Effect of Zeolite Incorporation on the Ion Release Properties of Silver-Reinforced Glass Ionomer Cement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Silver–Zeolite Powder
2.2. Preparation of GIC Discs
2.3. Ion Release Measurement
2.4. Statistical Analysis
3. Results
3.1. Ion Release from Zeolite-Incorporated GIC vs. Silver–Zeolite-Incorporated GIC
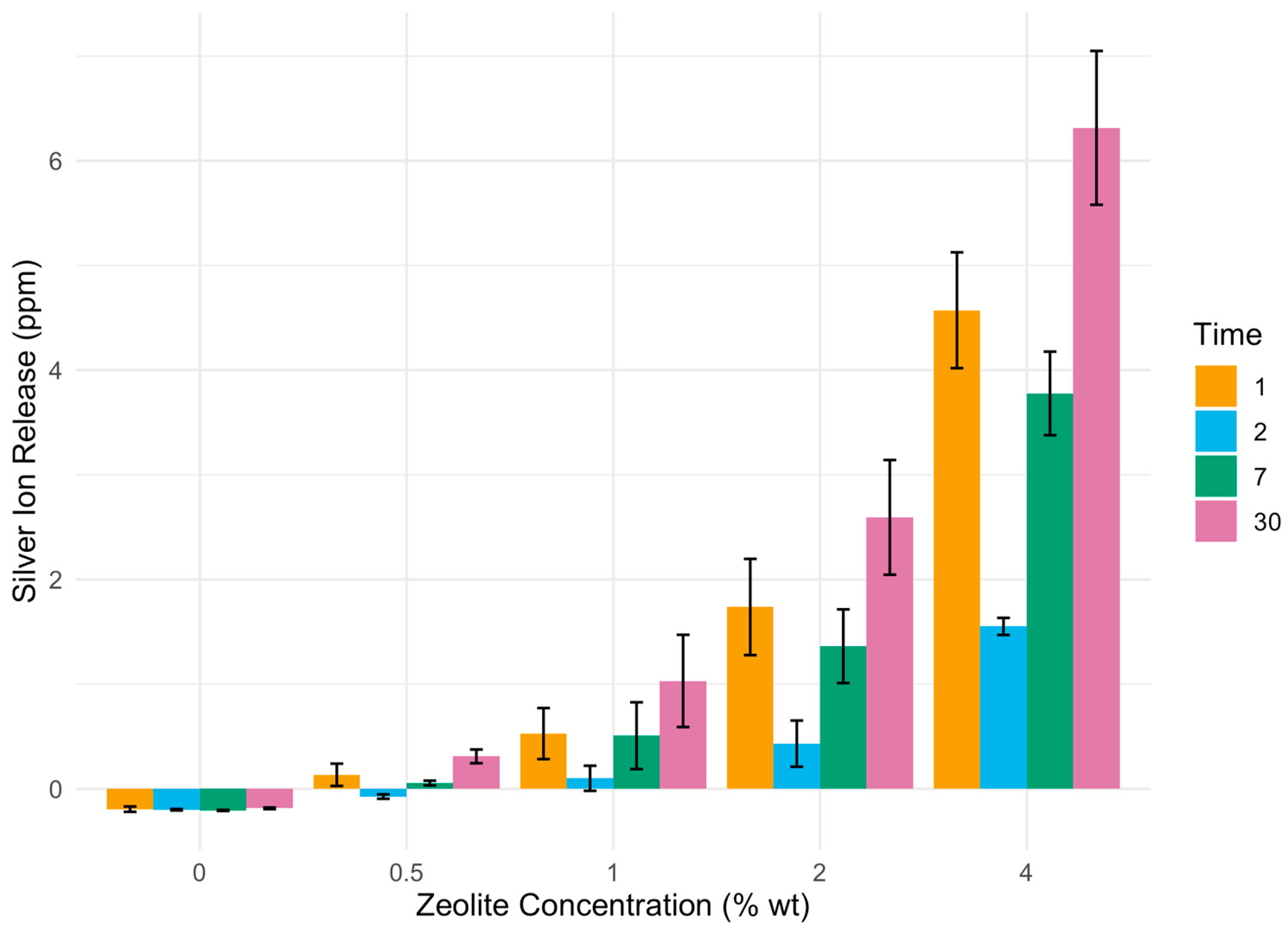
3.2. Ion Release from Silver–Zeolite-Incorporated GIC over Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sidhu, S.; Nicholson, J. A Review of Glass-Ionomer Cements for Clinical Dentistry. J. Funct. Biomater. 2016, 7, 16. [Google Scholar] [CrossRef]
- Schlafer, S.; Bornmann, T.; Paris, S.; Göstemeyer, G. The Impact of Glass Ionomer Cement and Composite Resin on Microscale PH in Cariogenic Biofilms and Demineralization of Dental Tissues. Dent. Mater. 2021, 37, 1576–1583. [Google Scholar] [CrossRef]
- Almuhaiza, M. Glass-Ionomer Cements in Restorative Dentistry: A Critical Appraisal. J. Contemp. Dent. Pract. 2016, 17, 331–336. [Google Scholar] [CrossRef]
- Nicholson, J.W. Maturation Processes in Glass-Ionomer Dental Cements. Acta Biomater. Odontol. Scand. 2018, 4, 63–71. [Google Scholar] [CrossRef]
- Swift, E.J.; Bader, J.D.; Shugars, D.A. Glass-Ionomer Cement Restorations and Secondary Caries. Quintessence Int. 1996, 27, 581–582. [Google Scholar]
- Zafar, M.S.; Ahmedb, N. Therapeutic Roles of Fluordide Released from Restorative Dental Materials. Fluoride 2015, 48, 184–194. [Google Scholar]
- Farrugia, C.; Camilleri, J. Antimicrobial Properties of Conventional Restorative Filling Materials and Advances in Antimicrobial Properties of Composite Resins and Glass Ionomer Cements—A Literature Review. Dent. Mater. 2015, 31, e89–e99. [Google Scholar] [CrossRef]
- Amin, F.; Rahman, S.; Khurshid, Z.; Zafar, M.S.; Sefat, F.; Kumar, N. Effect of Nanostructures on the Properties of Glass Ionomer Dental Restoratives/Cements: A Comprehensive Narrative Review. Materials 2021, 14, 6260. [Google Scholar] [CrossRef]
- Hao, J.; Lang, S.; Mante, F.; Pavelić, K.; Ozer, F. Antimicrobial and Mechanical Effects of Zeolite Use in Dental Materials: A Systematic Review. Acta Stomatol. Croat. 2021, 55, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Rolim, F.G.; de Araújo Lima, A.D.; Lima Campos, I.C.; de Sousa Ferreira, R.; da Cunha Oliveira-Júnior, C.; Gomes Prado, V.L.; Vale, G.C. Fluoride Release of Fresh and Aged Glass Ionomer Cements after Recharging with High-Fluoride Dentifrice. Int. J. Dent. 2019, 2019, 9785364. [Google Scholar] [CrossRef] [PubMed]
- De Witte, A.M.J.C.; De Maeyer, E.A.P.; Verbeeck, R.M.H.; Martens, L.C. Fluoride Release Profiles of Mature Restorative Glass Ionomer Cements after Fluoride Application. Biomaterials 2000, 21, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Weng, Y.; Guo, X.; Zhao, J.; Gregory, R.L.; Zheng, C. Preparation and Evaluation of a Novel Glass-Ionomer Cement with Antibacterial Functions. Dent. Mater. 2011, 27, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Kopperud, S.E.; Tveit, A.B.; Gaarden, T.; Sandvik, L.; Espelid, I. Longevity of Posterior Dental Restorations and Reasons for Failure. Eur. J. Oral Sci. 2012, 120, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.; Kheur, S.; Kheur, M.; Eyüboğlu, T.F.; Özcan, M. A Review on Zeolites and Their Applications in Dentistry. Curr. Oral Health Rep. 2023, 10, 36–42. [Google Scholar] [CrossRef]
- Tran, Y.T.; Lee, J.; Kumar, P.; Kim, K.-H.; Lee, S.S. Natural Zeolite and Its Application in Concrete Composite Production. Compos. Part B Eng. 2019, 165, 354–364. [Google Scholar] [CrossRef]
- Dutta, P.; Wang, B. Zeolite-Supported Silver as Antimicrobial Agents. Coord. Chem. Rev. 2019, 383, 1–29. [Google Scholar] [CrossRef]
- Ge, K.X.; Ying, C.; Lam, W.Y.H.; Chu, C.-H.; Yu, O.Y. A Novel Glass Ionomer Cement with Silver Zeolite for Restorative Dentistry. J. Dent. 2023, 133, 104524. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, K.; Tsuruda, K.; Morishita, M.; Uchida, M. Antibacterial Effect of Silver-Zeolite on Oral Bacteria under Anaerobic Conditions. Dent. Mater. 2000, 16, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Yoshikata, K.; Kunisaki, S.; Tsuchido, T. Mode of Bactericidal Action of Silver Zeolite and Its Comparison with that of Silver Nitrate. Appl. Environ. Microbiol. 2003, 69, 4278–4281. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.B.; Kim, K.N.; Kim, K.M.; Lee, Y.K. Antibacterial Effect of Silver-Zeolites in Glass-Ionomer Cements. Key Eng. Mater. 2007, 330–332, 831–834. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhao, I.S.; Mei, M.L.; Li, Q.; Yu, O.Y.; Chu, C.H. Use of Silver Nanomaterials for Caries Prevention: A Concise Review. Int. J. Nanomed. 2020, 15, 3181–3191. [Google Scholar] [CrossRef] [PubMed]
- El-Wassefy, N.A.; El-Mahdy, R.H.; El-Kholany, N.R. The Impact of Silver Nanoparticles Integration on Biofilm Formation and Mechanical Properties of Glass Ionomer Cement. J. Esthet. Restor. Dent. 2017, 30, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Farhadian, N.; Usefi Mashoof, R.; Khanizadeh, S.; Ghaderi, E.; Farhadian, M.; Miresmaeili, A. Streptococcus mutans Counts in Patients Wearing Removable Retainers with Silver Nanoparticles vs Those Wearing Conventional Retainers: A Randomized Clinical Trial. Am. J. Orthod. Dentofac. Orthop. 2016, 149, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Kędziora, A.; Speruda, M.; Krzyżewska, E.; Rybka, J.; Łukowiak, A.; Bugla-Płoskońska, G. Similarities and Differences between Silver Ions and Silver in Nanoforms as Antibacterial Agents. Int. J. Mol. Sci. 2018, 19, 444. [Google Scholar] [CrossRef]
- Fernandez, C.C.; Sokolonski, A.R.; Fonseca, M.S.; Stanisic, D.; Araújo, D.B.; Azevedo, V.; Portela, R.D.; Tasic, L. Applications of Silver Nanoparticles in Dentistry: Advances and Technological Innovation. Int. J. Mol. Sci. 2021, 22, 2485. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Díaz, M.A.; Boegli, L.; James, G.A.; Velasquillo, C.; Sánchez-Sánchez, R.; Martínez-Martínez, R.E.; Martínez-Castañón, G.A.; Martinez-Gutierrez, F. Silver Nanoparticles with Antimicrobial Activities against Streptococcus mutans and Their Cytotoxic Effect. Mater. Sci. Eng. C 2015, 55, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Mallineni, S.K.; Sakhamuri, S.; Kotha, S.L.; AlAsmari, A.R.G.M.; AlJefri, G.H.; Almotawah, F.N.; Mallineni, S.; Sajja, R. Silver Nanoparticles in Dental Applications: A Descriptive Review. Bioengineering 2023, 10, 327. [Google Scholar] [CrossRef]
- Teixeira, J.A.; e Silva, A.V.C.; Santos Júnior, V.E.D.; de Melo Júnior, P.C.; Arnaud, M.; Lima, M.G.; Flores, M.A.P.; Stamford, T.C.M.; Dias Pereira, J.R.; Ribeiro Targino, A.G.; et al. Effects of a New Nano-Silver Fluoride-Containing Dentifrice on Demineralization of Enamel and Streptococcus mutans Adhesion and Acidogenicity. Int. J. Dent. 2018, 2018, e1351925. [Google Scholar] [CrossRef]
- Bürgers, R.; Eidt, A.; Frankenberger, R.; Rosentritt, M.; Schweikl, H.; Handel, G.; Hahnel, S. The Anti-Adherence Activity and Bactericidal Effect of Microparticulate Silver Additives in Composite Resin Materials. Arch. Oral Biol. 2009, 54, 595–601. [Google Scholar] [CrossRef]
- Alshehri, T.D.; Kotha, S.B.; Abed, F.M.; Barry, M.J.; AlAsmari, A.; Mallineni, S.K. Effect of the Addition of Varying Concentrations of Silver Nanoparticles on the Fluoride Uptake and Recharge of Glass Ionomer Cement. Nanomaterials 2022, 12, 1971. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N.K.; El-Mallakh, B.; Graves, R. Silver Release from Metal-Reinforced Glass Ionomers. Dent. Mater. 1988, 4, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Lohbauer, U. Dental Glass Ionomer Cements as Permanent Filling Materials?—Properties, Limitations and Future Trends. Materials 2009, 3, 76–96. [Google Scholar] [CrossRef]
- Williams, J.A.; Billington, R.W.; Pearson, G. Silver and Fluoride Ion Release from Metal-Reinforced Glass-Ionomer Filling Materials. J. Oral Rehabil. 2008, 24, 369–375. [Google Scholar] [CrossRef]
- Odabaş, M.E.; Çinar, Ç.; Akça, G.; Araz, İ.; Ulusu, T.; Yücel, H. Short-Term Antimicrobial Properties of Mineral Trioxide Aggregate with Incorporated Silver-Zeolite. Dent. Traumatol. 2011, 27, 189–194. [Google Scholar] [CrossRef]
- Çınar, Ç.; Ulusu, T.; Özçelik, B.; Karamüftüoğlu, N.; Antibacterial, H.Y. Effect of Silver-Zeolite Containing Root-Canal Filling Material. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90B, 592–595. [Google Scholar] [CrossRef]
- Colella, C. Ion Exchange Equilibria in Zeolite Minerals. Miner. Depos. 1996, 31, 554–562. [Google Scholar] [CrossRef]
- Lang, S.; Hao, J.; Mante, F.K.; Pavelić, K.; Özer, F. The Effect of Zeolite Incorporation on the Physical Properties of Silver-Reinforced Glass Ionomer Cement. J. Mater. Sci. Mater. Med. 2022, 33, 38. [Google Scholar] [CrossRef]
- Freedman, R.; Diefenderfer, K.E. Effects of Daily Fluoride Exposures on Fluoride Release by Glass Ionomer-Based Restoratives. Oper. Dent. 2003, 28, 178–185. [Google Scholar]
- Al-Taee, L.; Deb, S.; Banerjee, A. An in Vitro Assessment of the Physical Properties of Manually- Mixed and Encapsulated Glass-Ionomer Cements. BDJ Open 2020, 6, 12. [Google Scholar] [CrossRef]
- Panayotova, M.I. Kinetics and Thermodynamics of Copper Ions Removal from Wastewater by Use of Zeolite. Waste Manag. 2001, 21, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Akgül, M.; Karabakan, A.; Acar, O.; Yürüm, Y. Removal of Silver (I) from Aqueous Solutions with Clinoptilolite. Microporous Mesoporous Mater. 2006, 94, 99–104. [Google Scholar] [CrossRef]
- Hotta, M.; Nakajima, H.; Yamamoto, K.; Aono, M. Antibacterial Temporary Filling Materials: The Effect of Adding Various Ratios of Ag-Zn-Zeolite. J. Oral Rehabil. 1998, 25, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Hoshino, M.; Takahashi, H.; Noguchi, T.; Murata, T.; Kanzaki, Y.; Hamashima, H.; Sasatsu, M. Bactericidal Activity of Ag–Zeolite Mediated by Reactive Oxygen Species under Aerated Conditions. J. Inorg. Biochem. 2002, 92, 37–42. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Guo, S.; Zhang, J.; Song, Y.; Dong, X.; Wang, X.; Yu, J. Antibacterial and Anti-Adhesive Zeolite Coatings on Titanium Alloy Surface. Microporous Mesoporous Mater. 2011, 146, 216–222. [Google Scholar] [CrossRef]
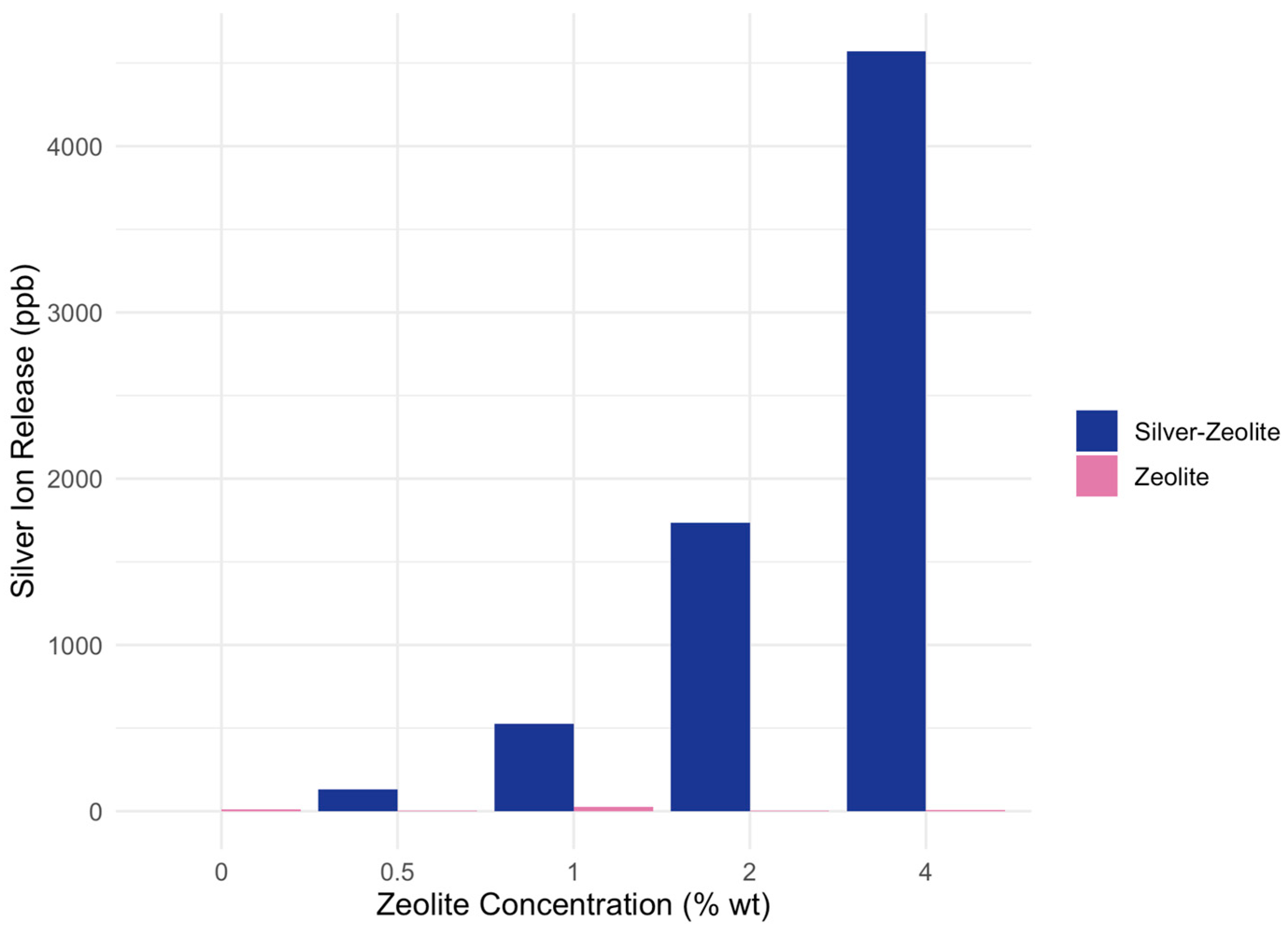
| C (0%) | 0.5% | 1% | 2% | 4% | |
|---|---|---|---|---|---|
| Zeolite | 10 | 4 | 25 | 5 | 6 |
| Silver–zeolite | 0 | 113 * | 527 * | 1737 * | 4572 * |
| C (0%) | 0.5% | 1% | 2% | 4% | |
|---|---|---|---|---|---|
| 24 h | −0.1945 | 0.1335 * | 0.5274 * | 1.7366 * | 4.5722 * |
| 48 h | −0.2000 | −0.0745 * | 0.1000 * | 0.4212 * | 1.5512 * |
| 7 days | −0.2069 | −0.0547 * | 0.5075 * | 1.3620 * | 3.7776 * |
| 30 days | −0.1867 | 0.3097 * | 1.0305 * | 2.5932 * | 6.3150 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, J.; Hao, J.; Vann, D.; Pavelić, K.; Ozer, F. Effect of Zeolite Incorporation on the Ion Release Properties of Silver-Reinforced Glass Ionomer Cement. Biomimetics 2024, 9, 365. https://doi.org/10.3390/biomimetics9060365
Tan J, Hao J, Vann D, Pavelić K, Ozer F. Effect of Zeolite Incorporation on the Ion Release Properties of Silver-Reinforced Glass Ionomer Cement. Biomimetics. 2024; 9(6):365. https://doi.org/10.3390/biomimetics9060365
Chicago/Turabian StyleTan, Jessica, Jessica Hao, David Vann, Krešimir Pavelić, and Fusun Ozer. 2024. "Effect of Zeolite Incorporation on the Ion Release Properties of Silver-Reinforced Glass Ionomer Cement" Biomimetics 9, no. 6: 365. https://doi.org/10.3390/biomimetics9060365
APA StyleTan, J., Hao, J., Vann, D., Pavelić, K., & Ozer, F. (2024). Effect of Zeolite Incorporation on the Ion Release Properties of Silver-Reinforced Glass Ionomer Cement. Biomimetics, 9(6), 365. https://doi.org/10.3390/biomimetics9060365








