Particulate 3D Hydrogels of Silk Fibroin-Pluronic to Deliver Curcumin for Infection-Free Wound Healing
Abstract
1. Introduction
2. Materials and Methods
2.1. Silk Fibroin
2.2. Synthesis of the Scaffolds
2.3. Characterization of Materials Properties
2.4. Degradation, Swelling, and Drug Release
2.5. Cytotoxicity
2.6. Antibacterial Study
2.7. Statistical Analysis
3. Results and Discussion
3.1. Hydrogel Characterization
3.2. Degradation and Drug Release
3.3. Cytotoxicity
3.4. Antibacterial Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rousselle, P.; Braye, F.; Dayan, G. Re-epithelialization of adult skin wounds: Cellular mechanisms and therapeutic strategies. Adv. Drug Deliv. Rev. 2019, 146, 344–365. [Google Scholar] [CrossRef] [PubMed]
- Mai, B.; Jia, M.; Liu, S.; Sheng, Z.; Li, M.; Gao, Y.; Wang, X.; Liu, Q.; Wang, P. Smart Hydrogel-Based DVDMS/bFGF Nanohybrids for Antibacterial Phototherapy with Multiple Damaging Sites and Accelerated Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 10156–10169. [Google Scholar] [CrossRef]
- Schmidt, M.; Gutknecht, D.; Simon, J.C.; Schulz, J.-N.; Eckes, B.; Anderegg, U.; Saalbach, A. Controlling the Balance of Fibroblast Proliferation and Differentiation: Impact of Thy-1. J. Investig. Dermatol. 2015, 135, 1893–1902. [Google Scholar] [CrossRef]
- Hosseini, M.; Shafiee, A. Engineering Bioactive Scaffolds for Skin Regeneration. Small 2021, 17, 2101384. [Google Scholar] [CrossRef]
- Castangia, I.; Nácher, A.; Caddeo, C.; Valenti, D.; Fadda, A.M.; Díez-Sales, O.; Ruiz-Saurí, A.; Manconi, M. Fabrication of quercetin and curcumin bionanovesicles for the prevention and rapid regeneration of full-thickness skin defects on mice. Acta Biomater. 2014, 10, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Xu, X.; Sun, D.; Hong, Y.; Wen, C.; Xie, Y.; Yan, B.; Zhang, H.; Ge, Q.; Li, W.; et al. Biomimetic macroporous hydrogel with a triple-network structure for full-thickness skin regeneration. Appl. Mater. Today 2022, 27, 101442. [Google Scholar] [CrossRef]
- Keirouz, A.; Chung, M.; Kwon, J.; Fortunato, G.; Radacsi, N. 2D and 3D electrospinning technologies for the fabrication of nanofibrous scaffolds for skin tissue engineering: A review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1626. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, S.; Wang, L.; You, R.; Yan, S.; Zhang, Q.; Li, M. Bioactive silk fibroin scaffold with nanoarchitecture for wound healing. Compos. Part B Eng. 2021, 224, 109165. [Google Scholar] [CrossRef]
- Hilmi, A.B.M. Vital roles of stem cells and biomaterials in skin tissue engineering. World J. Stem Cells 2015, 7, 428. [Google Scholar] [CrossRef]
- Percival, S.L.; Chen, R.; Mayer, D.; Salisbury, A.-M. Mode of action of poloxamer-based surfactants in wound care and efficacy on biofilms. Int. Wound J. 2018, 15, 749–755. [Google Scholar] [CrossRef]
- Romić, M.D.; Klarić, M.Š.; Lovrić, J.; Pepić, I.; Cetina-Čižmek, B.; Filipović-Grčić, J.; Hafner, A. Melatonin-loaded chitosan/Pluronic®® F127 microspheres as in situ forming hydrogel: An innovative antimicrobial wound dressing. Eur. J. Pharm. Biopharm. 2016, 107, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Wesenberg-Ward, K.E.; Tyler, B.J.; Sears, J.T. Adhesion and biofilm formation of Candida albicans on native and Pluronic-treated polystyrene. Biofilms 2005, 2, 63–71. [Google Scholar] [CrossRef]
- Sarkar, N.; Bose, S. Liposome-Encapsulated Curcumin-Loaded 3D Printed Scaffold for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 17184–17192. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, G.; Calamak, S.; Ulubayram, K.; Guven, E. Curcumin-loaded electrospun PHBV nanofibers as potential wound-dressing material. J. Drug Deliv. Sci. Technol. 2018, 43, 185–193. [Google Scholar] [CrossRef]
- Kamar, S.S.; Abdel-Kader, D.H.; Rashed, L.A. Beneficial effect of Curcumin Nanoparticles-Hydrogel on excisional skin wound healing in type-I diabetic rat: Histological and immunohistochemical studies. Ann. Anat.—Anat. Anz. 2019, 222, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Chakravorty, A.; Biswas, B.; Sankar Sana, S.; Rayan, R.A.; Lala, N.L.; Ramakrishna, S. A review on toxicity of turmeric derived Nano-Formulates against bacterial and fungal cells with special emphasis on electrospun nanofibers. Mater. Today Proc. 2021, 46, 6358–6362. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Li, W.; Ma, L.; Wang, E.; Xing, M.; Zhou, Y.; Huan, Z.; Guo, F.; Chang, J. Curcumin/Fe-SiO2 nano composites with multi-synergistic effects for scar inhibition and hair follicle regeneration during burn wound healing. Appl. Mater. Today 2021, 23, 101065. [Google Scholar] [CrossRef]
- Panja, S.; Behera, S.; Kundu, S.C.; Halder, M. Optical Spectroscopic and Morphological Characterizations of Curcuminized Silk Biomaterials: A Perspective from Drug Stabilization. ACS Omega 2017, 2, 6755–6767. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wang, H.; Wei, K.; Yang, Y.; Zheng, R.-Y.; Kim, I.; Zhang, K.-Q. A Review of Structure Construction of Silk Fibroin Biomaterials from Single Structures to Multi-Level Structures. Int. J. Mol. Sci. 2017, 18, 237. [Google Scholar] [CrossRef]
- Khodaei, A.; Jahanmard, F.; Madaah Hosseini, H.R.; Bagheri, R.; Dabbagh, A.; Weinans, H.; Amin Yavari, S. Controlled temperature-mediated curcumin release from magneto-thermal nanocarriers to kill bone tumors. Bioact. Mater. 2022, 11, 107–117. [Google Scholar] [CrossRef]
- La, W.-G.; Yang, H.S. Heparin-Conjugated Poly(Lactic-Co-Glycolic Acid) Nanospheres Enhance Large-Wound Healing by Delivering Growth Factors in Platelet-Rich Plasma. Artif. Organs 2015, 39, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Blaker, J.J.; Lyngstadaas, S.P.; Mano, J.F.; Haugen, H.J. Designing multigradient biomaterials for skin regeneration. Mater. Today Adv. 2020, 5, 100051. [Google Scholar] [CrossRef]
- Song, J.E.; Sim, B.R.; Jeon, Y.S.; Kim, H.S.; Shin, E.Y.; Carlomagno, C.; Khang, G. Characterization of surface modified glycerol/silk fibroin film for application to corneal endothelial cell regeneration. J. Biomater. Sci. Polym. Ed. 2019, 30, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Yodmuang, S.; McNamara, S.L.; Nover, A.B.; Mandal, B.B.; Agarwal, M.; Kelly, T.-A.N.; Chao, P.G.; Hung, C.; Kaplan, D.L.; Vunjak-Novakovic, G. Silk microfiber-reinforced silk hydrogel composites for functional cartilage tissue repair. Acta Biomater. 2015, 11, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zhou, J.; Liu, B.; Liang, C.; Pan, X.; Zhang, Y.; Zhang, Y.; Wang, Y.; Shao, L.; Zhu, B.; et al. Delivery of miR-675 by stem cell-derived exosomes encapsulated in silk fibroin hydrogel prevents aging-induced vascular dysfunction in mouse hindlimb. Mater. Sci. Eng. C 2019, 99, 322–332. [Google Scholar] [CrossRef]
- Karaji, Z.G.; Jahanmard, F.; Mirzaei, A.H.; van der Wal, B.; Amin Yavari, S. A multifunctional silk coating on additively manufactured porous titanium to prevent implant-associated infection and stimulate bone regeneration. Biomed. Mater. 2020, 15, 065016. [Google Scholar] [CrossRef]
- Choi, J.; McGill, M.; Raia, N.R.; Hasturk, O.; Kaplan, D.L. Silk Hydrogels Crosslinked by the Fenton Reaction. Adv. Healthc. Mater. 2019, 8, 1900644. [Google Scholar] [CrossRef] [PubMed]
- Koh, L.-D.; Cheng, Y.; Teng, C.-P.; Khin, Y.-W.; Loh, X.-J.; Tee, S.-Y.; Low, M.; Ye, E.; Yu, H.-D.; Zhang, Y.-W.; et al. Structures, mechanical properties and applications of silk fibroin materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Fatahi, Y.; Khademhosseini, A.; Kaplan, D.L. Overview of Silk Fibroin Use in Wound Dressings. Trends Biotechnol. 2018, 36, 907–922. [Google Scholar] [CrossRef]
- Johari, N.; Moroni, L.; Samadikuchaksaraei, A. Tuning the conformation and mechanical properties of silk fibroin hydrogels. Eur. Polym. J. 2020, 134, 109842. [Google Scholar] [CrossRef]
- Zhong, T.; Deng, C.; Gao, Y.; Chen, M.; Zuo, B. Studies of in situ-forming hydrogels by blending PLA-PEG-PLA copolymer with silk fibroin solution. J. Biomed. Mater. Res. A 2012, 100, 1983–1989. [Google Scholar] [CrossRef]
- Lee, O.J.; Kim, J.-H.; Ju, H.W.; Moon, B.M.; Park, H.J.; Sheikh, F.A.; Park, C.H. Fabrication and Characterization of Silk/PVA Hydrogels by Sonication and Freezing-Thawing Technique. Polym. Korea 2013, 37, 717–721. [Google Scholar] [CrossRef][Green Version]
- Singh, Y.P.; Bhardwaj, N.; Mandal, B.B. Potential of Agarose/Silk Fibroin Blended Hydrogel for in Vitro Cartilage Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 21236–21249. [Google Scholar] [CrossRef]
- Gil, E.S.; Frankowski, D.J.; Bowman, M.K.; Gozen, A.O.; Hudson, S.M.; Spontak, R.J. Mixed Protein Blends Composed of Gelatin and Bombyx m ori Silk Fibroin: Effects of Solvent-Induced Crystallization and Composition. Biomacromolecules 2006, 7, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Lu, Q.; Sun, L.; Cebe, P.; Wang, X.; Zhang, X.; Kaplan, D.L. Biomaterials from Ultrasonication-Induced Silk Fibroin−Hyaluronic Acid Hydrogels. Biomacromolecules 2010, 11, 3178–3188. [Google Scholar] [CrossRef]
- Johari, N.; Hosseini, H.R.M.; Samadikuchaksaraei, A. Optimized composition of nanocomposite scaffolds formed from silk fibroin and nano-TiO2 for bone tissue engineering. Mater. Sci. Eng. C 2017, 79, 783–792. [Google Scholar] [CrossRef]
- Choi, J.H.; Jeon, H.; Song, J.E. Biofunctionalized Lysophosphatidic Acid/Silk Fibroin Film for Cornea Endothelial Cell Regeneration. Nanomaterials 2018, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zou, L.-Q.; Niu, J.; Liu, W.; Peng, S.-F.; Liu, C.-M. The Stability, Sustained Release and Cellular Antioxidant Activity of Curcumin Nanoliposomes. Molecules 2015, 20, 14293–14311. [Google Scholar] [CrossRef] [PubMed]
- Mohan, P.R.K.; Sreelakshmi, G.; Muraleedharan, C.V.; Joseph, R. Water soluble complexes of curcumin with cyclodextrins: Characterization by FT-Raman spectroscopy. Vib. Spectrosc. 2012, 62, 77–84. [Google Scholar] [CrossRef]
- Sharma, V.; Pathak, K. Effect of hydrogen bond formation/replacement on solubility characteristics, gastric permeation and pharmacokinetics of curcumin by application of powder solution technology. Acta Pharm. Sin. B 2016, 6, 600–613. [Google Scholar] [CrossRef]
- Hu, X.; Kaplan, D.; Cebe, P. Determining Beta-Sheet Crystallinity in Fibrous Proteins by Thermal Analysis and Infrared Spectroscopy. Macromolecules 2006, 39, 6161–6170. [Google Scholar] [CrossRef]
- Motta, A.; Fambri, L.; Migliaresi, C. Regenerated silk fibroin films: Thermal and dynamic mechanical analysis. Macromol. Chem. Phys. 2002, 203, 1658–1665. [Google Scholar] [CrossRef]
- Müller, I. Entropy and Energy,—A Universal Competition. Entropy 2008, 10, 462–476. [Google Scholar] [CrossRef]
- Herminghaus, S. Dynamics of wet granular matter. Adv. Phys. 2005, 54, 221–261. [Google Scholar] [CrossRef]
- Vu, T.-L.; Nezamabadi, S.; Mora, S. Effects of particle compressibility on structural and mechanical properties of compressed soft granular materials. J. Mech. Phys. Solids 2021, 146, 104201. [Google Scholar] [CrossRef]
- Johari, N.; Madaah Hosseini, H.R.; Samadikuchaksaraei, A. Novel fluoridated silk fibroin/TiO2 nanocomposite scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2018, 82, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Norahan, M.H.; Pedroza-González, S.C.; Sánchez-Salazar, M.G.; Álvarez, M.M.; Trujillo de Santiago, G. Structural and biological engineering of 3D hydrogels for wound healing. Bioact. Mater. 2023, 24, 197–235. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Guo, L.; Sigen, A.; Gao, Y.; Zhou, D.; Greiser, U.; Creagh-Flynn, J.; Zhang, H.; Dong, Y.; Cutlar, L.; et al. Injectable hyperbranched poly(β-amino ester) hydrogels with on-demand degradation profiles to match wound healing processes. Chem. Sci. 2018, 9, 2179–2187. [Google Scholar] [CrossRef]
- Grillo, R.; de Melo, N.F.S.; de Lima, R.; Lourenço, R.W.; Rosa, A.H.; Fraceto, L.F. Characterization of Atrazine-Loaded Biodegradable Poly(Hydroxybutyrate-Co-Hydroxyvalerate) Microspheres. J. Polym. Environ. 2010, 18, 26–32. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Sapru, S.; Samadikuchaksaraei, A.; Reis, R.L.; Kaplan, D.L.; Kundu, S.C. Silk fibroin for skin injury repair: Where do things stand? Adv. Drug Deliv. Rev. 2020, 153, 28–53. [Google Scholar] [CrossRef]
- Johari, N.; Khodaei, A.; Samadikuchaksaraei, A.; Reis, R.L.; Kundu, S.C.; Moroni, L. Ancient fibrous biomaterials from silkworm protein fibroin and spider silk blends: Biomechanical patterns. Acta Biomater. 2022, 153, 38–67. [Google Scholar] [CrossRef]
- Vashi, A.V.; Keramidaris, E.; Abberton, K.M.; Morrison, W.A.; Wilson, J.L.; O’Connor, A.J.; Cooper-White, J.J.; Thompson, E.W. Adipose differentiation of bone marrow-derived mesenchymal stem cells using Pluronic F-127 hydrogel in vitro. Biomaterials 2008, 29, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Beard, M.C.; Cobb, L.H.; Grant, C.S.; Varadarajan, A.; Henry, T.; Swanson, E.A.; Kundu, S.; Priddy, L.B. Autoclaving of Poloxamer 407 hydrogel and its use as a drug delivery vehicle. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 338–347. [Google Scholar] [CrossRef]
- Wang, K.; Shi, W.; Yang, Y.; Guo, L.; Xu, L.; Leng, S.; Li, Q.; Tan, Y. Dopamine-functionalized poloxamers for antibacterial coating. Mater. Lett. 2021, 291, 129591. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, T.; Mei, N.; Li, B.; Xu, Z.; Wang, L.; Wang, X.; Tang, S. LED 209 conjugated chitosan as a selective antimicrobial and potential anti-adhesion material. Carbohydr. Polym. 2019, 206, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Feng, Y.; Jia, X.; Ma, X.; Chen, W.; Yang, L.; Li, J. Cotton fiber-based dressings with wireless electrical stimulation and antibacterial activity for wound repair. Int. J. Biol. Macromol. 2024, 256, 128496. [Google Scholar] [CrossRef]
- Teow, S.-Y.; Liew, K.; Ali, S.A.; Khoo, A.S.-B.; Peh, S.-C. Antibacterial Action of Curcumin against Staphylococcus aureus: A Brief Review. J. Trop. Med. 2016, 2016, 2853045. [Google Scholar] [CrossRef]
- Zheng, D.; Huang, C.; Huang, H.; Zhao, Y.; Khan, M.R.U.; Zhao, H.; Huang, L. Antibacterial Mechanism of Curcumin: A Review. Chem. Biodivers. 2020, 17, e2000171. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.R.; Shanholtzer, C.J. Tests for bactericidal effects of antimicrobial agents: Technical performance and clinical relevance. Clin. Microbiol. Rev. 1992, 5, 420–432. [Google Scholar] [CrossRef]
- Brackman, G.; Coenye, T. In Vitro and In Vivo Biofilm Wound Models and Their Application. Adv. Exp. Med. Biol. 2016, 897, 15–32. [Google Scholar] [CrossRef]
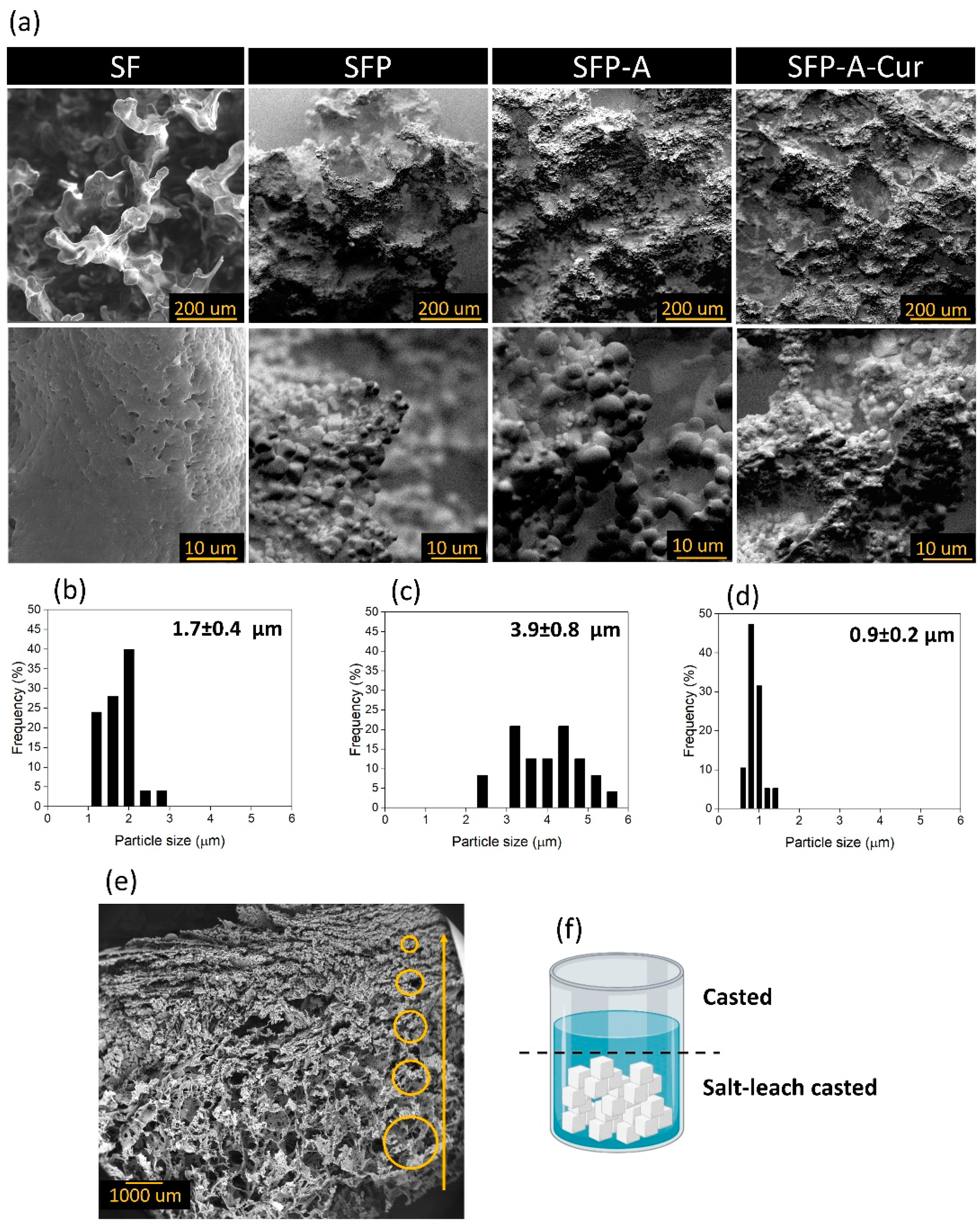
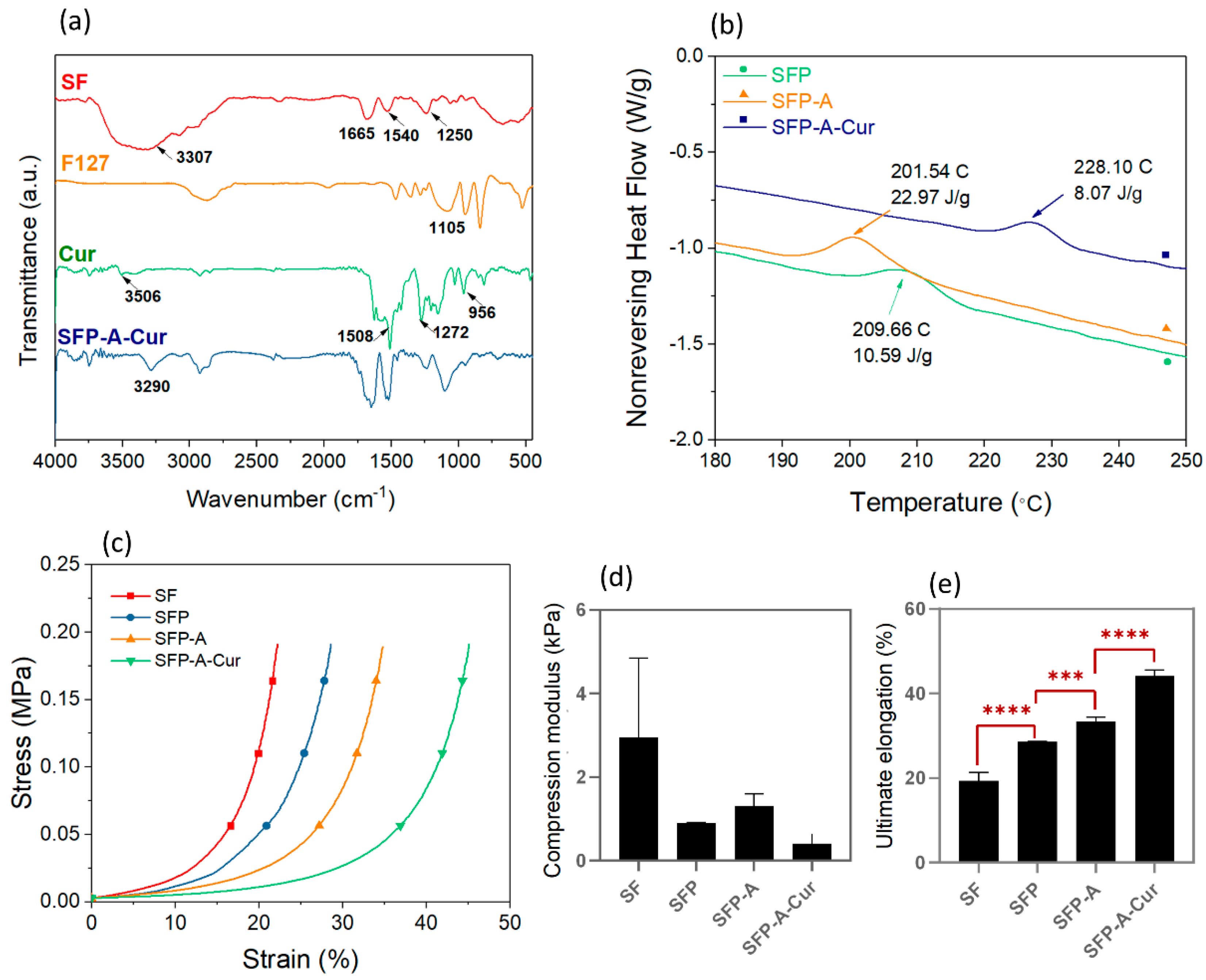


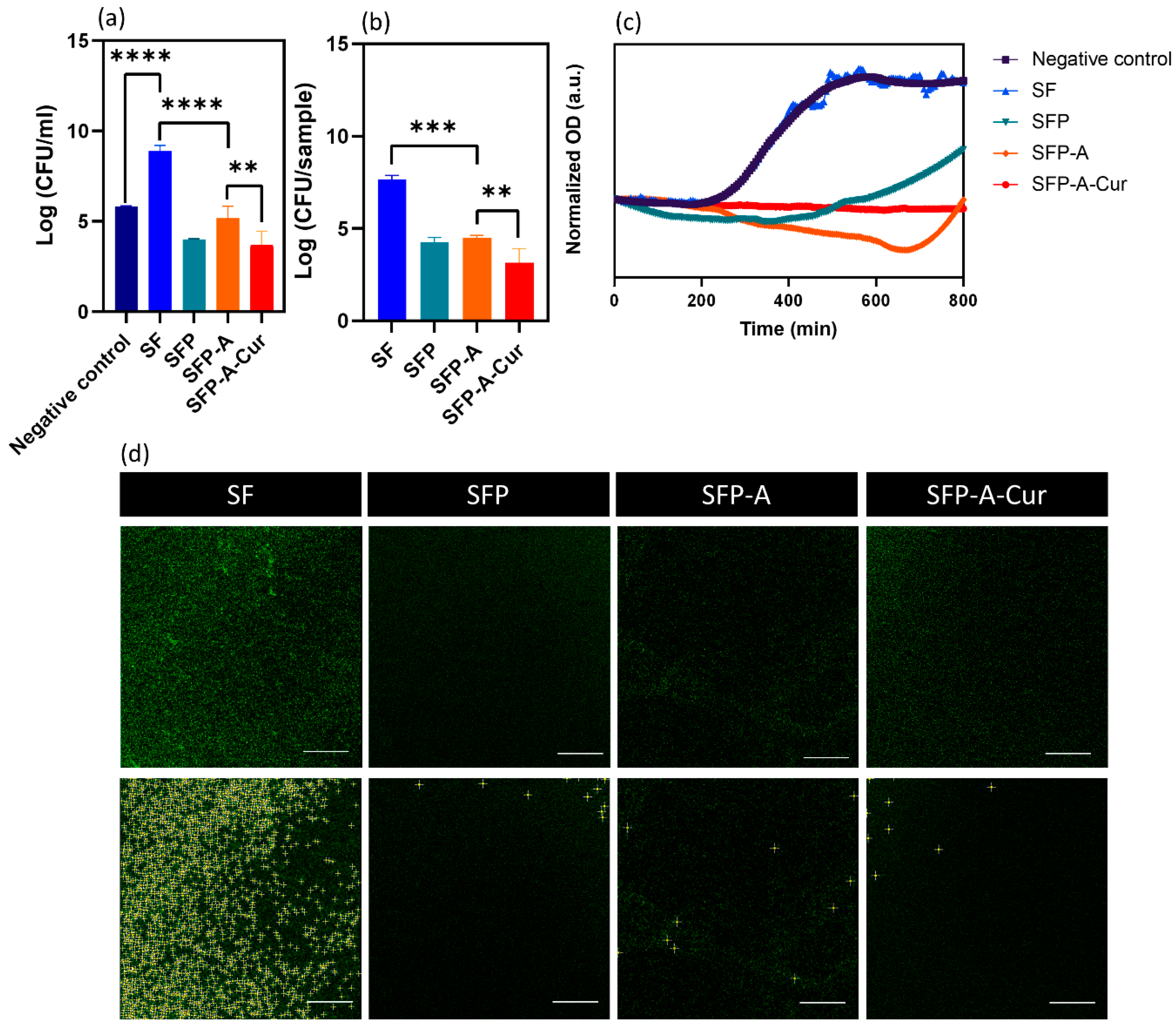
| Sample Code | Solution Composition (%w/v: g/mL) |
|---|---|
| SF | 3% SF |
| SFP | 3% SF–3% F127 |
| SFP-A | 3% SF–3% F127–5.3% acetone |
| SFP-A-Cur | 3% SF–3% F127–5.3% acetone–0.04% curcumin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khodaei, A.; Johari, N.; Jahanmard, F.; Cecotto, L.; Khosravimelal, S.; Madaah Hosseini, H.R.; Bagheri, R.; Samadikuchaksaraei, A.; Amin Yavari, S. Particulate 3D Hydrogels of Silk Fibroin-Pluronic to Deliver Curcumin for Infection-Free Wound Healing. Biomimetics 2024, 9, 483. https://doi.org/10.3390/biomimetics9080483
Khodaei A, Johari N, Jahanmard F, Cecotto L, Khosravimelal S, Madaah Hosseini HR, Bagheri R, Samadikuchaksaraei A, Amin Yavari S. Particulate 3D Hydrogels of Silk Fibroin-Pluronic to Deliver Curcumin for Infection-Free Wound Healing. Biomimetics. 2024; 9(8):483. https://doi.org/10.3390/biomimetics9080483
Chicago/Turabian StyleKhodaei, Azin, Narges Johari, Fatemeh Jahanmard, Leonardo Cecotto, Sadjad Khosravimelal, Hamid Reza Madaah Hosseini, Reza Bagheri, Ali Samadikuchaksaraei, and Saber Amin Yavari. 2024. "Particulate 3D Hydrogels of Silk Fibroin-Pluronic to Deliver Curcumin for Infection-Free Wound Healing" Biomimetics 9, no. 8: 483. https://doi.org/10.3390/biomimetics9080483
APA StyleKhodaei, A., Johari, N., Jahanmard, F., Cecotto, L., Khosravimelal, S., Madaah Hosseini, H. R., Bagheri, R., Samadikuchaksaraei, A., & Amin Yavari, S. (2024). Particulate 3D Hydrogels of Silk Fibroin-Pluronic to Deliver Curcumin for Infection-Free Wound Healing. Biomimetics, 9(8), 483. https://doi.org/10.3390/biomimetics9080483









