration in implant dentistry: Which are the factors affecting the clinical outcome? Periodontol. 2000 2023, 93, 26–55. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Urban, I.; Monje, A.; Kunrath, M.F.; Dahlin, C. Guided bone regeneration in implant dentistry: Basic principle, progress over 35 years, and recent research activities. Periodontol. 2000 2023, 93, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Laney, W. Glossary of Oral and Maxillofacial Implants. Int. J. Oral Maxillofac. Implants 2017, 32, Gi-G200. [Google Scholar] [CrossRef] [PubMed]
- Pilipchuk, S.P.; Plonka, A.B.; Monje, A.; Taut, A.D.; Lanis, A.; Kang, B.; Giannobile, W.V. Tissue Engineering for Bone Regeneration and Osseointegration in the Oral Cavity. Dent. Mater. 2015, 31, 317–338. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-M.; Jin, Y. Periodontal Tissue Engineering and Regeneration: Current Approaches and Expanding Opportunities. Tissue Eng. Part B Rev. 2010, 16, 219–255. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.M.S.; Cortez, A.L.V.; Moreira, R.W.F.; Mazzonetto, R. Complications of Intraoral Donor Site for Bone Grafting Prior to Implant Placement. Implant. Dent. 2006, 15, 420–426. [Google Scholar] [CrossRef]
- Sculean, A.; Nikolidakis, D.; Schwarz, F. Regeneration of Periodontal Tissues: Combinations of Barrier Membranes and Grafting Materials—Biological Foundation and Preclinical Evidence: A Systematic Review. J. Clin. Periodontol. 2008, 35, 106–116. [Google Scholar] [CrossRef]
- Mudda, J.; Bajaj, M. Stem Cell Therapy: A Challenge to Periodontist. Indian J. Dent. Res. 2011, 22, 132–139. [Google Scholar] [CrossRef]
- Gatou, M.-A.; Vagena, I.-A.; Lagopati, N.; Pippa, N.; Gazouli, M.; Pavlatou, E.A. Functional MOF-Based Materials for Environmental and Biomedical Applications: A Critical Review. Nanomaterials 2023, 13, 2224. [Google Scholar] [CrossRef]
- Funda, G.; Taschieri, S.; Bruno, G.A.; Grecchi, E.; Paolo, S.; Girolamo, D.; Del Fabbro, M. Nanotechnology Scaffolds for Alveolar Bone Regeneration. Materials 2020, 13, 201. [Google Scholar] [CrossRef]
- Rios, H.F.; Lin, Z.; Oh, B.; Park, C.H.; Giannobile, W.V. Cell- and Gene-Based Therapeutic Strategies for Periodontal Regenerative Medicine. J. Periodontol. 2011, 82, 1223–1237. [Google Scholar] [CrossRef]
- Göker, F.; Ersanlı, S.; Arısan, V.; Cevher, E.; Güzel, E.E.; İşsever, H.; Ömer, B.; Durmuş Altun, G.; Morina, D.; Ekiz Yılmaz, T.; et al. Combined Effect of Parathyroid Hormone and Strontium Ranelate on Bone Healing in Ovariectomized Rats. Oral. Dis. 2018, 24, 1255–1269. [Google Scholar] [CrossRef]
- Saiz, E.; Zimmermann, E.A.; Lee, J.S.; Wegst, U.G.K.; Tomsia, A.P. Perspectives on the Role of Nanotechnology in Bone Tissue Engineering. Dent. Mater. 2013, 29, 103–115. [Google Scholar] [CrossRef]
- Walmsley, G.G.; McArdle, A.; Tevlin, R.; Momeni, A.; Atashroo, D.; Hu, M.S.; Feroze, A.H.; Wong, V.W.; Lorenz, P.H.; Longaker, M.T.; et al. Nanotechnology in Bone Tissue Engineering. Nanomedicine 2015, 11, 1253–1263. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Fu, X.; Li, Y.; Li, R. Aligned carbon nanofibers for nanofibers-guided bone regeneration and orthopedic applications: A pilot study. Arab. J. Chem. 2023, 16, 105075. [Google Scholar] [CrossRef]
- Alavi, S.E.; Cabot, P.J.; Raza, A.; Moyle, P.M. Developing GLP-1 conjugated self-assembling nanofibers using copper-catalyzed alkyne–azide cycloaddition and evaluation of their biological activity. Bioconjug. Chem. 2021, 32, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.E.; Gholami, M.; Shahmabadi, H.E.; Reher, P. Resorbable GBR Scaffolds in Oral and Maxillofacial Tissue Engineering: Design, Fabrication, and Applications. J. Clin. Med. 2023, 12, 6962. [Google Scholar] [CrossRef] [PubMed]
- Carbone, E.J.; Jiang, T.; Nelson, C.; Henry, N.; Lo, K.W.-H. Small Molecule Delivery through Nanofibrous Scaffolds for Musculoskeletal Regenerative Engineering. Nanomedicine 2014, 10, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour, S.; Ghazizadeh Ahsaie, M.; Rezai Rad, M.; Baghani, M.T.; Motamedian, S.R.; Khojasteh, A. Application of Selected Scaffolds for Bone Tissue Engineering: A Systematic Review. Oral Maxillofac. Surg. 2017, 21, 109–129. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Su, Y.; Kucine, A.J.; Cheng, K.; Zhu, D. Guided Bone Regeneration Using Barrier Membrane in Dental Applications. ACS Biomater. Sci. Eng. 2023, 9, 5457–5478. [Google Scholar] [CrossRef] [PubMed]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Patil, S.; Bhandi, S.; Bakri, M.M.H.; Albar, D.H.; Alzahrani, K.J.; Al-Ghamdi, M.S.; Alnfiai, M.M.; Tovani-Palone, M.R. Evaluation of Efficacy of Non-Resorbable Membranes Compared to Resorbable Membranes in Patients Undergoing Guided Bone Regeneration. Heliyon 2023, 9, e13488. [Google Scholar] [CrossRef]
- Deng, Y.; Liang, Y.; Liu, X. Biomaterials for Periodontal Regeneration. Dent. Clin. N. Am. 2022, 66, 659–672. [Google Scholar] [CrossRef]
- Hollister, S.J.; Pilipchuk, S.P.; Bartold, P.M.; Hutmacher, D.W.; Giannobile, W.V.; Ivanovski, S. Tissue Engineered Constructs for Periodontal Regeneration: Current Status and Future Perspectives. Adv. Healthc. Mater. 2018, 7, 1800457. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, Y.; Xue, Y.; Shi, J.; Zhang, X.; Liu, Y.; Midgley, A.C.; Wang, S. Multifunctional triple-layered composite scaffolds combining platelet-rich fibrin promote bone regeneration. ACS Biomater. Sci. Eng. 2019, 5, 6691–6702. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, M.; He, J. A review of biomimetic scaffolds for bone regeneration: Toward a cell-free strategy. Bioeng. Transl. Med. 2020, 6, e10206. [Google Scholar] [CrossRef] [PubMed]
- Ivanovski, S.; Vaquette, C.; Gronthos, S.; Hutmacher, D.W.; Bartold, P.M. Multiphasic Scaffolds for Periodontal Tissue Engineering. J. Dent. Res. 2014, 93, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Ivanovski, S. Periodontal Regeneration. Aust. Dent. J. 2009, 54, S118–S128. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zeng, X.; Zou, S.; Xu, Y.; Duan, P. Recent Advances in Horizontal Alveolar Bone Regeneration. Biomed. Mater. 2023, 18, 052004. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Mitchell, J.; Ivanovski, S. Recent Advances in Vertical Alveolar Bone Augmentation Using Additive Manufacturing Technologies. Front. Bioeng. Biotechnol. 2022, 9, 798393. [Google Scholar] [CrossRef]
- Urban, I.A.; Montero, E.; Amerio, E.; Palombo, D.; Monje, A. Techniques on Vertical Ridge Augmentation: Indications and Effectiveness. Periodontol. 2000 2023, 93, 153–182. [Google Scholar] [CrossRef]
- Benic, G.I.; Hämmerle, C.H. Horizontal bone augmentation by means of guided bone regeneration. Periodontol. 2000 2014, 66, 13–40. [Google Scholar] [CrossRef]
- Chen, K.; Zhao, L.; Huang, C.; Yin, X.; Zhang, X.; Li, P.; Gu, X.; Fan, Y. Recent Advances in the Development of Magnesium-Based Alloy Guided Bone Regeneration (GBR) Membrane. Metals 2022, 12, 2074. [Google Scholar] [CrossRef]
- Fok, M.R.; Pelekos, G.; Tonetti, M.S. Feasibility and Needs for Simultaneous or Staged Bone Augmentation to Place Prosthetically Guided Dental Implants after Extraction or Exfoliation of First Molars Due to Severe Periodontitis. J. Clin. Periodontol. 2020, 47, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- EzEldeen, M.; Moroni, L.; Nejad, Z.M.; Jacobs, R.; Mota, C. Biofabrication of Engineered Dento-Alveolar Tissue. Biomater. Adv. 2023, 148, 213371. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yu, Y.; Han, L.; Ma, S.; Zhao, J.; Chen, H.; Yang, Z.; Zhang, F.; Xia, Y.; Zhou, Y. Biocompatibility and Osteogenic Activity of Guided Bone Regeneration Membrane Based on Chitosan-Coated Magnesium Alloy. Mater. Sci. Eng. C. 2019, 100, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Ku, J.-K. Guided Bone Regeneration. J. Korean Assoc. Oral Maxillofac. Surg. 2020, 46, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M.S. Chitosan as a Vehicle for Growth Factor Delivery: Various Preparations and Their Applications in Bone Tissue Regeneration. Int. J. Biol. Macromol. 2017, 104, 1383–1397. [Google Scholar] [CrossRef] [PubMed]
- Omar, O.; Elgali, I.; Dahlin, C.; Thomsen, P. Barrier Membranes: More than the Barrier Effect? J. Clin. Periodontol. 2019, 46, 103–123. [Google Scholar] [CrossRef]
- Saito, E.; Saito, A.; Kuboki, Y.; Kimura, M.; Honma, Y.; Takahashi, T.; Kawanami, M. Periodontal Repair Following Implantation of Beta-Tricalcium Phosphate with Different Pore Structures in Class III Furcation Defects in Dogs. Dent. Mater. J. 2012, 31, 681–688. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The Effect of Mean Pore Size on Cell Attachment, Proliferation and Migration in Collagen–Glycosaminoglycan Scaffolds for Bone Tissue Engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone Grafts: Which Is the Ideal Biomaterial? J. Clin. Periodontol. 2019, 46, 92–102. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Finkemeier, C.G. Bone-Grafting and bone-graft subtitutes. J. Bone Jt. Surg. Am. 2002, 84, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Winkler, T.; Sass, F.A.; Duda, G.N.; Schmidt-Bleek, K. A Review of Biomaterials in Bone Defect Healing, Remaining Shortcomings and Future Opportunities for Bone Tissue Engineering. Bone Jt. Res. 2018, 7, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.-M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone Substitutes: A Review of Their Characteristics, Clinical Use, and Perspectives for Large Bone Defects Management. J. Tissue Eng. 2018, 9, 204173141877681. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.E.; Mosheiff, R. Tissue Engineering Approaches for Bone Repair: Concepts and Evidence. Injury 2011, 42, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, G.; Moghaddam, A. Allograft Bone Matrix versus Synthetic Bone Graft Substitutes. Injury 2011, 42, S16–S21. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Sánchez, I.; Ortiz-Vigón, A.; Sanz-Martín, I.; Figuero, E.; Sanz, M. Effectiveness of Lateral Bone Augmentation on the Alveolar Crest Dimension. J. Dent. Res. 2015, 94, 128S–142S. [Google Scholar] [CrossRef] [PubMed]
- Fukuba, S.; Okada, M.; Nohara, K.; Iwata, T. Alloplastic Bone Substitutes for Periodontal and Bone Regeneration in Dentistry: Current Status and Prospects. Materials 2021, 14, 1096. [Google Scholar] [CrossRef]
- Yip, I.; Ma, L.; Mattheos, N.; Dard, M.; Lang, N.P. Defect healing with various bone substitutes. Clin. Oral Implants Res. 2015, 26, 606–614. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef] [PubMed]
- Mayer, Y.; Zigdon-Giladi, H.; Machtei, E.E. Ridge Preservation Using Composite Alloplastic Materials: A Randomized Control Clinical and Histological Study in Humans. Clin. Implant Dent. Relat. Res. 2016, 18, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Rajula, M.P.B.; Narayanan, V.; Venkatasubbu, G.D.; Mani, R.C.; Sujana, A. Nano-hydroxyapatite: A Driving Force for Bone Tissue Engineering. J. Pharm. Bioallied Sci. 2021, 13 (Suppl. S1), S11–S14. [Google Scholar] [CrossRef]
- Saito, H.; Couso-Queiruga, E.; Shiau, H.J.; Stuhr, S.; Prasad, H.; Allareddy, T.V.; Reynolds, M.A.; Avila-Ortiz, G. Evaluation of poly lactic-co-glycolic acid-coated β-tricalcium phosphate for alveolar ridge preservation: A multicenter randomized controlled trial. J. Periodontol. 2021, 92, 524–535. [Google Scholar] [CrossRef]
- Sanz, M.; Dahlin, C.; Apatzidou, D.; Artzi, Z.; Bozic, D.; Calciolari, E.; De Bruyn, H.; Dommisch, H.; Donos, N.; Eickholz, P.; et al. Biomaterials and Regenerative Technologies Used in Bone Regeneration in the Craniomaxillofacial Region: Consensus Report of Group 2 of the 15th European Workshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46, 82–91. [Google Scholar] [CrossRef]
- Mizraji, G.; Davidzohn, A.; Gursoy, M.; Gursoy, U.K.; Shapira, L.; Wilensky, A. Membrane Barriers for Guided Bone Regeneration: An Overview of Available Biomaterials. Periodontol. 2000 2023, 93, 56–76. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, D. Recent Advances in GTR Scaffolds. Bioinformation 2022, 18, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.M.G.; Kowolik, M.J.; Janowski, G.M. Recent Advances in the Development of GTR/GBR Membranes for Periodontal Regeneration—A Materials Perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef]
- Reise, M.; Wyrwa, R.; Müller, U.; Zylinski, M.; Völpel, A.; Schnabelrauch, M.; Berg, A.; Jandt, K.D.; Watts, D.C.; Sigusch, B.W. Release of Metronidazole from Electrospun Poly(l-Lactide-Co-d/l-Lactide) Fibers for Local Periodontitis Treatment. Dent. Mater. 2012, 28, 179–188. [Google Scholar] [CrossRef]
- Ferracane, J.L.; Giannobile, W.V. Novel Biomaterials and Technologies for the Dental, Oral, and Craniofacial Structures. J. Dent. Res. 2014, 93, 1185–1186. [Google Scholar] [CrossRef]
- Fisher, S.; Franz-Odendaal, T. Evolution of the Bone Gene Regulatory Network. Curr. Opin. Genet. Dev. 2012, 22, 390–397. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous Scaffold Design for Tissue Engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Yamada, S.; Shanbhag, S.; Mustafa, K. Scaffolds in Periodontal Regenerative Treatment. Dent. Clin. N. Am. 2022, 66, 111–130. [Google Scholar] [CrossRef]
- Marin, E.; Boschetto, F.; Pezzotti, G. Biomaterials and Biocompatibility: An Historical Overview. J. Biomed. Mater. Res. A 2020, 108, 1617–1633. [Google Scholar] [CrossRef] [PubMed]
- Schulze, F.; Lang, A.; Schoon, J.; Wassilew, G.I.; Reichert, J. Scaffold Guided Bone Regeneration for the Treatment of Large Segmental Defects in Long Bones. Biomedicines 2023, 11, 325. [Google Scholar] [CrossRef]
- Mitra, D.; Whitehead, J.; Yasui, O.W.; Leach, J.K. Bioreactor Culture Duration of Engineered Constructs Influences Bone Formation by Mesenchymal Stem Cells. Biomaterials 2017, 146, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczak, P.; Przekora, A. Osteoconductive and Osteoinductive Surface Modifications of Biomaterials for Bone Regeneration: A Concise Review. Coatings 2020, 10, 971. [Google Scholar] [CrossRef]
- Overmann, A.L.; Aparicio, C.; Richards, J.T.; Mutreja, I.; Fischer, N.G.; Wade, S.M.; Potter, B.K.; Davis, T.A.; Bechtold, J.E.; Forsberg, J.A.; et al. Orthopaedic Osseointegration: Implantology and Future Directions. J. Orthop. Res. 2020, 38, 1445–1454. [Google Scholar] [CrossRef]
- Pawelec, K.M.; Planell, J.A. Bone Repair Biomaterials; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780081024515. [Google Scholar]
- Yannas, I.V. Regeneration of Skin. In Tissue and Organ Regeneration in Adults; Springer: New York, NY, USA, 2015; pp. 89–136. [Google Scholar]
- Latimer, J.M.; Maekawa, S.; Yao, Y.; Wu, D.T.; Chen, M.; Giannobile, W.V. Regenerative Medicine Technologies to Treat Dental, Oral, and Craniofacial Defects. Front. Bioeng. Biotechnol. 2021, 9, 704048. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The Development of Collagen Based Composite Scaffolds for Bone Regeneration. Bioact. Mater. 2018, 3, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Scheinpflug, J.; Pfeiffenberger, M.; Damerau, A.; Schwarz, F.; Textor, M.; Lang, A.; Schulze, F. Journey into Bone Models: A Review. Genes 2018, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & Scaffolds for Tissue Engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.A. Blood Clots and Tissue Regeneration of 3D Printed Dual Scale Porous Polymeric Scaffolds. Mater. Lett. 2021, 285, 129184. [Google Scholar] [CrossRef]
- Li, Z.; Lv, X.; Chen, S.; Wang, B.; Feng, C.; Xu, Y.; Wang, H. Improved Cell Infiltration and Vascularization of Three-Dimensional Bacterial Cellulose Nanofibrous Scaffolds by Template Biosynthesis. RSC Adv. 2016, 6, 42229–42239. [Google Scholar] [CrossRef]
- Hong, W.X.; Hu, M.S.; Esquivel, M.; Liang, G.Y.; Rennert, R.C.; McArdle, A.; Paik, K.J.; Duscher, D.; Gurtner, G.C.; Lorenz, H.P.; et al. The Role of Hypoxia-Inducible Factor in Wound Healing. Adv. Wound Care 2014, 3, 390–399. [Google Scholar] [CrossRef]
- Darby, I.A.; Hewitson, T.D. Hypoxia in Tissue Repair and Fibrosis. Cell Tissue Res. 2016, 365, 553–562. [Google Scholar] [CrossRef]
- Klenke, F.M.; Liu, Y.; Yuan, H.; Hunziker, E.B.; Siebenrock, K.A.; Hofstetter, W. Impact of Pore Size on the Vascularization and Osseointegration of Ceramic Bone Substitutes in Vivo. J. Biomed. Mater. Res. A 2008, 85A, 777–786. [Google Scholar] [CrossRef]
- Murphy, C.M.; O’Brien, F.J. Understanding the Effect of Mean Pore Size on Cell Activity in Collagen-Glycosaminoglycan Scaffolds. Cell Adh. Migr. 2010, 4, 377–381. [Google Scholar] [CrossRef]
- Jin, H.; Zhuo, Y.; Sun, Y.; Fu, H.; Han, Z. Microstructure Design and Degradation Performance in Vitro of Three-Dimensional Printed Bioscaffold for Bone Tissue Engineering. Adv. Mech. Eng. 2019, 11, 168781401988378. [Google Scholar] [CrossRef]
- Botchwey, E.A.; Dupree, M.A.; Pollack, S.R.; Levine, E.M.; Laurencin, C.T. Tissue Engineered Bone: Measurement of Nutrient Transport in Three-dimensional Matrices. J. Biomed. Mater. Res. A 2003, 67A, 357–367. [Google Scholar] [CrossRef]
- Mastrullo, V.; Cathery, W.; Velliou, E.; Madeddu, P.; Campagnolo, P. Angiogenesis in Tissue Engineering: As Nature Intended? Front. Bioeng. Biotechnol. 2020, 8, 188. [Google Scholar] [CrossRef]
- Dutta, R.C.; Dey, M.; Dutta, A.K.; Basu, B. Competent Processing Techniques for Scaffolds in Tissue Engineering. Biotechnol. Adv. 2017, 35, 240–250. [Google Scholar] [CrossRef]
- Nakayama, K.H.; Hou, L.; Huang, N.F. Role of Extracellular Matrix Signaling Cues in Modulating Cell Fate Commitment for Cardiovascular Tissue Engineering. Adv. Healthc. Mater. 2014, 3, 628–641. [Google Scholar] [CrossRef] [PubMed]
- Tavelli, L.; McGuire, M.K.; Zucchelli, G.; Rasperini, G.; Feinberg, S.E.; Wang, H.; Giannobile, W.V. Extracellular Matrix-based Scaffolding Technologies for Periodontal and Peri-implant Soft Tissue Regeneration. J. Periodontol. 2020, 91, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.; Vaquette, C.; Theodoropoulos, C.; Hamlet, S.M.; Hutmacher, D.W.; Ivanovski, S. Decellularized Periodontal Ligament Cell Sheets with Recellularization Potential. J. Dent. Res. 2014, 93, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.; Vaquette, C.; Hutmacher, D.W.; Bartold, P.M.; Ivanovski, S. Fabrication and Characterization of Decellularized Periodontal Ligament Cell Sheet Constructs. Methods Mol. Biol. 2017, 1537, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.; Vaquette, C.; Hutmacher, D.W.; Bartold, P.M.; Ivanovski, S. Fabrication and Characterization of Decellularized Periodontal Ligament Cell Sheet Constructs. Methods Mol. Biol. 2023, 2588, 429–438. [Google Scholar] [CrossRef]
- Son, H.; Jeon, M.; Choi, H.-J.; Lee, H.-S.; Kim, I.-H.; Kang, C.-M.; Song, J.S. Decellularized Human Periodontal Ligament for Periodontium Regeneration. PLoS ONE 2019, 14, e0221236. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, E.; Sun, Y.; Jacobs, R.; Politis, C. Three-Dimensional Printed Final Occlusal Splint for Orthognathic Surgery: Design and Validation. Int. J. Oral Maxillofac. Surg. 2017, 46, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, N.; Endo, F.; Maeda, T.; Hotta, A. Electrospinning and Surface Modification Methods for Functionalized Cell Scaffolds. In Nanostructures for Novel Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 201–225. [Google Scholar]
- Lannutti, J.; Reneker, D.; Ma, T.; Tomasko, D.; Farson, D. Electrospinning for Tissue Engineering Scaffolds. Mater. Sci. Eng. C 2007, 27, 504–509. [Google Scholar] [CrossRef]
- Farag, A.; Hashimi, S.M.; Vaquette, C.; Bartold, P.M.; Hutmacher, D.W.; Ivanovski, S. The Effect of Decellularized Tissue Engineered Constructs on Periodontal Regeneration. J. Clin. Periodontol. 2018, 45, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Cortez Tornello, P.R.; Caracciolo, P.C.; Igartúa Roselló, J.I.; Abraham, G.A. Electrospun Scaffolds with Enlarged Pore Size: Porosimetry Analysis. Mater. Lett. 2018, 227, 191–193. [Google Scholar] [CrossRef]
- Jeon, J.E.; Vaquette, C.; Klein, T.J.; Hutmacher, D.W. Perspectives in Multiphasic Osteochondral Tissue Engineering. Anat. Rec. 2014, 297, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.; Camarero-Espinosa, S.; Baker, M.B.; Wieringa, P.; Moroni, L. Bioprinting: From Tissue and Organ Development to in Vitro Models. Chem. Rev. 2020, 120, 10547–10607. [Google Scholar] [CrossRef] [PubMed]
- Washio, K.; Tsutsumi, Y.; Tsumanuma, Y.; Yano, K.; Srithanyarat, S.S.; Takagi, R.; Ichinose, S.; Meinzer, W.; Yamato, M.; Okano, T.; et al. In Vivo Periodontium Formation Around Titanium Implants Using Periodontal Ligament Cell Sheet. Tissue Eng. Part. A 2018, 24, 1273–1282. [Google Scholar] [CrossRef]
- Monteiro, N.; Smith, E.E.; Angstadt, S.; Zhang, W.; Khademhosseini, A.; Yelick, P.C. Dental Cell Sheet Biomimetic Tooth Bud Model. Biomaterials 2016, 106, 167–179. [Google Scholar] [CrossRef]
- White, J.; Foley, M.; Rowley, A. A Novel Approach to 3D-Printed Fabrics and Garments. 3D Print. Addit. Manuf. 2015, 2, 145–149. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Rao Kummara, M.; Kamal, T.; Alghyamah, A.-A.A.; Jan Iftikhar, F.; Bano, B.; Khan, N.; Amjid Afridi, M.; Soo Han, S.; et al. Advances in the Scaffolds Fabrication Techniques Using Biocompatible Polymers and Their Biomedical Application: A Technical and Statistical Review. J. Saudi Chem. Soc. 2020, 24, 186–215. [Google Scholar] [CrossRef]
- Farag, M.M. Recent Trends on Biomaterials for Tissue Regeneration Applications: Review. J. Mater. Sci. 2023, 58, 527–558. [Google Scholar] [CrossRef]
- Li, Z.; Xie, M.-B.; Li, Y.; Ma, Y.; Li, J.-S.; Dai, F.-Y. Recent Progress in Tissue Engineering and Regenerative Medicine. J. Biomater. Tissue Eng. 2016, 6, 755–766. [Google Scholar] [CrossRef]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludağ, H. Current State of Fabrication Technologies and Materials for Bone Tissue Engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Arslan, Y.E.; Sezgin Arslan, T.; Derkus, B.; Emregul, E.; Emregul, K.C. Fabrication of Human Hair Keratin/Jellyfish Collagen/Eggshell-Derived Hydroxyapatite Osteoinductive Biocomposite Scaffolds for Bone Tissue Engineering: From Waste to Regenerative Medicine Products. Colloids Surf. B Biointerfaces 2017, 154, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Canciani, E.; Gagliano, N.; Paino, F.; Amler, E.; Divin, R.; Denti, L.; Henin, D.; Fiorati, A.; Dellavia, C. Polyblend Nanofibers to Regenerate Gingival Tissue: A Preliminary In Vitro Study. Front. Mater. 2021, 8, 670010. [Google Scholar] [CrossRef]
- Ghavimi, M.A.; Bani Shahabadi, A.; Jarolmasjed, S.; Memar, M.Y.; Maleki Dizaj, S.; Sharifi, S. Nanofibrous Asymmetric Collagen/Curcumin Membrane Containing Aspirin-Loaded PLGA Nanoparticles for Guided Bone Regeneration. Sci. Rep. 2020, 10, 18200. [Google Scholar] [CrossRef]
- Lim, J.; Jang, K.-J.; Son, H.; Park, S.; Kim, J.; Kim, H.; Seonwoo, H.; Choung, Y.-H.; Lee, M.; Chung, J. Aligned Nanofiber-Guided Bone Regeneration Barrier Incorporated with Equine Bone-Derived Hydroxyapatite for Alveolar Bone Regeneration. Polymers 2020, 13, 60. [Google Scholar] [CrossRef]
- Boda, S.K.; Almoshari, Y.; Wang, H.; Wang, X.; Reinhardt, R.A.; Duan, B.; Wang, D.; Xie, J. Mineralized Nanofiber Segments Coupled with Calcium-Binding BMP-2 Peptides for Alveolar Bone Regeneration. Acta Biomater. 2019, 85, 282–293. [Google Scholar] [CrossRef]
- Pouponneau, P.; Perrey, O.; Brunon, C.; Grossiord, C.; Courtois, N.; Salles, V.; Alves, A. Electrospun Bioresorbable Membrane Eluting Chlorhexidine for Dental Implants. Polymers 2020, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Watcharajittanont, N.; Putson, C.; Pripatnanont, P.; Meesane, J. Electrospun Polyurethane Fibrous Membranes of Mimicked Extracellular Matrix for Periodontal Ligament: Molecular Behavior, Mechanical Properties, Morphology, and Osseointegration. J. Biomater. Appl. 2020, 34, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Fan, W.; Xiao, Y.; Hamlet, S.; Hutmacher, D.W.; Ivanovski, S. A Biphasic Scaffold Design Combined with Cell Sheet Technology for Simultaneous Regeneration of Alveolar Bone/Periodontal Ligament Complex. Biomaterials 2012, 33, 5560–5573. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; de Malheiro, A.B.F.B.; van Blitterswijk, C.; Mota, C.; Wieringa, P.A.; Moroni, L. Direct Writing Electrospinning of Scaffolds with Multidimensional Fiber Architecture for Hierarchical Tissue Engineering. ACS Appl. Mater. Interfaces 2017, 9, 38187–38200. [Google Scholar] [CrossRef]
- Moonesi Rad, R.; Atila, D.; Evis, Z.; Keskin, D.; Tezcaner, A. Development of a Novel Functionally Graded Membrane Containing Boron-modified Bioactive Glass Nanoparticles for Guided Bone Regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 1331–1345. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Mitchell, J.; Fernandez-Medina, T.; Kumar, S.; Ivanovski, S. Resorbable Additively Manufactured Scaffold Imparts Dimensional Stability to Extraskeletally Regenerated Bone. Biomaterials 2021, 269, 120671. [Google Scholar] [CrossRef] [PubMed]
- Sudheesh Kumar, P.T.; Hashimi, S.; Saifzadeh, S.; Ivanovski, S.; Vaquette, C. Additively Manufactured Biphasic Construct Loaded with BMP-2 for Vertical Bone Regeneration: A Pilot Study in Rabbit. Mater. Sci. Eng. C 2018, 92, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.; Puppi, D.; Chiellini, F.; Chiellini, E. Additive Manufacturing Techniques for the Production of Tissue Engineering Constructs. J. Tissue Eng. Regen. Med. 2015, 9, 174–190. [Google Scholar] [CrossRef]
- Kaliampakou, C.; Lagopati, N.; Charitidis, C.A. Direct Ink Writing of Alginate–Gelatin Hydrogel: An Optimization of Ink Property Design and Printing Process Efficacy. Appl. Sci. 2023, 13, 8261. [Google Scholar] [CrossRef]
- Verykokou, S.; Ioannidis, C.; Angelopoulos, C. CBCT-Based Design of Patient-Specific 3D Bone Grafts for Periodontal Regeneration. J. Clin. Med. 2023, 12, 5023. [Google Scholar] [CrossRef]
- Yang, W.; Chen, D.; Wang, C.; Apicella, D.; Apicella, A.; Huang, Y.; Li, L.; Zheng, L.; Ji, P.; Wang, L.; et al. The Effect of Bone Defect Size on the 3D Accuracy of Alveolar Bone Augmentation Performed with Additively Manufactured Patient-Specific Titanium Mesh. BMC Oral Health 2022, 22, 557. [Google Scholar] [CrossRef]
- Chi, C.-Y.; Chen, C.-Y.; Huang, J.-Y.; Kuan, C.-Y.; Lin, Y.-Y.; Li, C.-H.; Yang, C.-C.; Lin, F.-H. Preparation and In-Vitro Evaluation of Fe2O3-Doped DP-Bioglass in Combination with 3D-Printing and Selective Laser Sintering Process (3DP-SLS) for Alveolar Bone Augmentation. Ceram. Int. 2021, 47, 12725–12734. [Google Scholar] [CrossRef]
- Rasperini, G.; Pilipchuk, S.P.; Flanagan, C.L.; Park, C.H.; Pagni, G.; Hollister, S.J.; Giannobile, W.V. 3D-Printed Bioresorbable Scaffold for Periodontal Repair. J. Dent. Res. 2015, 94, 153S–157S. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, C.; Li, X.; Fu, G.; Chen, D.; Huang, Y. Research on the Dimensional Accuracy of Customized Bone Augmentation Combined with 3D-printing Individualized Titanium Mesh: A Retrospective Case Series Study. Clin. Implant Dent. Relat. Res. 2021, 23, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, C.H.; Kim, B.K.; Mao, J.J. Anatomically Shaped Tooth and Periodontal Regeneration by Cell Homing. J. Dent. Res. 2010, 89, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Kaliampakou, C.; Lagopati, N.; Pavlatou, E.A.; Charitidis, C.A. Alginate–Gelatin Hydrogel Scaffolds; An Optimization of Post-Printing Treatment for Enhanced Degradation and Swelling Behavior. Gels 2023, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Gillispie, G.J.; Copus, J.S.; Zhang, W.; Atala, A.; Yoo, J.J.; Yelick, P.C.; Lee, S.J. The Effect of BMP-Mimetic Peptide Tethering Bioinks on the Differentiation of Dental Pulp Stem Cells (DPSCs) in 3D Bioprinted Dental Constructs. Biofabrication 2020, 12, 035029. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, X. Synthetic Polymers for Organ 3D Printing. Polymers 2020, 12, 1765. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Kundu, S.; Nag, A.; Xu, Y. 3D Printed Sensors for Biomedical Applications: A Review. Sensors 2019, 19, 1706. [Google Scholar] [CrossRef] [PubMed]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, S.F.; Young, F.A.; Mathews, R.S.; Klawitter, J.J.; Talbert, C.D.; Stelling, F.H. Potential of ceramic materials as permanently implantable skeletal prostheses. J. Biomed. Mater. Res. 1970, 4, 433–456. [Google Scholar] [CrossRef]
- Iviglia, G.; Kargozar, S.; Baino, F. Biomaterials, Current Strategies, and Novel Nano-Technological Approaches for Periodontal Regeneration. J. Funct. Biomater. 2019, 10, 3. [Google Scholar] [CrossRef]
- Jin, Q.M.; Takita, H.; Kohgo, T.; Atsumi, K.; Itoh, H.; Kuboki, Y. Effects of geometry of hydroxyapatite as a cell substratum in BMP-induced ectopic bone formation. J. Biomed. Mater. Res. 2000, 52, 491–499. [Google Scholar] [CrossRef]
- Kuboki, Y.; Jin, Q.; Kikuchi, M.; Mamood, J.; Takita, H. Geometry of artificial ECM: Sizes of pores controlling phenotype expression in BMP-induced osteogenesis and chondrogenesis. Connect. Tissue Res. 2002, 43, 529–534. [Google Scholar] [CrossRef]
- Kuboki, Y.; Jin, Q.; Takita, H. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. J. Bone Jt. Surg. Am. 2001, 83, S105–S115. [Google Scholar] [CrossRef]
- Groll, J.; Burdick, J.A.; Cho, D.-W.; Derby, B.; Gelinsky, M.; Heilshorn, S.C.; Jüngst, T.; Malda, J.; Mironov, V.A.; Nakayama, K.; et al. A Definition of Bioinks and Their Distinction from Biomaterial Inks. Biofabrication 2018, 11, 013001. [Google Scholar] [CrossRef] [PubMed]
- Thattaruparambil Raveendran, N.; Vaquette, C.; Meinert, C.; Samuel Ipe, D.; Ivanovski, S. Optimization of 3D Bioprinting of Periodontal Ligament Cells. Dent. Mater. 2019, 35, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.; Moroni, L. High Throughput Screening with Biofabrication Platforms. In Essentials of 3D Biofabrication and Translation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 187–213. [Google Scholar]
- Narazaki, A.; Oyane, A.; Miyaji, H. Laser-Induced Forward Transfer with Optical Stamp of a Protein-Immobilized Calcium Phosphate Film Prepared by Biomimetic Process to a Human Dentin. Appl. Sci. 2020, 10, 7984. [Google Scholar] [CrossRef]
- Catros, S.; Fricain, J.-C.; Guillotin, B.; Pippenger, B.; Bareille, R.; Remy, M.; Lebraud, E.; Desbat, B.; Amédée, J.; Guillemot, F. Laser-Assisted Bioprinting for Creating on-Demand Patterns of Human Osteoprogenitor Cells and Nano-Hydroxyapatite. Biofabrication 2011, 3, 025001. [Google Scholar] [CrossRef]
- Ventura, R.D. An Overview of Laser-Assisted Bioprinting (LAB) in Tissue Engineering Applications. Med. Lasers 2021, 10, 76–81. [Google Scholar] [CrossRef]
- Kérourédan, O.; Ribot, E.J.; Fricain, J.-C.; Devillard, R.; Miraux, S. Magnetic Resonance Imaging for Tracking Cellular Patterns Obtained by Laser-Assisted Bioprinting. Sci. Rep. 2018, 8, 15777. [Google Scholar] [CrossRef]
- Preethi Soundarya, S.; Haritha Menon, A.; Viji Chandran, S.; Selvamurugan, N. Bone Tissue Engineering: Scaffold Preparation Using Chitosan and Other Biomaterials with Different Design and Fabrication Techniques. Int. J. Biol. Macromol. 2018, 119, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Lagopati, N.; Pippa, N.; Gatou, M.-A.; Papadopoulou-Fermeli, N.; Gorgoulis, V.G.; Gazouli, M.; Pavlatou, E.A. Marine-Originated Materials and Their Potential Use in Biomedicine. Appl. Sci. 2023, 13, 9172. [Google Scholar] [CrossRef]
- Shrestha, S.; Shrestha, B.K.; Ko, S.W.; Kandel, R.; Park, C.H.; Kim, C.S. Engineered cellular microenvironments from functionalized multiwalled carbon nanotubes integrating Zein/Chitosan @Polyurethane for bone cell regeneration. Carbohydr. Polym. 2021, 251, 117035. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.X.; Langer, R. Fabrication of Biodegradable Polymer Foams for Cell Transplantation and Tissue Engineering. In Tissue Engineering; Humana Press: Totowa, NJ, USA, 1999; pp. 47–56. [Google Scholar]
- Zimina, A.; Senatov, F.; Choudhary, R.; Kolesnikov, E.; Anisimova, N.; Kiselevskiy, M.; Orlova, P.; Strukova, N.; Generalova, M.; Manskikh, V.; et al. Biocompatibility and Physico-Chemical Properties of Highly Porous PLA/HA Scaffolds for Bone Reconstruction. Polymers 2020, 12, 2938. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-T.; Ramesh, N.-S. Polymeric Foams; Lee, S.-T., Ramesh, N.S., Eds.; CRC Press: Boca Raton, FL, USA, 2004; ISBN 9780203506141. [Google Scholar]
- Fanovich, M.A.; Di Maio, E.; Salerno, A. Current Trend and New Opportunities for Multifunctional Bio-Scaffold Fabrication via High-Pressure Foaming. J. Funct. Biomater. 2023, 14, 480. [Google Scholar] [CrossRef] [PubMed]
- Chinnasami, H.; Dey, M.K.; Devireddy, R. Three-Dimensional Scaffolds for Bone Tissue Engineering. Bioengineering 2023, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.I. Polymer Synthesis and Processing Using Supercritical Carbon Dioxide. J. Mater. Chem. 2000, 10, 207–234. [Google Scholar] [CrossRef]
- Sukpaita, T.; Chirachanchai, S.; Pimkhaokham, A.; Ampornaramveth, R.S. Chitosan-Based Scaffold for Mineralized Tissues Regeneration. Mar. Drugs 2021, 19, 551. [Google Scholar] [CrossRef]
- Gilpin, A.; Yang, Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. BioMed Res. Int. 2017, 2017, 9831534. [Google Scholar] [CrossRef]
- Santoso, E.G.; Yoshida, K.; Hirota, Y.; Aizawa, M.; Yoshino, O.; Kishida, A.; Osuga, Y.; Saito, S.; Ushida, T.; Furukawa, K.S. Application of Detergents or High Hydrostatic Pressure as Decellularization Processes in Uterine Tissues and Their Subsequent Effects on In Vivo Uterine Regeneration in Murine Models. PLoS ONE 2014, 9, e103201. [Google Scholar] [CrossRef] [PubMed]
- Smoak, M.M.; Han, A.; Watson, E.; Kishan, A.; Grande-Allen, K.J.; Cosgriff-Hernandez, E.; Mikos, A.G. Fabrication and Characterization of Electrospun Decellularized Muscle-Derived Scaffolds. Tissue Eng. Part C Methods 2019, 25, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.S.; Cordeiro, R.; Moura, C.S.; Cabral, J.M.S.; Castelo Ferreira, F.; Silva, J.C.; Carvalho, M.S. Bioactive Nanofibrous Scaffolds Incorporating Decellularized Cell-Derived Extracellular Matrix for Periodontal Tissue Engineering. ACS Appl. Nano Mater. 2024, 7, 4501–4517. [Google Scholar] [CrossRef]
- Leong, K.F.; Cheah, C.M.; Chua, C.K. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials 2003, 24, 2363–2378. [Google Scholar] [CrossRef]
- Yuan, B.; Zhou, S.Y.; Chen, X.S. Rapid prototyping technology and its application in bone tissue engineering. J. Zhejiang Univ. Sci. B. 2017, 18, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Rider, P.; Kačarević, Ž.P.; Alkildani, S.; Retnasingh, S.; Schnettler, R.; Barbeck, M. Additive Manufacturing for Guided Bone Regeneration: A Perspective for Alveolar Ridge Augmentation. Int. J. Mol. Sci. 2018, 19, 3308. [Google Scholar] [CrossRef] [PubMed]
- Tom, T.; Sreenilayam, S.P.; Brabazon, D.; Jose, J.P.; Joseph, B.; Madanan, K.; Thomas, S. Additive manufacturing in the biomedical field-recent research developments. Results Eng. 2022, 16, 100661. [Google Scholar] [CrossRef]
- Alqahtani, A.M. Guided Tissue and Bone Regeneration Membranes: A Review of Biomaterials and Techniques for Periodont Treatments. Polymers 2023, 15, 3355. [Google Scholar] [CrossRef]
- Stafin, K.; Śliwa, P.; Piątkowski, M. Towards Polycaprolactone-Based Scaffolds for Alveolar Bone Tissue Engineering: A Biomimetic Approach in a 3D Printing Technique. Int. J. Mol. Sci. 2023, 24, 16180. [Google Scholar] [CrossRef]
- Khalaf, A.T.; Wei, Y.; Wan, J.; Zhu, J.; Peng, Y.; Abdul Kadir, S.Y.; Zainol, J.; Oglah, Z.; Cheng, L.; Shi, Z. Bone Tissue Engineering through 3D Bioprinting of Bioceramic Scaffolds: A Review and Update. Life 2022, 12, 903. [Google Scholar] [CrossRef]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 3429527, 2019. [Google Scholar] [CrossRef]
- Anjum, S.; Rahman, F.; Pandey, P.; Arya, D.K.; Alam, M.; Rajinikanth, P.S.; Ao, Q. Electrospun Biomimetic Nanofibrous Scaffolds: A Promising Prospect for Bone Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 9206. [Google Scholar] [CrossRef] [PubMed]
- Kumbar, S.G.; James, R.; Nukavarapu, S.P.; Laurencin, C.T. Electrospun nanofiber scaffolds: Engineering soft tissues. Biomed. Mater. 2008, 3, 034002. [Google Scholar] [CrossRef]
- Khoramgah, M.S.; Ranjbari, J.; Abbaszadeh, H.A.; Tabatabaei Mirakabad, F.S.; Hatami, S.; Hosseinzadeh, S.; Ghanbarian, H. Freeze-dried multiscale porous nanofibrous three dimensional scaffolds for bone regenerations. Bioimpacts 2020, 10, 73–85. [Google Scholar] [CrossRef]
- Biscaia, S.; Branquinho, M.V.; Alvites, R.D.; Fonseca, R.; Sousa, A.C.; Pedrosa, S.S.; Caseiro, A.R.; Guedes, F.; Patrício, T.; Viana, T.; et al. 3D Printed Poly(ε-caprolactone)/Hydroxyapatite Scaffolds for Bone Tissue Engineering: A Comparative Study on a Composite Preparation by Melt Blending or Solvent Casting Techniques and the Influence of Bioceramic Content on Scaffold Properties. Int. J. Mol. Sci. 2022, 23, 2318. [Google Scholar] [CrossRef] [PubMed]
- Naghieh, S.; Sarker, M.; Izadifar, M.; Chen, X. Dispensing-based bioprinting of mechanically-functional hybrid scaffolds with vessel-like channels for tissue engineering applications—A brief review. J. Mech. Behav. Biomed. Mater. 2018, 78, 298–314. [Google Scholar] [CrossRef]
- Kim, D.; Lee, H.; Lee, G.-H.; Hoang, T.-H.; Kim, H.-R.; Kim, G.H. Fabrication of bone-derived decellularized extracellular matrix/ceramic-based biocomposites and their osteo/odontogenic differentiation ability for dentin regeneration. Bioeng. Transl. Med. 2022, 7, e10317. [Google Scholar] [CrossRef]
- Ostrovidov, S.; Ramalingam, M.; Bae, H.; Orive, G.; Fujie, T.; Shi, X.; Kaji, H. Bioprinting and biomaterials for dental alveolar tissue regeneration. Front. Bioeng. Biotechnol. 2023, 11, 991821. [Google Scholar] [CrossRef]
- Ananth, K.P.; Jayram, N.D. A comprehensive review of 3D printing techniques for biomaterial-based scaffold fabrication in bone tissue engineering. Ann. 3D Print. Med. 2024, 13, 100141. [Google Scholar] [CrossRef]
- Logeshwaran, A.; Elsen, R.; Nayak, S. Artificial Intelligence-Based 3D Printing Strategies for Bone Scaffold Fabrication and Its Application in Preclinical and Clinical Investigations. ACS Biomater. Sci. Eng. 2024, 10, 677–696. [Google Scholar] [CrossRef]
- Rahmati, M.; Pennisi, C.P.; Budd, E.; Mobasheri, A.; Mozafari, M. Biomaterials for Regenerative Medicine: Historical Perspectives and Current Trends. In Cell Biology and Translational Medicine; Springer: Cham, Switzerland, 2018; pp. 1–19. [Google Scholar]
- Geevarghese, R.; Sajjadi, S.S.; Hudecki, A.; Sajjadi, S.; Jalal, N.R.; Madrakian, T.; Ahmadi, M.; Włodarczyk-Biegun, M.K.; Ghavami, S.; Likus, W.; et al. Biodegradable and Non-Biodegradable Biomaterials and Their Effect on Cell Differentiation. Int. J. Mol. Sci. 2022, 23, 16185. [Google Scholar] [CrossRef]
- Singh, A.; Elisseeff, J. Biomaterials for Stem Cell Differentiation. J. Mater. Chem. 2010, 20, 8832. [Google Scholar] [CrossRef]
- Dawson, E.; Mapili, G.; Erickson, K.; Taqvi, S.; Roy, K. Biomaterials for Stem Cell Differentiation. Adv. Drug Deliv. Rev. 2008, 60, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Prato, G.P.; Cortellini, P. Factors Affecting the Healing Response of Intrabony Defects Following Guided Tissue Regeneration and Access Flap Surgery. J. Clin. Periodontol. 1996, 23, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Sufaru, I.-G.; Macovei, G.; Stoleriu, S.; Martu, M.-A.; Luchian, I.; Kappenberg-Nitescu, D.-C.; Solomon, S.M. 3D Printed and Bioprinted Membranes and Scaffolds for the Periodontal Tissue Regeneration: A Narrative Review. Membranes 2022, 12, 902. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.-S.D.; Costa, P.F.; Vaquette, C.; Ivanovski, S.; Hutmacher, D.W.; Malda, J. Additive Biomanufacturing: An Advanced Approach for Periodontal Tissue Regeneration. Ann. Biomed. Eng. 2017, 45, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Daghrery, A.; de Souza Araújo, I.J.; Castilho, M.; Malda, J.; Bottino, M.C. Unveiling the Potential of Melt Electrowriting in Regenerative Dental Medicine. Acta Biomater. 2023, 156, 88–109. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Tai, W.; Ho, M.; Chang, P. Combination of a Biomolecule-aided Biphasic Cryogel Scaffold with a Barrier Membrane Adhering PDGF-encapsulated Nanofibers to Promote Periodontal Regeneration. J. Periodontal Res. 2020, 55, 529–538. [Google Scholar] [CrossRef]
- Costa, P.F.; Vaquette, C.; Zhang, Q.; Reis, R.L.; Ivanovski, S.; Hutmacher, D.W. Advanced Tissue Engineering Scaffold Design for Regeneration of the Complex Hierarchical Periodontal Structure. J. Clin. Periodontol. 2014, 41, 283–294. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, S.; Zhou, C.; Cheng, L.; Gao, X.; Xie, X.; Sun, J.; Wang, H.; Weir, M.D.; Reynolds, M.A.; et al. Advanced Smart Biomaterials and Constructs for Hard Tissue Engineering and Regeneration. Bone Res. 2018, 6, 31. [Google Scholar] [CrossRef]
- Motamedian, S.R.; Hosseinpour, S.; Ahsaie, M.G.; Khojasteh, A. Smart Scaffolds in Bone Tissue Engineering: A Systematic Review of Literature. World J. Stem Cells 2015, 7, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Tanaka, M. Designing Smart Biomaterials for Tissue Engineering. Int. J. Mol. Sci. 2017, 19, 17. [Google Scholar] [CrossRef]
- Mittal, A.; Negi, P.; Garkhal, K.; Verma, S.; Kumar, N. Integration of Porosity and Bio-Functionalization to Form a 3D Scaffold: Cell Culture Studies and in Vitro Degradation. Biomed. Mater. 2010, 5, 045001. [Google Scholar] [CrossRef]
- Zeng, D.; Zhang, X.; Wang, X.; Huang, Q.; Wen, J.; Miao, X.; Peng, L.; Li, Y.; Jiang, X. The Osteoimmunomodulatory Properties of MBG Scaffold Coated with Amino Functional Groups. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, K.; Gong, T.; Song, J.; Bao, C.; Luo, E.; Weng, J.; Zhou, S. Delivery of Growth Factors Using a Smart Porous Nanocomposite Scaffold to Repair a Mandibular Bone Defect. Biomacromolecules 2014, 15, 1019–1030. [Google Scholar] [CrossRef]
- Damaraju, S.M.; Shen, Y.; Elele, E.; Khusid, B.; Eshghinejad, A.; Li, J.; Jaffe, M.; Arinzeh, T.L. Three-Dimensional Piezoelectric Fibrous Scaffolds Selectively Promote Mesenchymal Stem Cell Differentiation. Biomaterials 2017, 149, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Dan, P.; Sosnik, A.; Kalarikkal, N.; Tran, N.; Vincent, B.; Thomas, S.; Menu, P.; Rouxel, D. Electrospun Poly(Vinylidene Fluoride-Trifluoroethylene)/Zinc Oxide Nanocomposite Tissue Engineering Scaffolds with Enhanced Cell Adhesion and Blood Vessel Formation. Nano Res. 2017, 10, 3358–3376. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent Advances in 3D Printing of Biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef]
- Yu, N.; Nguyen, T.; Cho, Y.D.; Kavanagh, N.M.; Ghassib, I.; Giannobile, W.V. Personalized Scaffolding Technologies for Alveolar Bone Regenerative Medicine. Orthod. Craniofac. Res. 2019, 22, 69–75. [Google Scholar] [CrossRef]
- Yazdanian, M.; Arefi, A.H.; Alam, M.; Abbasi, K.; Tebyaniyan, H.; Tahmasebi, E.; Ranjbar, R.; Seifalian, A.; Rahbar, M. Decellularized and biological scaffolds in dental and craniofacial tissue engineering: A comprehensive overview. J. Mater. Res. Technol. 2021, 15, 1217–1251. [Google Scholar] [CrossRef]
- Park, S.-H.; Kang, B.-K.; Lee, J.E.; Chun, S.W.; Jang, K.; Kim, Y.H.; Jeong, M.A.; Kim, Y.; Kang, K.; Lee, N.K.; et al. Design and Fabrication of a Thin-Walled Free-Form Scaffold on the Basis of Medical Image Data and a 3D Printed Template: Its Potential Use in Bile Duct Regeneration. ACS Appl. Mater. Interfaces 2017, 9, 12290–12298. [Google Scholar] [CrossRef]
- Hollister, S.J.; Lin, C.Y.; Saito, E.; Lin, C.Y.; Schek, R.D.; Taboas, J.M.; Williams, J.M.; Partee, B.; Flanagan, C.L.; Diggs, A.; et al. Engineering Craniofacial Scaffolds. Orthod. Craniofac. Res. 2005, 8, 162–173. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Zielińska, A.; Karczewski, J.; Eder, P.; Kolanowski, T.; Szalata, M.; Wielgus, K.; Szalata, M.; Kim, D.; Shin, S.R.; Słomski, R.; et al. Scaffolds for Drug Delivery and Tissue Engineering: The Role of Genetics. J. Control. Release 2023, 359, 207–223. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Kantorski, K.Z.; Dubey, N.; Daghrery, A.; Fenno, J.C.; Mishina, Y.; Chan, H.-L.; Mendonça, G.; Bottino, M.C. Personalized and Defect-Specific Antibiotic-Laden Scaffolds for Periodontal Infection Ablation. ACS Appl. Mater. Interfaces 2021, 13, 49642–49657. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Daghrery, A.; Dubey, N.; Li, C.; Mei, L.; Fenno, J.C.; Schwendeman, A.; Aytac, Z.; Bottino, M.C. Hybrid Antimicrobial Hydrogel as Injectable Therapeutics for Oral Infection Ablation. Biomacromolecules 2020, 21, 3945–3956. [Google Scholar] [CrossRef] [PubMed]
- Van Dyke, T.E. The Management of Inflammation in Periodontal Disease. J. Periodontol. 2008, 79, 1601–1608. [Google Scholar] [CrossRef]
- Hu, Z.; Ma, C.; Rong, X.; Zou, S.; Liu, X. Immunomodulatory ECM-like Microspheres for Accelerated Bone Regeneration in Diabetes Mellitus. ACS Appl. Mater. Interfaces 2018, 10, 2377–2390. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Luan, X.; Liu, X. Recent Advances in Periodontal Regeneration: A Biomaterial Perspective. Bioact. Mater. 2020, 5, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Yar, M.; Farooq, A.; Shahzadi, L.; Khan, A.S.; Mahmood, N.; Rauf, A.; Chaudhry, A.A.; ur Rehman, I. Novel Meloxicam Releasing Electrospun Polymer/Ceramic Reinforced Biodegradable Membranes for Periodontal Regeneration Applications. Mater. Sci. Eng. C 2016, 64, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gu, Z.; Chen, X.; Shi, C.; Liu, C.; Liu, M.; Wang, L.; Sun, M.; Zhang, K.; Liu, Q.; et al. An Injectable and Thermosensitive Hydrogel: Promoting Periodontal Regeneration by Controlled-Release of Aspirin and Erythropoietin. Acta Biomater. 2019, 86, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Batool, F.; Morand, D.-N.; Thomas, L.; Bugueno, I.; Aragon, J.; Irusta, S.; Keller, L.; Benkirane-Jessel, N.; Tenenbaum, H.; Huck, O. Synthesis of a Novel Electrospun Polycaprolactone Scaffold Functionalized with Ibuprofen for Periodontal Regeneration: An In Vitro and In Vivo Study. Materials 2018, 11, 580. [Google Scholar] [CrossRef] [PubMed]
- Lagopati, N.; Kotsinas, A.; Veroutis, D.; Evangelou, K.; Papaspyropoulos, A.; Arfanis, M.; Falaras, P.; Kitsiou, P.V.; Pateras, I.; Bergonzini, A.; et al. Effect of Silver-modified Nanostructured Titanium Dioxide in Cancer. Cancer Genom. Proteom. 2021, 18 (Suppl. S3), 425–439. [Google Scholar] [CrossRef] [PubMed]
- Pantelis, P.; Theocharous, G.; Lagopati, N.; Veroutis, D.; Thanos, D.-F.; Lampoglou, G.-P.; Pippa, N.; Gatou, M.-A.; Tremi, I.; Papaspyropoulos, A.; et al. The Dual Role of Oxidative-Stress-Induced Autophagy in Cellular Senescence: Comprehension and Therapeutic Approaches. Antioxidants 2023, 12, 169. [Google Scholar] [CrossRef]
- Lagopati, N.; Valamvanos, T.-F.; Proutsou, V.; Karachalios, K.; Pippa, N.; Gatou, M.-A.; Vagena, I.-A.; Cela, S.; Pavlatou, E.A.; Gazouli, M.; et al. The Role of Nano-Sensors in Breath Analysis for Early and Non-Invasive Disease Diagnosis. Chemosensors 2023, 11, 317. [Google Scholar] [CrossRef]
- Abedi, N.; Rajabi, N.; Kharaziha, M.; Nejatidanesh, F.; Tayebi, L. Layered Scaffolds in Periodontal Regeneration. J. Oral Biol. Craniofac. Res. 2022, 12, 782–797. [Google Scholar] [CrossRef]




| Fabrication Technique | Material Examples | Advantages | Disadvantages | Reference |
|---|---|---|---|---|
| Fused Deposition Modeling (FDM) | Thermoplastic polymers and their composites (PCL-TCP scaffold, PCL/poly(glycolic acid) (PGA)) | Low cost, simple to use, various lay-down patterns, good mechanical and thermal properties, high porosity, can control porosity and pore size, pore interconnectivity, macro shape control, solvent-free. | High processing temperature, inconsistency in pores, limited application just on PLA and PCL due to the required thermoplastic, materials in filament form, smooth surface, requires support structures for irregular shapes, pore occlusion at boundaries. | [171,172,173,174,175,176] |
| Direct ink writing (DIW) or microextrusion | Natural or synthetic based Hydrogels (alginate, chitosan, polyethylene glycol (PEG)) Bioceramics | Quick printing speed, low production cost, simple to use/operate, wide range of application, cells embedded into hydrogels, cellular and acellular printing. | Low printing accuracy compared to SLA, resolution approximately 100 μm. | [28,133,177] |
| Stereolithography (SLA) | poly(ethylene furandicarboxylate) (PEF), PCL | Highest resolution, enhanced versatility, fast speed of production, 5–300 μm accuracy and the smoothest surface finish among the other available techniques, complex 3D structures that may incorporate cells and bioactive agents, through heating it is easy to remove the photopolymer. | Photopolymerization of materials (it can be processed only into photo-crosslinked hydrogels and can be modified by adding photo-crosslinked groups), photocurable, high production and equipment cost, limited range of photosensitive materials. | [28,172,173,175] |
| Selective laser sintering (SLS) | Ceramics, Polymers (TCP, Hydroxyapatite (HA), PCL) Composites | Fabrication of highly detailed products with thin walls, complex structures with good mechanical strength, can control pore size and porosity independently, high porosity, solvent free, wide variety of materials can be used with the addition of any secondary binder system. | One of the poorest dimensional accuracies (150–180 μm) compared to the other AM fabrication methods, small pore size, unable to incorporate cells and growth factors during printing process, the thermal distortion shrinks and warps the produced scaffold, inability to use natural polymers due to the high temperatures generated by the laser beam, only thermally stable polymers can be used, materials in powder form, difficult to remove trapped materials. | [28,172,173,175,176] |
| Electrospinning | PLGA/PCL PCL/PEG Silk fibroin | The development of nanofibrous scaffolds is achieved through this technique, fiber homogenous mixture with high tensile strength, simple to use, cost efficient compared to other methods, continuous process, scalability, controllable fiber diameter from nm to microns. | Toxicity of solvents, packaging–shipping handling, jet instability. | [175,178,179,180] |
| Freeze Drying | Computer-aided design and computer-aided manufacturing (CAD/CAM) bone grafting PTFE/PVA polymers with/without graphene oxide nanoparticles | Solid porogen is not required, highly porous structures with enhanced interconnectivity, control pore size by altering the freezing method, capability to prevent high temperatures. | Organic solvents, limited to small pore size (15–35 μm), irregular porosity, long processing time, high energy consumption. | [171,175,178,181] |
| Solvent Casting-Particulate Leaching | PLA/HA scaffolds | Simple technique, scaffolds with regular to high porosity (50–90%), controlled pore size and composition, crystallinity can be tailored, low production cost. | The incorporation of biomolecules and cells into scaffolds is hindered due to the organic solvents used, difficulty to adequately control pore shape and interconnectivity, limited mechanical properties and thickness of structures developed, residual porogens and problems with residual solvent, widespread use of toxic solvents. | [161,171,175,182] |
| Gas-Foaming | Chitosan-based scaffolds | Chemical solvents are not required, and their use is prevented, porosity up to 85%, pore size between 30 and 700 μm, low production cost. | High pressurized technique that prohibits the incorporation of bioactive agents and cells into the scaffolds, difficult to control pore sizes and ensure their interconnectivity, low interconnectivity insufficient mechanical strength the denaturation of materials due to high temperatures during compression molding step can be observed. | [164,166,175,178] |
| Bioprinting | PCL and alginate Alginate, PCL/alginate mesh Collagen type I/bone dECM/ β-TCP | Low production cost, high degree of accuracy, great shape complexity, high printing speed, capability to support parallel high cell viability. | Depending on the existence of cells. | [179,183,184,185,186,187] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valamvanos, T.-F.; Dereka, X.; Katifelis, H.; Gazouli, M.; Lagopati, N. Recent Advances in Scaffolds for Guided Bone Regeneration. Biomimetics 2024, 9, 153. https://doi.org/10.3390/biomimetics9030153
Valamvanos T-F, Dereka X, Katifelis H, Gazouli M, Lagopati N. Recent Advances in Scaffolds for Guided Bone Regeneration. Biomimetics. 2024; 9(3):153. https://doi.org/10.3390/biomimetics9030153
Chicago/Turabian StyleValamvanos, Theodoros-Filippos, Xanthippi Dereka, Hector Katifelis, Maria Gazouli, and Nefeli Lagopati. 2024. "Recent Advances in Scaffolds for Guided Bone Regeneration" Biomimetics 9, no. 3: 153. https://doi.org/10.3390/biomimetics9030153
APA StyleValamvanos, T.-F., Dereka, X., Katifelis, H., Gazouli, M., & Lagopati, N. (2024). Recent Advances in Scaffolds for Guided Bone Regeneration. Biomimetics, 9(3), 153. https://doi.org/10.3390/biomimetics9030153







