Photorefraction Simulates Well the Plasticity of Neural Synaptic Connections
Abstract
1. Introduction
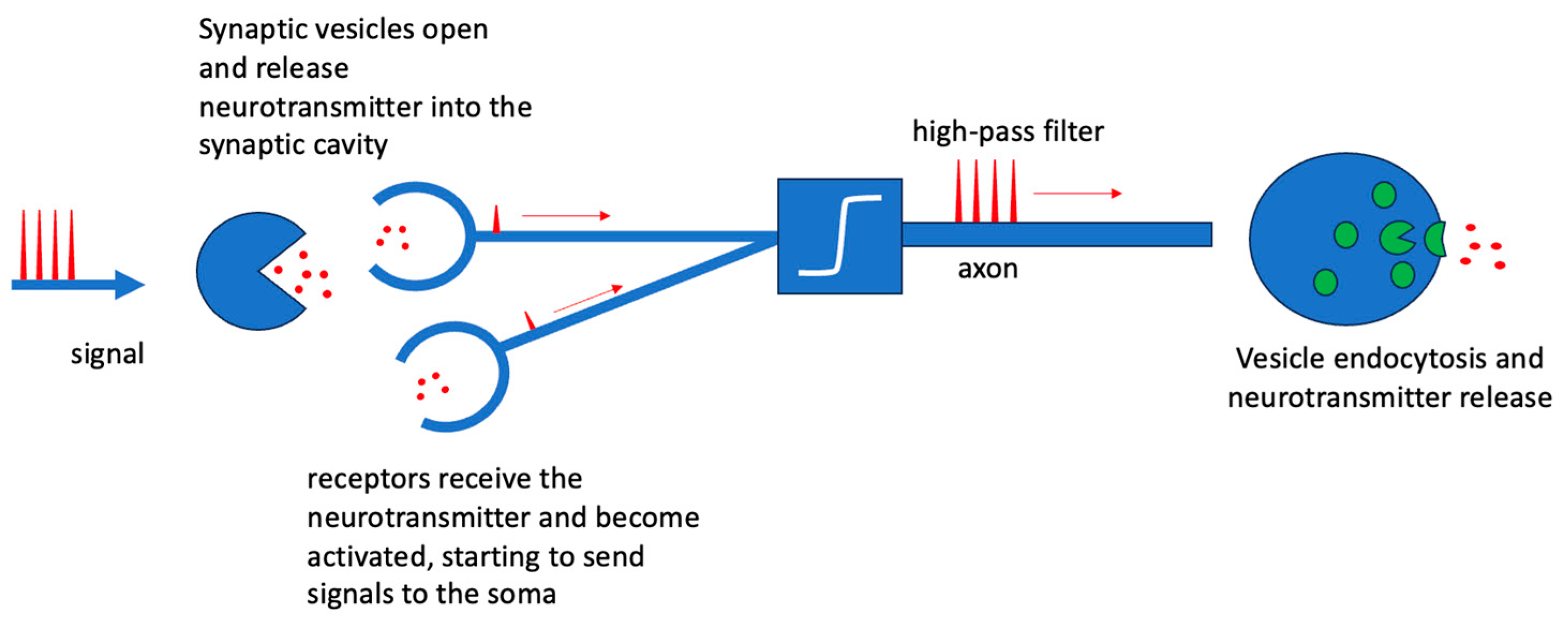
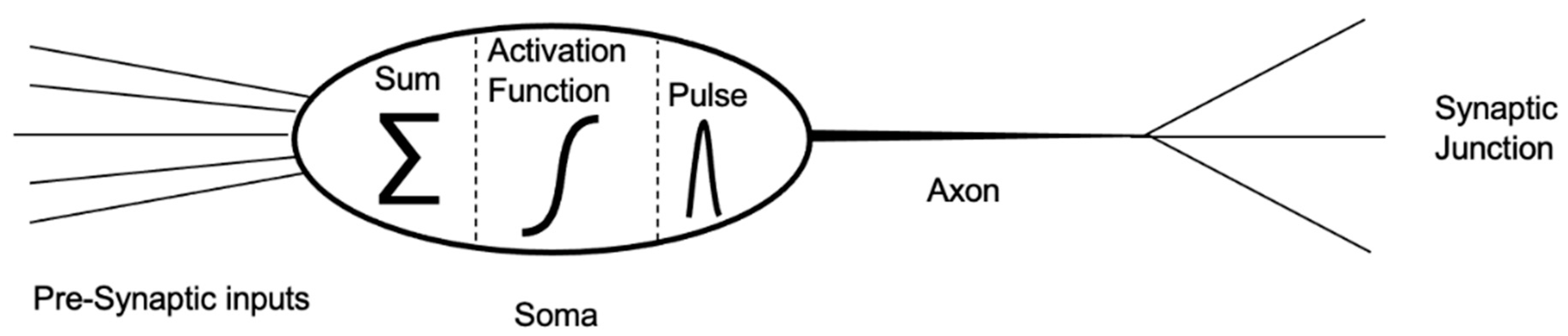
2. Biological Neurons and Neural Networks
3. Photonic Neuromorphics
- (1)
- the realization of a single neuron;
- (2)
- the realization of active connections between neurons.
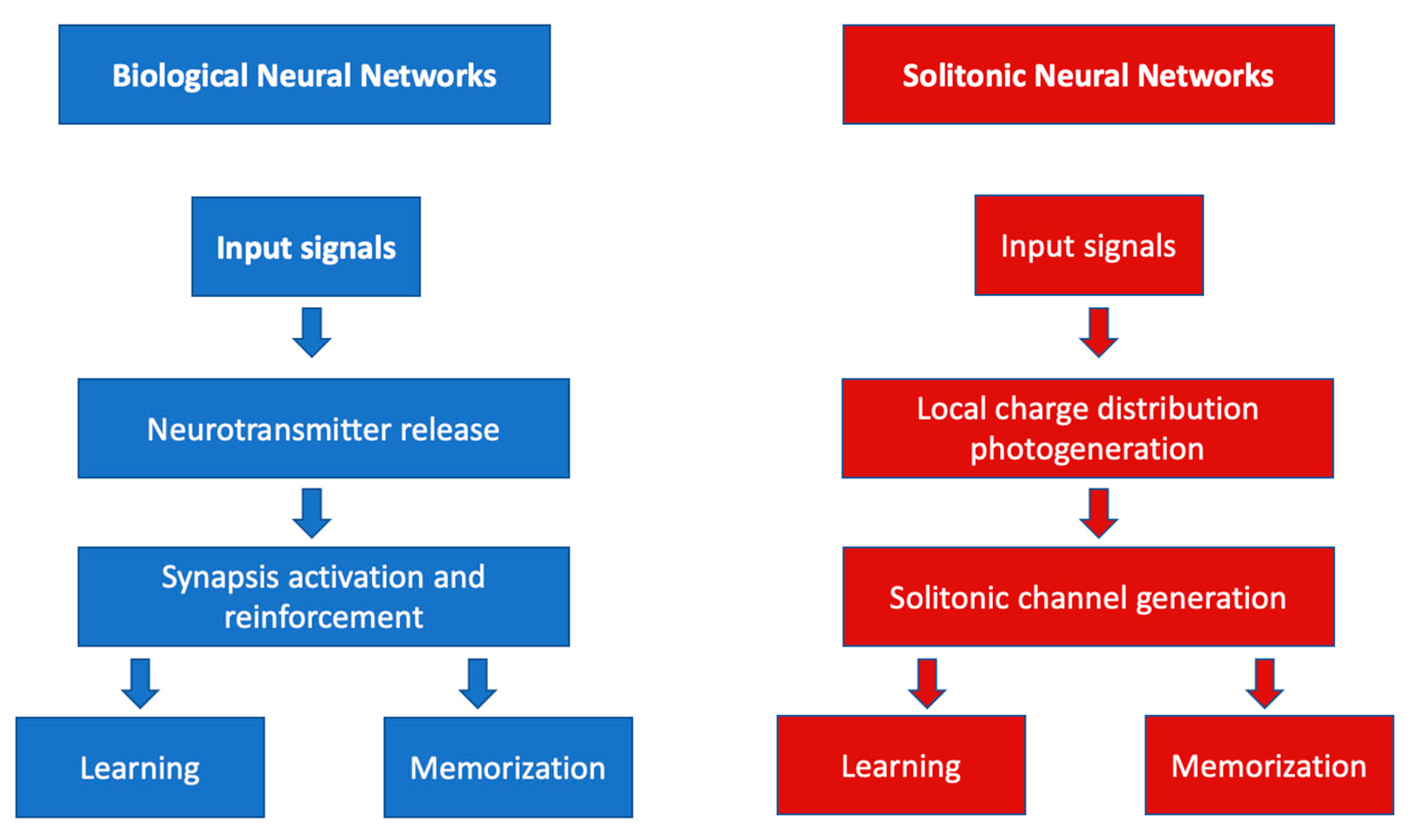
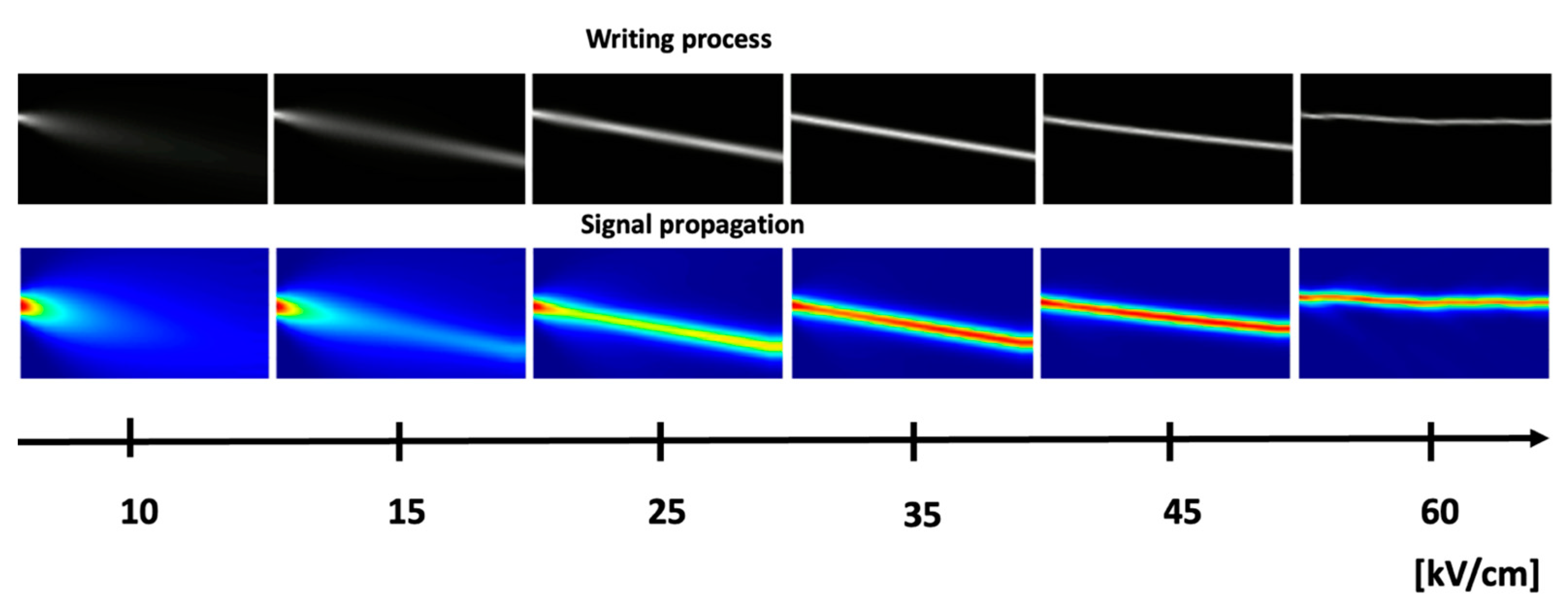
4. Photorefraction as a Basis for Optical Neural Networks
- -
- the intensity of incoming signals;
- -
- the frequency with which the signals occur in a specific pathway.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gerstner, W.; Kistler, W.M.; Naud, R.; Paninski, L. Neuronal Dynamics: From Single Neurons to Networks and Models of Cognition; Cambridge University Press: Cambridge, UK, 2014; ISBN -10: 978-1107060838. [Google Scholar]
- Ramachandran, V. Encyclopedia of the Human Brain; Elsevier: Amsterdam, The Netherlands, 2002; ISBN -10: 978-0080548036. [Google Scholar]
- Dupeyroux, J.; Hagenaars, J.; Paredes-Valls, J.; de Croon, G.C.H.E. Neuromorphic control for optic-flow-based landing of MAVs using the Loihi processor. In Proceedings of the IEEE International Conference on Robotics and Automation (ICRA), Xi’an, China, 30 May–5 June 2021; pp. 96–102. [Google Scholar]
- Hartline, D.K. What is myelin? Neuron Glia Biol. 2008, 4, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Wetzstein, G.; Ozcan, A.; Gigan, S.; Fan, S.; Englund, D.; Soljačić, M.; Psaltis, D. Inference in artificial intelligence with deep optics and photonics. Nature 2020, 588, 39–47. [Google Scholar] [CrossRef]
- Krauskopf, B.; Schneider, K.; Sieber, J.; Wieczorek, S.; Wolfrum, M. Excitability and self-pulsations near homoclinic bifurcations in seminconductor laser systems. Opt. Commun. 2003, 215, 367379. [Google Scholar] [CrossRef]
- Wunsche, H.J.; Brox, O.; Radziunas, M.; Henneberger, F. Excitability of a semiconductor laser by a two-mode homoclinic bifurcation. Phys. Rev. Lett. 2001, 88, 023901. [Google Scholar] [CrossRef] [PubMed]
- Shastri, B.J.; Nahmias, M.A.; Tait, A.N.; Rodriguez, A.W.; Wu, B.; Prucnal, P.R. Spike processing with a graphene excitable laser. Sci. Rep. 2016, 6, 19126. [Google Scholar] [CrossRef] [PubMed]
- Brunstein, M.; Yacomotti, A.M.; Sagnes, I.; Ranieri, F.; Bigot, L.; Levenson, A. Excitability and self-pulsing in a photonic crystal nanocavity. Phys. Rev. A 2012, 85, 031803. [Google Scholar] [CrossRef]
- Coomans, W.; Beri, S.; d. Sande, G.V.; Gelens, L.; Danckaert, J. Optical injection in semiconductor ring lasers. Phys. Rev. A 2010, 81, 033802. [Google Scholar] [CrossRef]
- Romeira, B. Delayed feedback dynamics of lienard-type resonant tunneling-photo-detector optoelectronic oscillators. IEEE J. Quantum Electron. 2013, 49, 3142. [Google Scholar] [CrossRef]
- Robertson, J.; Hejda, M.; Bueno, J.; Hurtado, A. Ultrafast optical integration and pattern classification for neuromorphic phton-ics based on spiking VCSEL neurons. Sci. Rep. 2020, 10, 6098. [Google Scholar] [CrossRef]
- Kandel, E.R. Alla Ricerca Della Memoria; Codice Ed.: Turin, Italy, 2017; ISBN 9788875786755. [Google Scholar]
- Bonabi, S.; Asgharian, H.; Safari, S.; Nili, A.M. PGA implementation of a biological neural network based on the Hodkin-Huxley neuron model. Front. Neurosci. 2014, 8, 00379. [Google Scholar]
- Williamson, I.A.D. Reprogrammable electro-optic nonlinear activation functions for optical neural networks. IEEE J. Sel. Top. Quantum Electron. 2020, 26, 7700412. [Google Scholar] [CrossRef]
- McCaughan, A.N.; Verma, V.B.; Buckley, S.M.; Allmaras, J.P.; Kozorezov, A.G.; Tait, A.N.; Shainline, J.M. A superconducting thermal switch with ultra- high impedance for interfacing superconductors to semiconductors. Nat. Electron. 2019, 2, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, J.; Youngblood, N.; Wright, C.D.; Bhaskaran, H.; Pernice, W.H.P. All-optical spiking neurosynaptic networks with self-learning capabilities. Nature 2019, 569, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Rios, C.; Pernice, W.H.; Wright, D.; Bhaskaran, H. On-chip photonic synapse. Sci. Adv. 2017, 3, 1700160. [Google Scholar] [CrossRef] [PubMed]
- Bile, A. Solitonic Neural Networks: An Innovative Photonic Neural Network Based on Solitonic Interconnections. In AI in Materials; Springer: Berlin/Heidelberg, Germany, 2023; ISBN -10: 3031486544. [Google Scholar]
- Bile, A.; Chauvet, M.; Tari, H.; Fazio, E. Supervised learning of soliton X-junctions in lithium niobate films on insulator. Opt. Lett. 2022, 47, 5893–5896. [Google Scholar] [CrossRef] [PubMed]
- Segev, M.; Shih, M.-F.; Valley, G.C. Photorefractive screening solitons of high and low intensity. J. Opt. Soc. Am. B 1996, 13, 706–718. [Google Scholar] [CrossRef]
- Fazio, E.; Renzi, F.; Rinaldi, R.; Bertolotti, M.; Chauvet, M.; Ramadan, W.; Vlad, V.I. Screening-photovoltaic bright solitons in lithium niobate and associated single-mode waveguides. Appl. Phys. Lett. 2004, 85, 2193–2195. [Google Scholar] [CrossRef]
- Ransom, B.R.; Sontheimer, H. The neurophysiology of glial cells. J. Clin. Neurophysiol. 1992, 9, 00004691. [Google Scholar] [CrossRef] [PubMed]
- Parpura, V.; Heneka, M.T.; Montana, V.; Oliet, S.H.; Schousboe, A.; Haydon, P.G.; Verkhratsky, A. Glial cells in (patho)physiology. J. Neurochem. 2012, 121, 4–27. [Google Scholar] [CrossRef]
- Nedergaard, M.; Takano, T.; Hansen, A. Beyond the role of glutamate as a neurotransmitter. Nat. Rev. Neurosci. 2002, 3, 748755. [Google Scholar] [CrossRef]
- Bile, A.; Tari, H.; Fazio, E. Episodic memory and information recognition using photorefractive-based solitonic neural networks. Appl. Sci. 2022, 12, 5585. [Google Scholar] [CrossRef]
- Lei, Z.-L.; Guo, B. 2D Material-Based Optical Biosensor: Status and Prospect. Adv. Sci. 2022, 9, 2102924. [Google Scholar] [CrossRef]
- Wei, L.; Wang, Z.; Chen, Y. Optical Biosensor for Ochratoxin a Detection in Grains Using an Enzyme-Mediated Click Reaction and Polystyrene Nanoparticles. J. Agric. Food Chem. 2022, 70, 14798–14804. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, X.; He, F.; Zhao, M.; Ling, L. Turn-off colorimetric sensor for sequence-specific recognition of single-stranded DNA based upon Y-shaped DNA structure. Sci. Rep. 2018, 8, 12021. [Google Scholar] [CrossRef] [PubMed]
- Westerveld, W.J.; Mahmud-Ul-Hasan, M.; Shnaiderman, R.; Ntziachristos, V.; Rottenberg, X.; Severi, S.; Rochus, V. Sensitive, small, broadband and scalable optomechanical ultrasound sensor in silicon photonics. Nat. Photonics 2021, 15, 341–345. [Google Scholar] [CrossRef]
- Tari, H.; Bile, A.; Nabizada, A.; Fazio, E. Ultra-broadband interconnection between two SPP nanostrips by a photorefractive soliton waveguide. Opt. Express 2023, 31, 26092–26103. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bile, A.; Tari, H.; Pepino, R.; Nabizada, A.; Fazio, E. Photorefraction Simulates Well the Plasticity of Neural Synaptic Connections. Biomimetics 2024, 9, 231. https://doi.org/10.3390/biomimetics9040231
Bile A, Tari H, Pepino R, Nabizada A, Fazio E. Photorefraction Simulates Well the Plasticity of Neural Synaptic Connections. Biomimetics. 2024; 9(4):231. https://doi.org/10.3390/biomimetics9040231
Chicago/Turabian StyleBile, Alessandro, Hamed Tari, Riccardo Pepino, Arif Nabizada, and Eugenio Fazio. 2024. "Photorefraction Simulates Well the Plasticity of Neural Synaptic Connections" Biomimetics 9, no. 4: 231. https://doi.org/10.3390/biomimetics9040231
APA StyleBile, A., Tari, H., Pepino, R., Nabizada, A., & Fazio, E. (2024). Photorefraction Simulates Well the Plasticity of Neural Synaptic Connections. Biomimetics, 9(4), 231. https://doi.org/10.3390/biomimetics9040231








