Bioinspired Synthesis and Characterization of Dual-Function Zinc Oxide Nanoparticles from Saccharopolyspora hirsuta: Exploring Antimicrobial and Anticancer Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Biosynthesis of Zinc Oxide Nanoparticles
2.3. Characterization of Zinc Oxide Nanoparticles
2.4. Determination of ZnO-NP Cytotoxic Activity
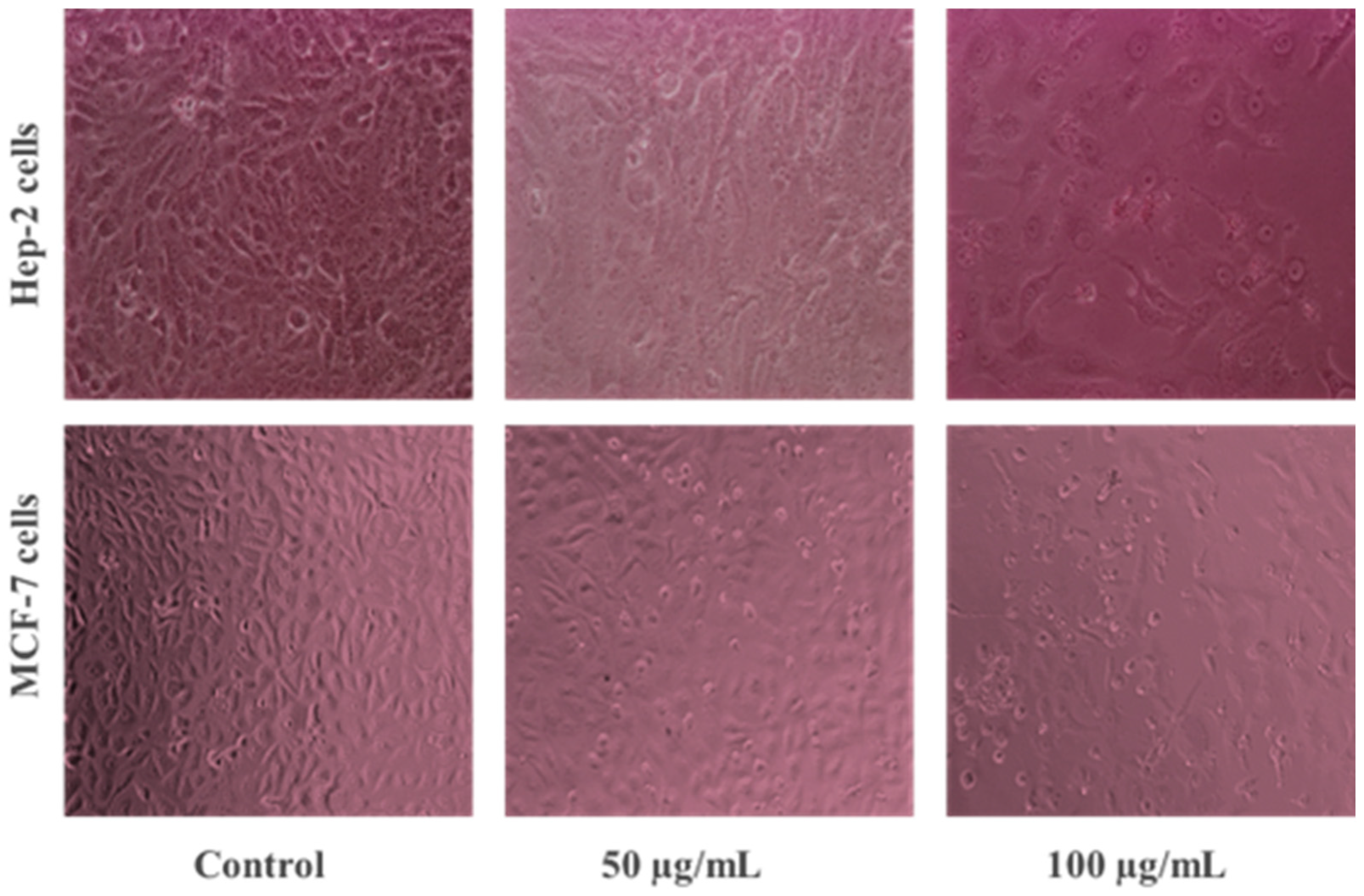
2.5. Morphological Analysis of ZnO-NP-Treated Cancer Cells
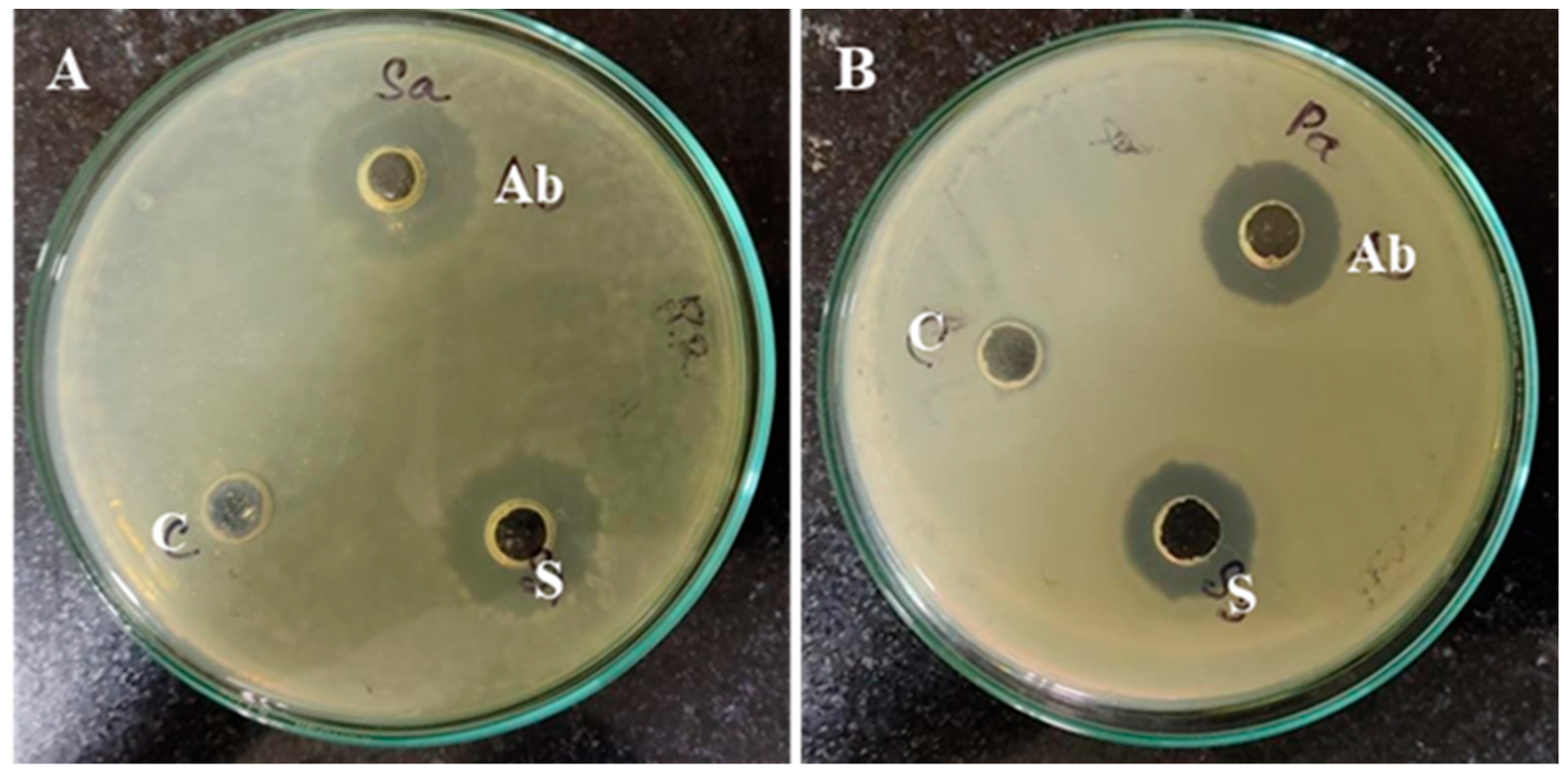
2.6. Antibacterial Activity and Minimum Inhibitory/Bactericidal Concentrations (MIC/MBC) of ZnO-NPs
2.7. Statistical Analysis
3. Results and Discussion
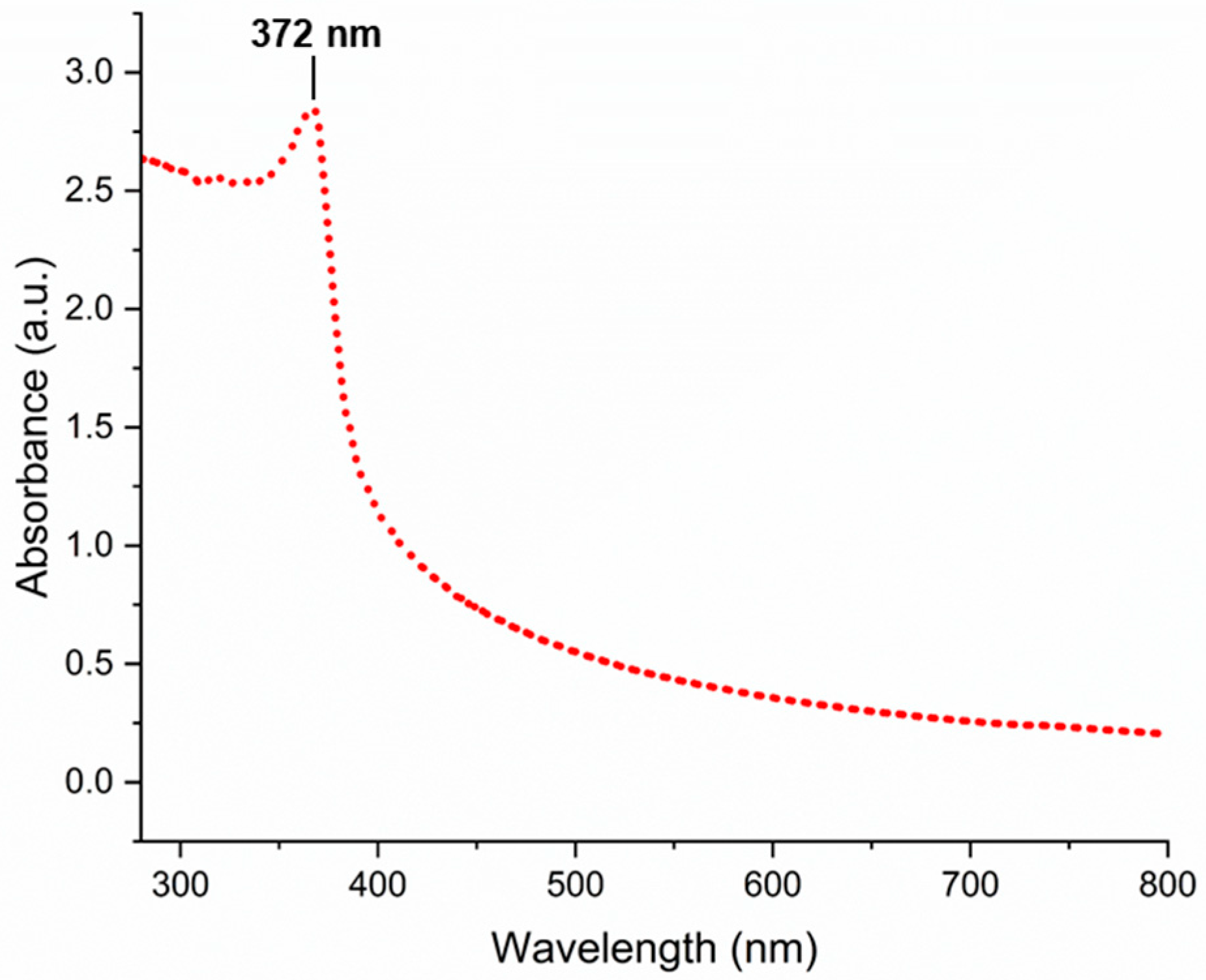
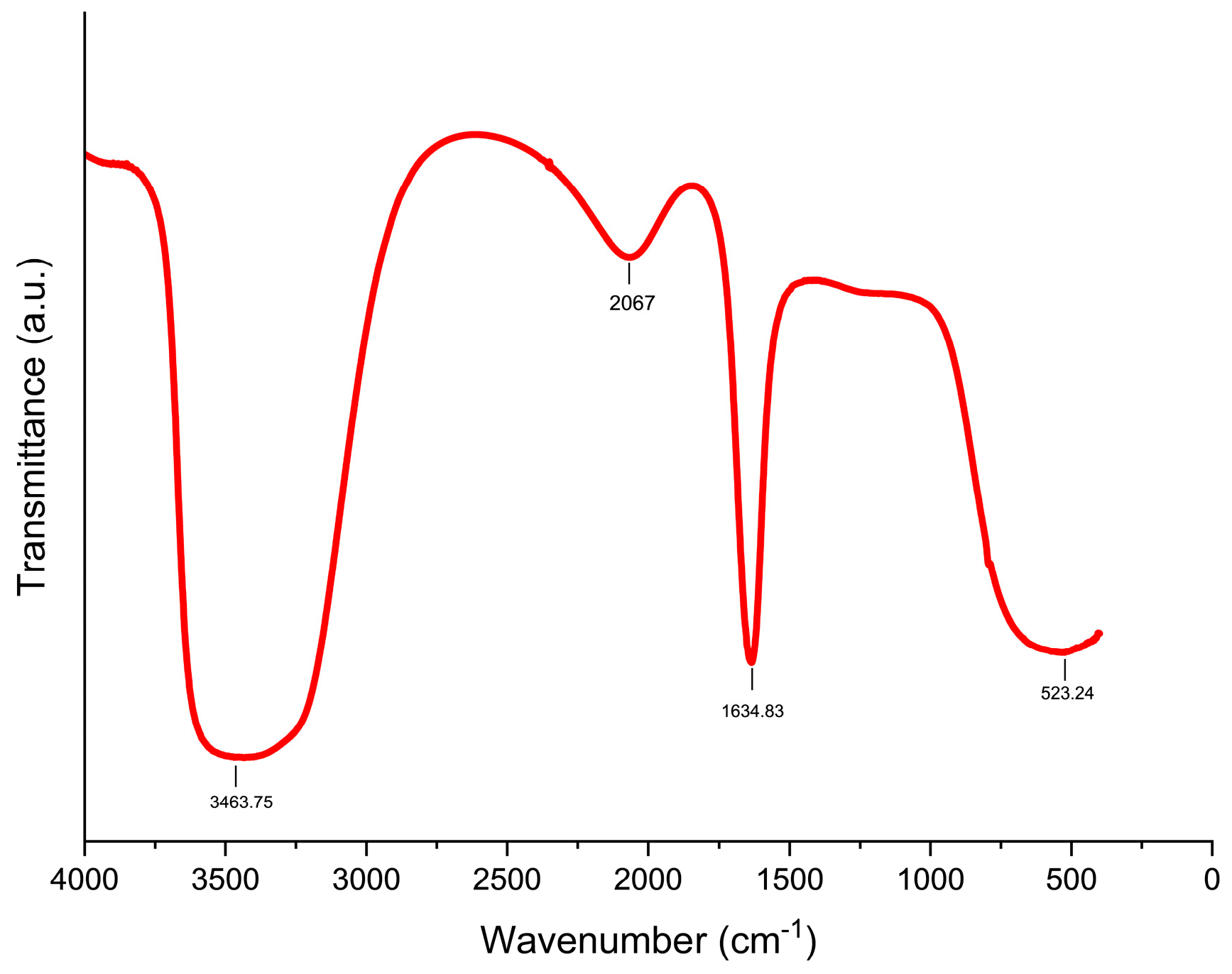
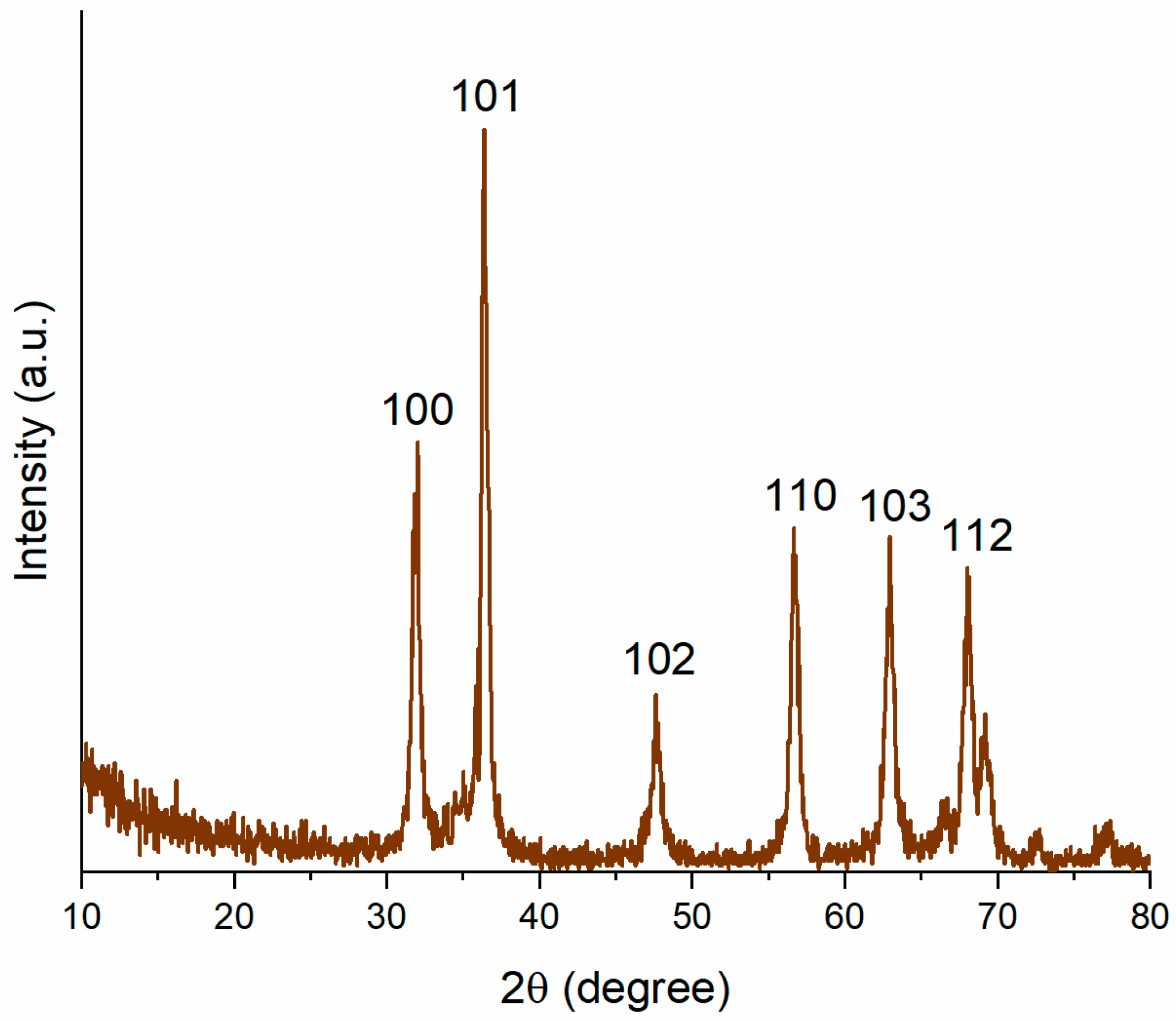
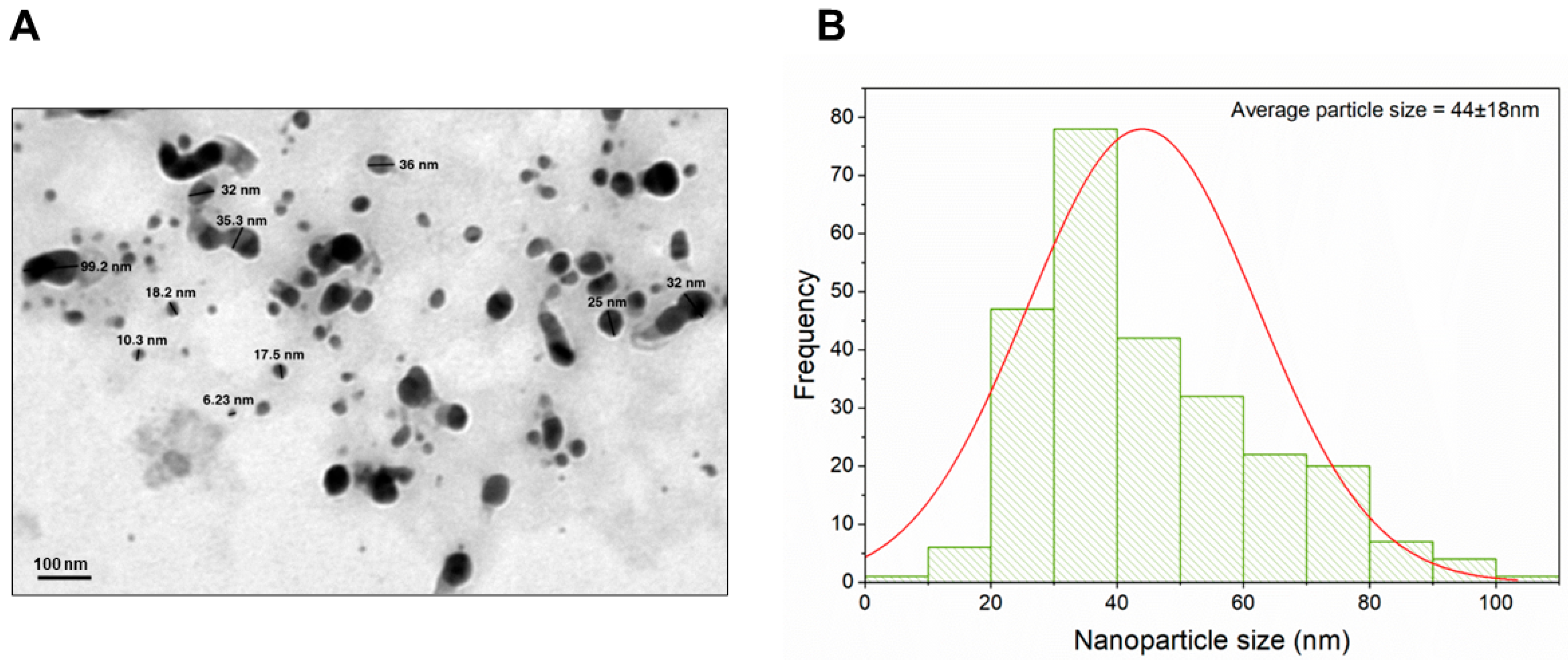
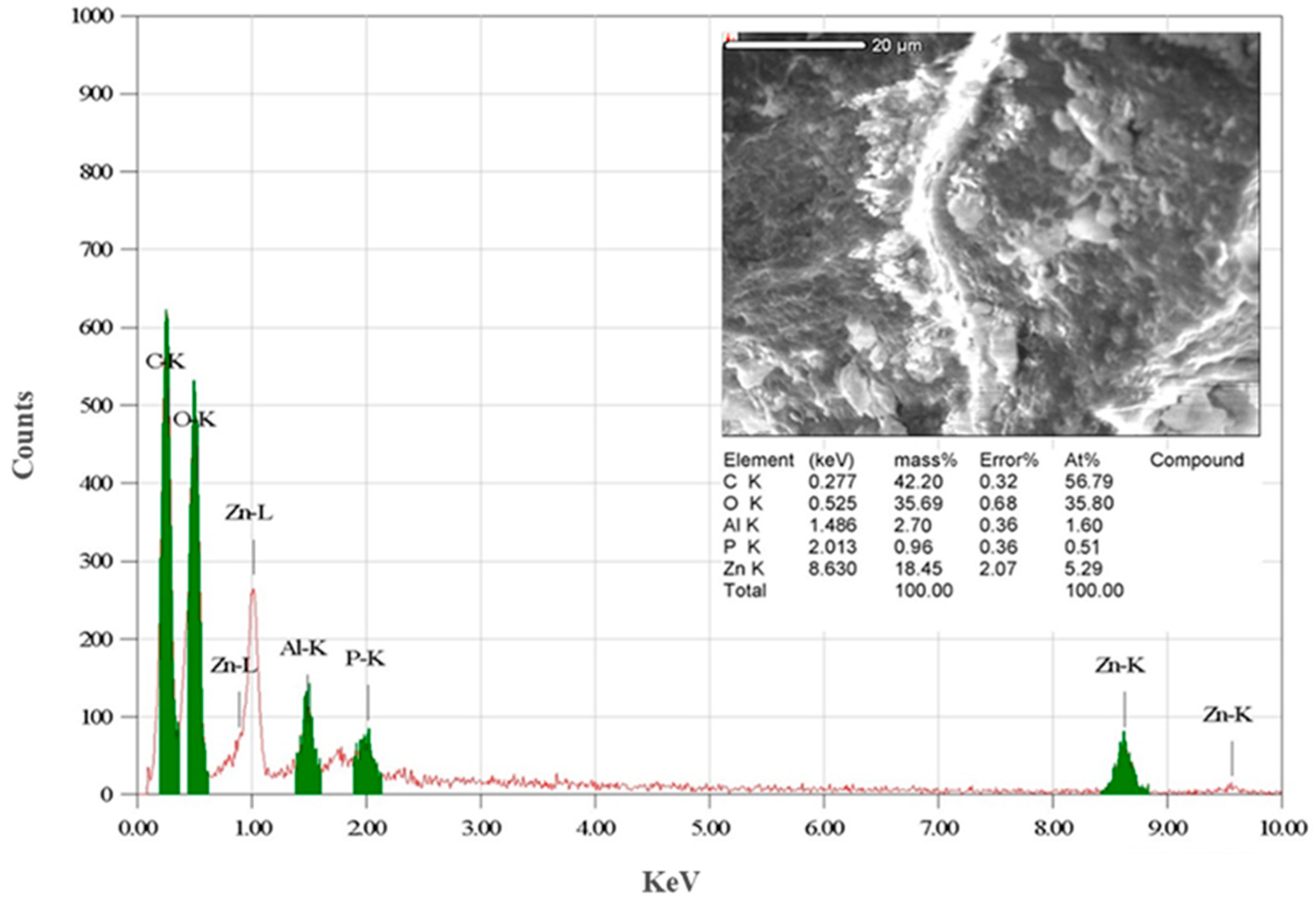
3.1. Characterization of Biogenically Synthesized ZnO-NPs Using S. hirsute
3.2. Evaluation of Biomedical Potential of S. hirsuta-Mediated ZnO-NPs
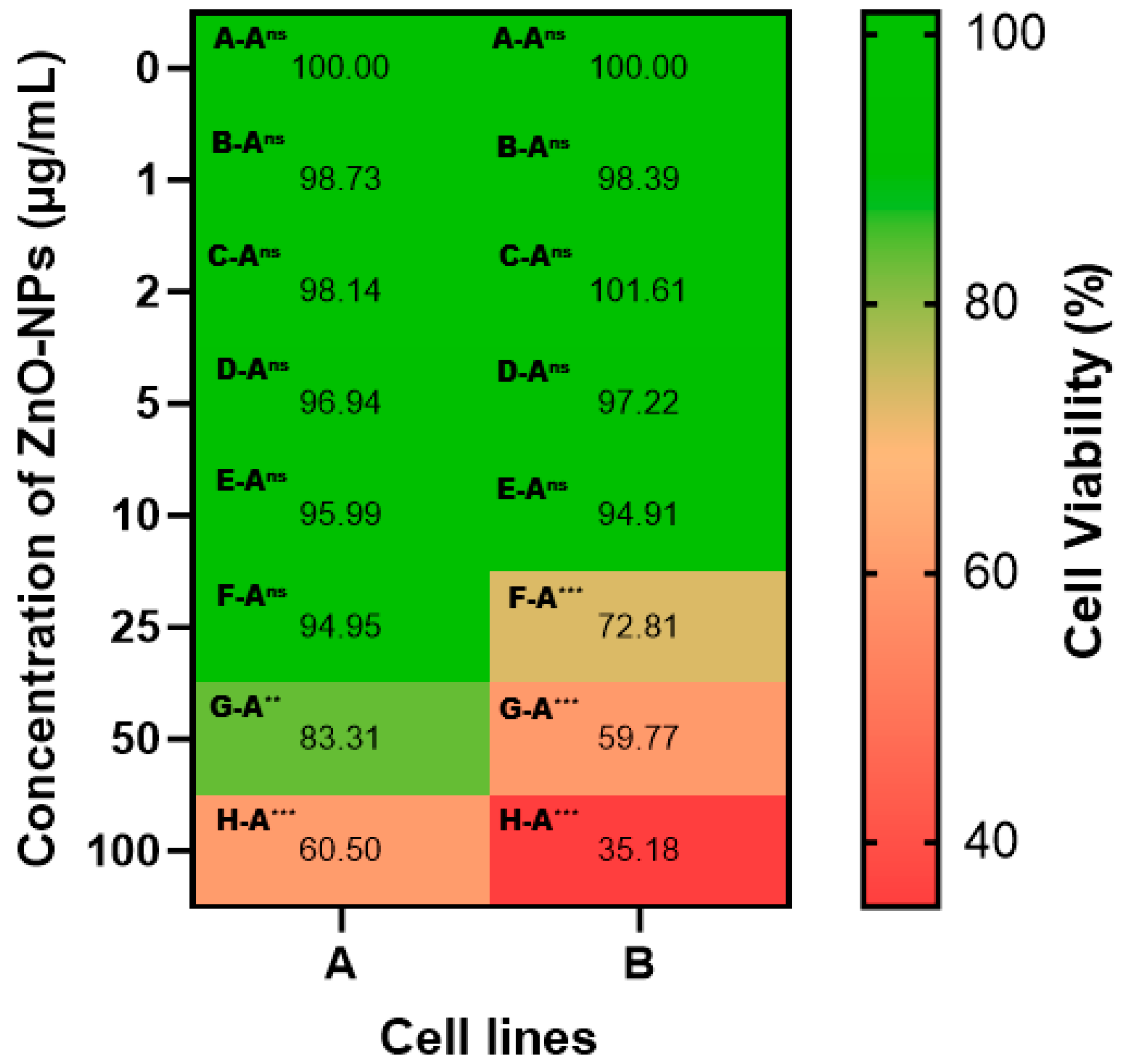
3.2.1. Anticancer Potential
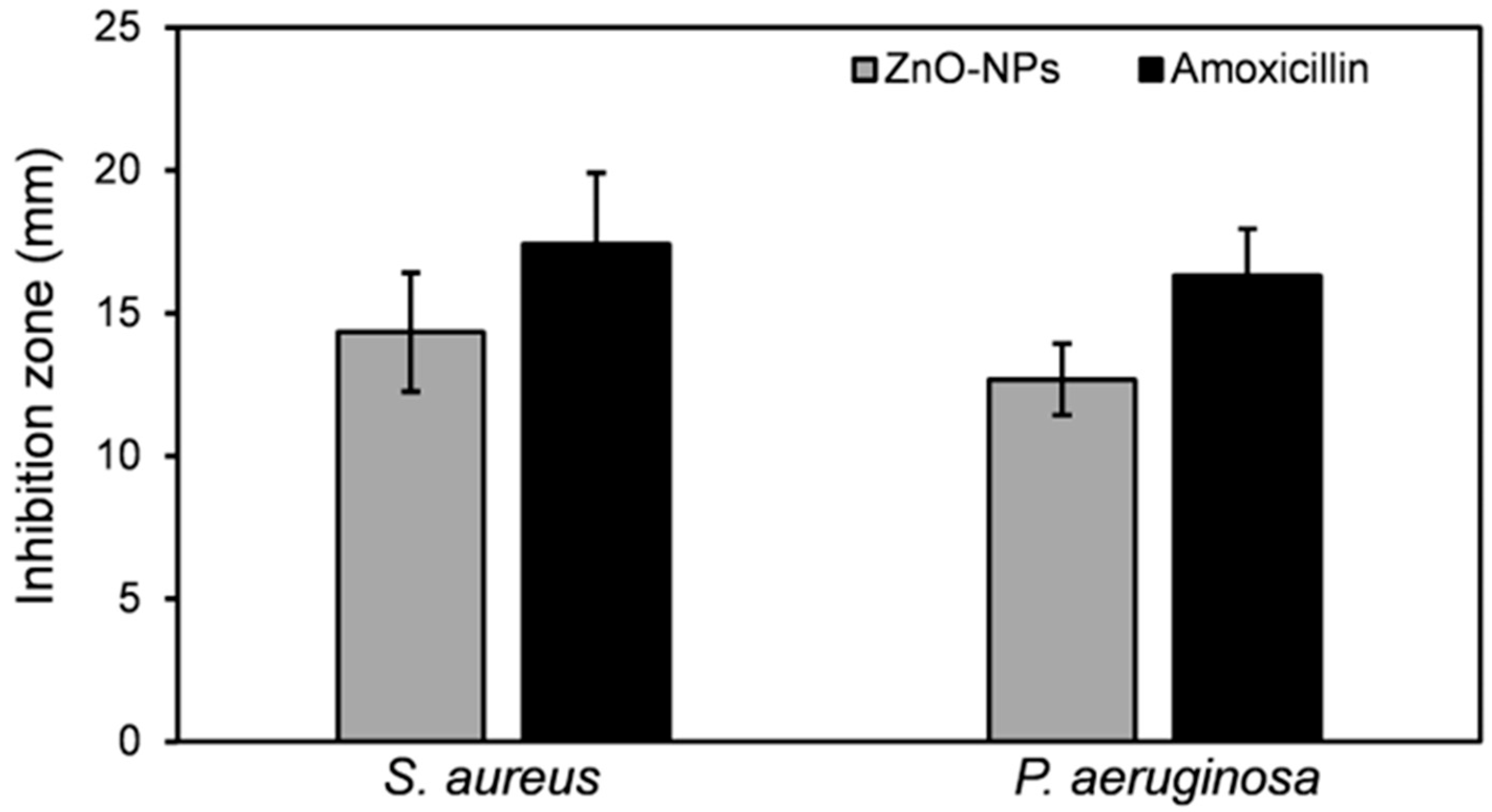
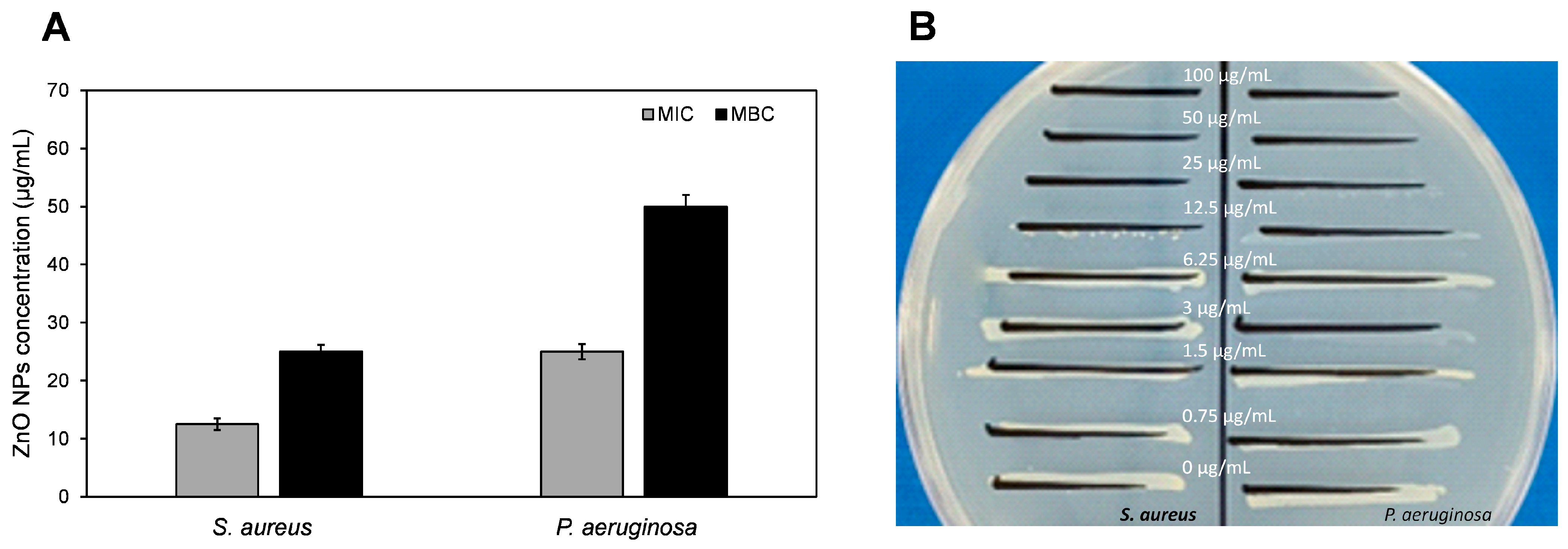
3.2.2. Antimicrobial Potential
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rana, A.; Pathak, S.; Lim, D.-K.; Kim, S.-K.; Srivastava, R.; Sharma, S.N.; Verma, R. Recent advancements in plant-and microbe-mediated synthesis of metal and metal oxide nanomaterials and their emerging antimicrobial applications. ACS Appl. Nano Mater. 2023, 6, 8106–8134. [Google Scholar] [CrossRef]
- Vadakkan, K.; Rumjit, N.P.; Ngangbam, A.K.; Vijayanand, S.; Nedumpillil, N.K. Novel advancements in the sustainable green synthesis approach of silver nanoparticles (AgNPs) for antibacterial therapeutic applications. Coord. Chem. Rev. 2024, 499, 215528. [Google Scholar] [CrossRef]
- Amin, K.N.M.; Abu, R.; Mohamad, S.F.S.; Hipeni, A.R.H. Microorganism-Based Synthesis of Nanomaterials and Their Applications. Green. Synth. Nanomater. Biol. Environ. Appl. 2024, 2024, 46–70. [Google Scholar]
- Min, K.H.; Shin, J.W.; Ki, M.-R.; Pack, S.P. Green synthesis of silver nanoparticles on biosilica diatomite: Well-dispersed particle formation and reusability. Process Biochem. 2023, 125, 232–238. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical-Physical Applications to Nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, J. Microbial synthesized nanoparticles in environment management. In Microbiome-Based Decontamination of Environmental Pollutants; Elsevier: Amsterdam, The Netherlands, 2024; pp. 381–401. [Google Scholar]
- Choudhary, A.; Musheer, N.; Ashraf, S.; Saeed, S. Microbe-mediated nanoparticles: Potential nanobiofungicides. In Nanofungicides; Elsevier: Amsterdam, The Netherlands, 2024; pp. 65–84. [Google Scholar]
- Rathnakumar, S.S.; Noluthando, K.; Kulandaiswamy, A.J.; Rayappan, J.B.B.; Kasinathan, K.; Kennedy, J.; Maaza, M. Stalling behaviour of chloride ions: A non-enzymatic electrochemical detection of α-Endosulfan using CuO interface. Sens. Actuat B-Chem. 2019, 293, 100–106. [Google Scholar] [CrossRef]
- Jagannathan, S.V.; Manemann, E.M.; Rowe, S.E.; Callender, M.C.; Soto, W. Marine actinomycetes, new sources of biotechnological products. Mar. Drugs 2021, 19, 365. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, S.N.; Thakur, D. Actinobacteria. In Beneficial Microbes in Agro-Ecology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 443–476. [Google Scholar]
- Paul, S.; Lakshmi, S.; Amala, T.; Akula, D.; Rao, M.; Mohapatra, P.; Thekkangil, A. Actinomycetes as Nanofactories: Synthesis and Therapeutic Applications. In Concepts in Pharmaceutical Biotechnology and Drug Development; Springer: Berlin/Heidelberg, Germany, 2024; pp. 139–155. [Google Scholar]
- Kaari, M.; Manikkam, R.; Annamalai, K.K.; Joseph, J. Actinobacteria as a source of biofertilizer/biocontrol agents for bio-organic agriculture. J. Appl. Microbiol. 2023, 134, lxac047. [Google Scholar] [CrossRef]
- Dua, A.; Nigam, A.; Saxena, A.; Dhingra, G.G.; Kumar, R. Microbial Bioproduction of Antiaging Molecules. Microb. Bioreact. Ind. Mol. 2023, 2023, 465–485. [Google Scholar]
- Subathra Devi, C.; Merlyn Keziah, S.; Jemimah Naine, S.; Mohanasrinivasan, V. Actinomycetes: Microbiology to systems biology. In Actinobacteria: Microbiology to Synthetic Biology; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–35. [Google Scholar]
- Kaltenpoth, M.; Engl, T. Defensive microbial symbionts in H ymenoptera. Funct. Ecol. 2014, 28, 315–327. [Google Scholar] [CrossRef]
- Yaradoddi, J.S.; Banapurmath, N.R.; Kontro, M.H.; Ganachari, S.V.; Hallad, S.; Soudagar, M.E.M.; Ramaswamy, V. Recent trends of actinomycetes in nanotechnology. In Actinobacteria: Ecology, Diversity, Classification and Extensive Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 199–212. [Google Scholar]
- Koul, B.; Poonia, A.K.; Yadav, D.; Jin, J.O. Microbe-Mediated Biosynthesis of Nanoparticles: Applications and Future Prospects. Biomolecules 2021, 11, 886. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, S.; Inbaneson, S.J.; Uthiraselvam, M.; Priya, S.R.; Ramu, A.; Banerjee, M.B. Diversity of Endophytic Actinomycetes from Karangkadu Mangrove Ecosystem and Its Antibacterial Potential against Bacterial Pathogens. J. Pharm. Res. 2011, 4, 294–296. [Google Scholar]
- Rajeswaran, S.; Veeraiyan, B.; Saravanan, V.; Manickam, E.; Danaraj, J. Biogenic facile green synthesis of actinobacterium exopolysaccharide-fabricated zinc oxide nanoparticles for the diverse biomedical applications. Biomass Convers. Biorefinery 2023, 2023, 1–15. [Google Scholar] [CrossRef]
- Rajabairavi, N.; Raju, C.S.; Karthikeyan, C.; Varutharaju, K.; Nethaji, S.; Hameed, A.S.H.; Shajahan, A. Biosynthesis of novel zinc oxide nanoparticles (ZnO NPs) using endophytic bacteria Sphingobacterium thalpophilum. In Recent Trends in Materials Science and Applications; Ebenezar, J., Ed.; Springer Proceedings in Physics; Springer: Cham, Switzerland, 4 May 2017; Volume 189, pp. 245–254. [Google Scholar]
- Golinska, P.; Wypij, M.; Ingle, A.; Gupta, I.; Dahm, H.; Rai, M. Biogenic synthesis of metal nanoparticles from actinomycetes: Biomedical applications and cytotoxicity. Appl. Microbiol. Biot. 2014, 98, 8083–8097. [Google Scholar] [CrossRef]
- Pal, G.; Rai, P.; Pandey, A. Chapter 1—Green synthesis of nanoparticles: A greener approach for a cleaner future. In Green Synthesis, Characterization and Applications of Nanoparticles; Shukla, A.K., Iravani, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–26. [Google Scholar]
- Salem, S.S. A mini review on green nanotechnology and its development in biological effects. Arch. Microbiol. 2023, 205, 128. [Google Scholar] [CrossRef]
- Manivasagan, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.-K. Actinobacteria mediated synthesis of nanoparticles and their biological properties: A review. Crit. Rev. Microbiol. 2016, 42, 209–221. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, B.; Yu, L.; Tong, Y.; Yan, M.; Zhang, R.; Han, W.; Hao, Y.; Shangguan, L.; Zhang, S. Research progresses in preparation methods and applications of zinc oxide nanoparticles. J. Alloys Compd. 2023, 2023, 170316. [Google Scholar] [CrossRef]
- Bhandari, K.P.; Sapkota, D.R.; Jamarkattel, M.K.; Stillion, Q.; Collins, R.W. Zinc Oxide Nanoparticles—Solution-Based Synthesis and Characterizations. Nanomaterials 2023, 13, 1795. [Google Scholar] [CrossRef]
- Soni, J.; Revathi, D.; Dhanraj, G.; Ramasubburayan, R. Bioinspired Green Synthesis of ZnO Nanoparticles by marine-derived Streptomyces plicatus and its multifaceted biomedicinal properties. Microb. Pathog. 2024, 193, 106758. [Google Scholar] [CrossRef]
- Rajivgandhi, G.N.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Kanisha, C.C.; Ramachandran, G.; Manoharan, N.; Alanzi, K.F. Identification of carbapenems resistant genes on biofilm forming K. Pneumoniae from urinary tract infection. Saudi J. Biol. Sci. 2021, 28, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Rajivgandhi, G.; Gnanamangai, B.M.; Prabha, T.H.; Poornima, S.; Maruthupandy, M.; Alharbi, N.S.; Kadaikunnan, S.; Li, W.-J. Biosynthesized zinc oxide nanoparticles (ZnO NPs) using actinomycetes enhance the anti-bacterial efficacy against K. Pneumoniae. J. King Saud. Univ. Sci. 2022, 34, 101731. [Google Scholar] [CrossRef]
- Sanjivkumar, M.; Silambarasan, T.; Ananthi, S.; ThangaTharani, K. Biosynthesis and characterization of zinc oxide nanoparticles from an estuarine-associated actinobacterium Streptomyces spp. and its biotherapeutic applications. Arch. Microbiol. 2022, 204, 17. [Google Scholar] [CrossRef] [PubMed]
- Rameshbabu, D.; Sarojini, K.; Sanjivkumar, M.; Ramasubburayan, R.; Prakash, S.; Punitha, M.J.; Immanuel, G. Investigation on characterization, antifouling and cytotoxic properties of zinc oxide nanoparticles biosynthesized by a mangrove-associated actinobacterium Streptomyces olivaceus (MSU3). Arch. Microbiol. 2022, 204, 386. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.B.; Moawad, R.; Abdallah, Y.; Abdel-Rasheed, M.; Zaher, A.M.A. Zinc oxide nanoparticles promise anticancer and antibacterial activity in ovarian cancer. Pharm. Res. 2023, 40, 2281–2290. [Google Scholar] [CrossRef] [PubMed]
- Sholkamy, E.N.; Ahamd, M.S.; Yasser, M.M.; Eslam, N. Anti-microbiological activities of bio-synthesized silver Nano-stars by Saccharopolyspora hirsuta. Saudi J. Biol. Sci. 2019, 26, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Mahadevan, S.; Arulmozhi, P.; Sriram, S.; Praseetha, P.K. Green synthesis of zinc oxide nanoparticles using Atalantia monophylla leaf extracts: Characterization and antimicrobial analysis. Mater. Sci. Semicond. Process. 2018, 82, 39–45. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Patra, H.K.; Banerjee, S.; Chaudhuri, U.; Lahiri, P.; Dasgupta, A.K. Cell selective response to gold nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 111–119. [Google Scholar] [CrossRef]
- Kumar SR, S.; Rao, K.V.B. In-vitro antimicrobial activity of marine actinobacteria against multidrug resistance Staphylococcus aureus. Asian Pac. J. Trop. Biomed. 2012, 2, 787–792. [Google Scholar]
- Mishra, M.; Paliwal, J.S.; Singh, S.K.; Selvarajan, E.; Subathradevi, C.; Mohanasrinivasan, V. Studies on the inhibitory activity of biologically synthesized and characterized zinc oxide nanoparticles using lactobacillus sporogens against Staphylococcus aureus. J. Pure Appl. Microbiol. 2013, 7, 1263–1268. [Google Scholar]
- Jain, D.; Shivani; Bhojiya, A.A.; Singh, H.; Daima, H.K.; Singh, M.; Mohanty, S.R.; Stephen, B.J.; Singh, A. Microbial Fabrication of Zinc Oxide Nanoparticles and Evaluation of Their Antimicrobial and Photocatalytic Properties. Front. Chem. 2020, 8, 106758. [Google Scholar] [CrossRef] [PubMed]
- Pachaiappan, R.; Rajendran, S.; Ramalingam, G.; Vo, D.-V.N.; Priya, P.M.; Soto-Moscoso, M. Green synthesis of zinc oxide nanoparticles by Justicia adhatoda leaves and their antimicrobial activity. Chem. Eng. Technol. 2021, 44, 551–558. [Google Scholar] [CrossRef]
- Sharma, D.; Rajput, J.; Kaith, B.; Kaur, M.; Sharma, S. Synthesis of ZnO nanoparticles and study of their antibacterial and antifungal properties. Thin Solid Film. 2010, 519, 1224–1229. [Google Scholar] [CrossRef]
- Tandon, N.; Patil, S.M.; Tandon, R.; Kumar, P. Magnetically recyclable silica-coated ferrite magnetite-K 10 montmorillonite nanocatalyst and its applications in O, N, and S-acylation reaction under solvent-free conditions. RSC Adv. 2021, 11, 21291–21300. [Google Scholar] [CrossRef] [PubMed]
- Mohd Basri, M.S.; Mustapha, F.; Mazlan, N.; Ishak, M.R. Rice husk ash-based geopolymer binder: Compressive strength, optimize composition, FTIR spectroscopy, microstructural, and potential as fire-retardant material. Polymers 2021, 13, 4373. [Google Scholar] [CrossRef]
- Hoffman, A.J.; Asokan, C.; Gadinas, N.; Kravchenko, P.; Getsoian, A.B.; Christopher, P.; Hibbitts, D. Theoretical and experimental characterization of adsorbed CO and NO on γ-Al2O3-supported Rh nanoparticles. J. Phys. Chem. C 2021, 125, 19733–19755. [Google Scholar] [CrossRef]
- Dappula, S.S.; Kandrakonda, Y.R.; Shaik, J.B.; Mothukuru, S.L.; Lebaka, V.R.; Mannarapu, M.; Amooru, G.D. Biosynthesis of zinc oxide nanoparticles using aqueous extract of Andrographis alata: Characterization, optimization and assessment of their antibacterial, antioxidant, antidiabetic and anti-Alzheimer’s properties. J. Mol. Struct. 2023, 1273, 134264. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, H.; Xie, F.; Ma, X.; Niu, B.; Chen, M.; Zhang, H.; Zhang, Y.; Long, D. General synthesis of ultrafine metal oxide/reduced graphene oxide nanocomposites for ultrahigh-flux nanofiltration membrane. Nat. Commun. 2022, 13, 471. [Google Scholar] [CrossRef]
- Lalithamba, H.S.; Raghavendra, M.; Uma, K.; Yatish, K.V.; Mousumi, D.; Nagendra, G. Capsicum annuum Fruit Extract: A Novel Reducing Agent for the Green Synthesis of ZnO Nanoparticles and Their Multifunctional Applications. Acta Chim. Slov. 2018, 65, 354–364. [Google Scholar] [CrossRef]
- Hussain, A.; Oves, M.; Alajmi, M.F.; Hussain, I.; Amir, S.; Ahmed, J.; Rehman, M.T.; El-Seedi, H.R.; Ali, I. Biogenesis of ZnO nanoparticles using Pandanus odorifer leaf extract: Anticancer and antimicrobial activities. RSC Adv. 2019, 9, 15357–15369. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Chimal, R.; García-Pérez, V.I.; Álvarez-Pérez, M.A.; Tavera-Hernández, R.; Reyes-Carmona, L.; Martínez-Hernández, M.; Arenas-Alatorre, J.Á. Influence of the particle size on the antibacterial activity of green synthesized zinc oxide nanoparticles using Dysphania ambrosioides extract, supported by molecular docking analysis. Arab. J. Chem. 2022, 15, 103804. [Google Scholar] [CrossRef]
- Yassin, M.T.; Mostafa, A.A.-F.; Al-Askar, A.A.; Al-Otibi, F.O. Facile green synthesis of zinc oxide nanoparticles with potential synergistic activity with common antifungal agents against multidrug-resistant candidal strains. Crystals 2022, 12, 774. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Guo, Y.; Lu, J.; Veeraraghavan, V.P.; Mohan, S.K.; Wang, C.; Yu, X. Synthesis of Zinc oxide nanoparticles from Marsdenia tenacissima inhibits the cell proliferation and induces apoptosis in laryngeal cancer cells (Hep-2). J. Photochem. Photobiol. B Biol. 2019, 201, 111624. [Google Scholar] [CrossRef] [PubMed]
- Wahab, R.; Siddiqui, M.A.; Saquib, Q.; Dwivedi, S.; Ahmad, J.; Musarrat, J.; Al-Khedhairy, A.A.; Shin, H.-S. ZnO nanoparticles induced oxidative stress and apoptosis in HepG2 and MCF-7 cancer cells and their antibacterial activity. Colloids Surf. B Biointerfaces 2014, 117, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Selvakumari, D.; Deepa, R.; Mahalakshmi, V.; Subhashini, P.; Lakshminarayan, N. Anti cancer activity of ZnO nanoparticles on MCF7 (breast cancer cell) and A549 (lung cancer cell). ARPN J. Eng. Appl. Sci. 2015, 10, 5418–5421. [Google Scholar]
- Boroumand Moghaddam, A.; Moniri, M.; Azizi, S.; Abdul Rahim, R.; Bin Ariff, A.; Navaderi, M.; Mohamad, R. Eco-friendly formulated zinc oxide nanoparticles: Induction of cell cycle arrest and apoptosis in the MCF-7 cancer cell line. Genes 2017, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Hashim, M.; Malik, S.A.; Khan, M.; Lorenzo, J.M.; Abbasi, B.H.; Hano, C. Recent advances in zinc oxide nanoparticles (ZnO NPs) for cancer diagnosis, target drug delivery, and treatment. Cancers 2021, 13, 4570. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.K.; Sharma, V.; Pandey, A.K.; Singh, S.; Sultana, S.; Dhawan, A. ROS-mediated genotoxicity induced by titanium dioxide nanoparticles in human epidermal cells. Toxicol. In Vitro 2011, 25, 231–241. [Google Scholar] [CrossRef]
- Chang, L.; Karin, M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef]
- Brunner, T.J.; Wick, P.; Manser, P.; Spohn, P.; Grass, R.N.; Limbach, L.K.; Bruinink, A.; Stark, W.J. In vitro cytotoxicity of oxide nanoparticles: Comparison to asbestos, silica, and the effect of particle solubility. Env. Sci. Technol. 2006, 40, 4374–4381. [Google Scholar] [CrossRef] [PubMed]
- Sawant, V.; Bamane, S. PEG-beta-cyclodextrin functionalized zinc oxide nanoparticles show cell imaging with high drug payload and sustained pH responsive delivery of curcumin in to MCF-7 cells. J. Drug Deliv. Sci. Technol. 2018, 43, 397–408. [Google Scholar] [CrossRef]
- Sharma, V.; Anderson, D.; Dhawan, A. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2). Apoptosis 2012, 17, 852–870. [Google Scholar] [CrossRef] [PubMed]
- Kanagamani, K.; Muthukrishnan, P.; Saravanakumar, K.; Shankar, K.; Kathiresan, A. Photocatalytic degradation of environmental perilous gentian violet dye using leucaena-mediated zinc oxide nanoparticle and its anticancer activity. Rare Met. 2019, 38, 277–286. [Google Scholar] [CrossRef]
- Ansari, M.A.; Murali, M.; Prasad, D.; Alzohairy, M.A.; Almatroudi, A.; Alomary, M.N.; Udayashankar, A.C.; Singh, S.B.; Asiri, S.M.M.; Ashwini, B.S. Cinnamomum verum bark extract mediated green synthesis of ZnO nanoparticles and their antibacterial potentiality. Biomolecules 2020, 10, 336. [Google Scholar] [CrossRef] [PubMed]
- Ki, M.-R.; Kim, S.H.; Park, T.I.; Pack, S.P. Self-Entrapment of Antimicrobial Peptides in Silica Particles for Stable and Effective Antimicrobial Peptide Delivery System. Int. J. Mol. Sci. 2023, 24, 16423. [Google Scholar] [CrossRef]
- Murali, M.; Mahendra, C.; Rajashekar, N.; Sudarshana, M.; Raveesha, K.; Amruthesh, K. Antibacterial and antioxidant properties of biosynthesized zinc oxide nanoparticles from Ceropegia candelabrum L.—An endemic species. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 179, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Alshahrani, M.Y.; Wahab, S.; Al-Harbi, A.I.; Nisar, N.; Alraey, Y.; Alqahtani, A.; Mir, M.A.; Irfan, S.; Saeed, M. Zinc oxide nanoparticle: An effective antibacterial agent against pathogenic bacterial isolates. J. King Saud. Univ. Sci. 2022, 34, 102110. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Ding, Y.; Daskalakis, N.; Jeuken, L.; Povey, M.; O’neill, A.J.; York, D.W. Mechanistic investigation into antibacterial behaviour of suspensions of ZnO nanoparticles against E. coli. J. Nanoparticle Res. 2010, 12, 1625–1636. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Gul, A.; Jehan, S.; Khan, Z.; Saeed, J.; Shirazi, R.R.; Raziq, A.; Waseem Khan, M.; Ullah, H. Biodiversity of Actinomycetes and Their Secondary Metabolites: A Comprehensive Review. J. Adv. Biomed. Pharm. Sci. 2023, 6, 36–48. [Google Scholar] [CrossRef]
- Djebbah, F.Z.; Belyagoubi, L.; Abdelouahid, D.E.; Kherbouche, F.; Al-Dhabi, N.A.; Arasu, M.V.; Ravindran, B. Isolation and characterization of novel Streptomyces strain from Algeria and its in-vitro antimicrobial properties against microbial pathogens. J. Infect. Public. Health 2021, 14, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sholkamy, E.N.; Abdelhamid, M.A.A.; Khalifa, H.O.; Ki, M.-R.; Pack, S.P. Bioinspired Synthesis and Characterization of Dual-Function Zinc Oxide Nanoparticles from Saccharopolyspora hirsuta: Exploring Antimicrobial and Anticancer Activities. Biomimetics 2024, 9, 456. https://doi.org/10.3390/biomimetics9080456
Sholkamy EN, Abdelhamid MAA, Khalifa HO, Ki M-R, Pack SP. Bioinspired Synthesis and Characterization of Dual-Function Zinc Oxide Nanoparticles from Saccharopolyspora hirsuta: Exploring Antimicrobial and Anticancer Activities. Biomimetics. 2024; 9(8):456. https://doi.org/10.3390/biomimetics9080456
Chicago/Turabian StyleSholkamy, Essam N., Mohamed A. A. Abdelhamid, Hazim O. Khalifa, Mi-Ran Ki, and Seung Pil Pack. 2024. "Bioinspired Synthesis and Characterization of Dual-Function Zinc Oxide Nanoparticles from Saccharopolyspora hirsuta: Exploring Antimicrobial and Anticancer Activities" Biomimetics 9, no. 8: 456. https://doi.org/10.3390/biomimetics9080456





