AI-Driven Data Analysis of Quantifying Environmental Impact and Efficiency of Shape Memory Polymers
Abstract
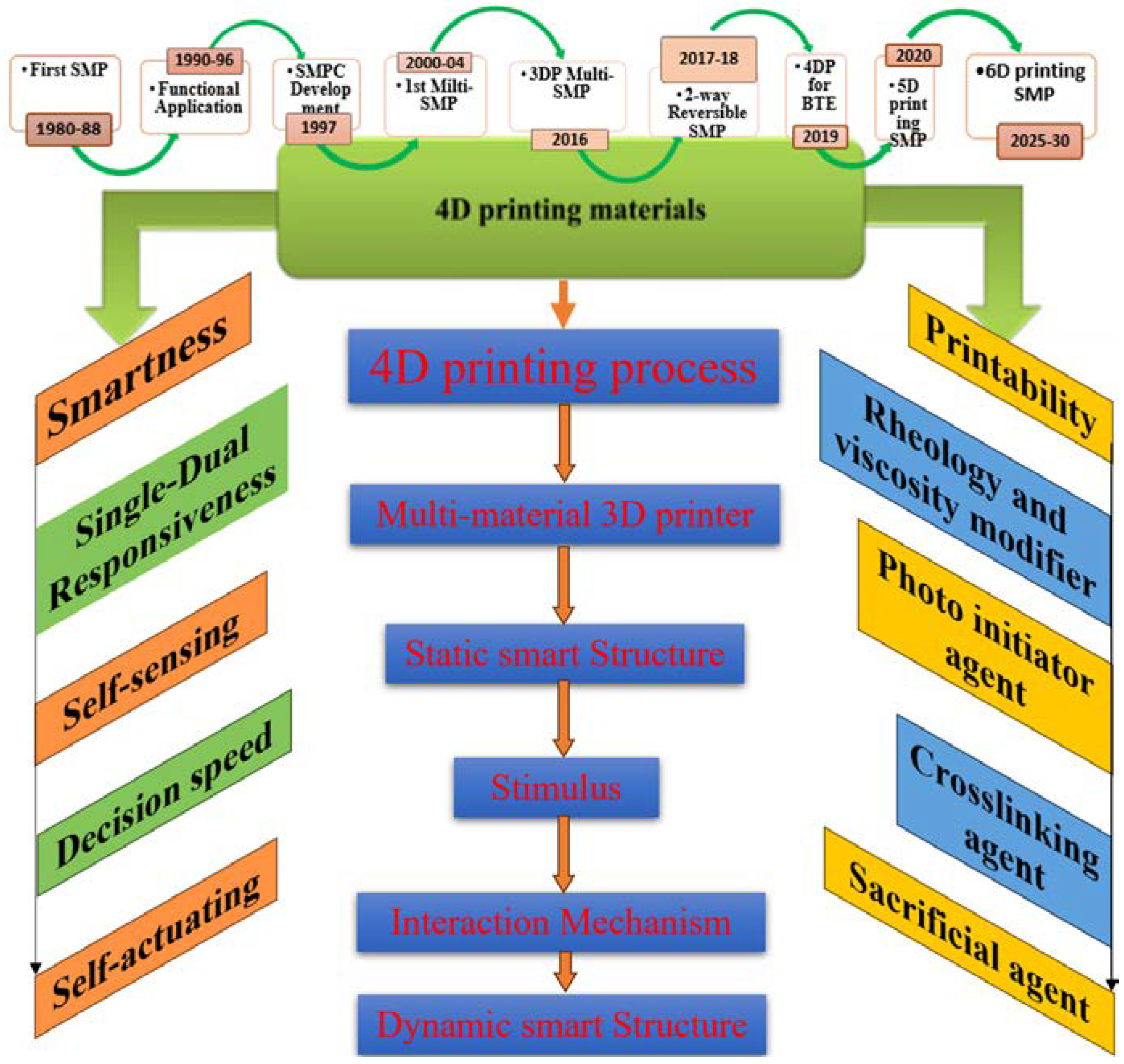
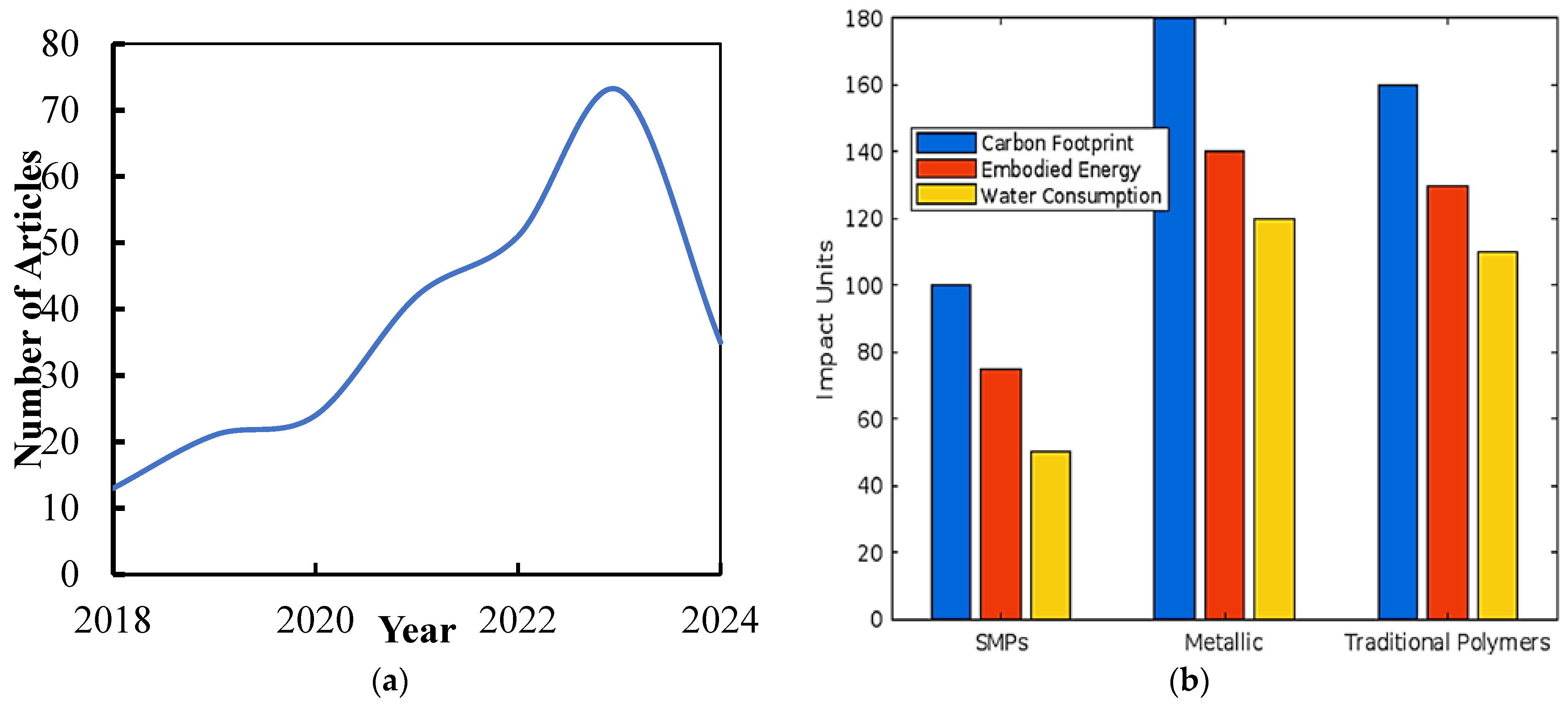
:1. Introduction
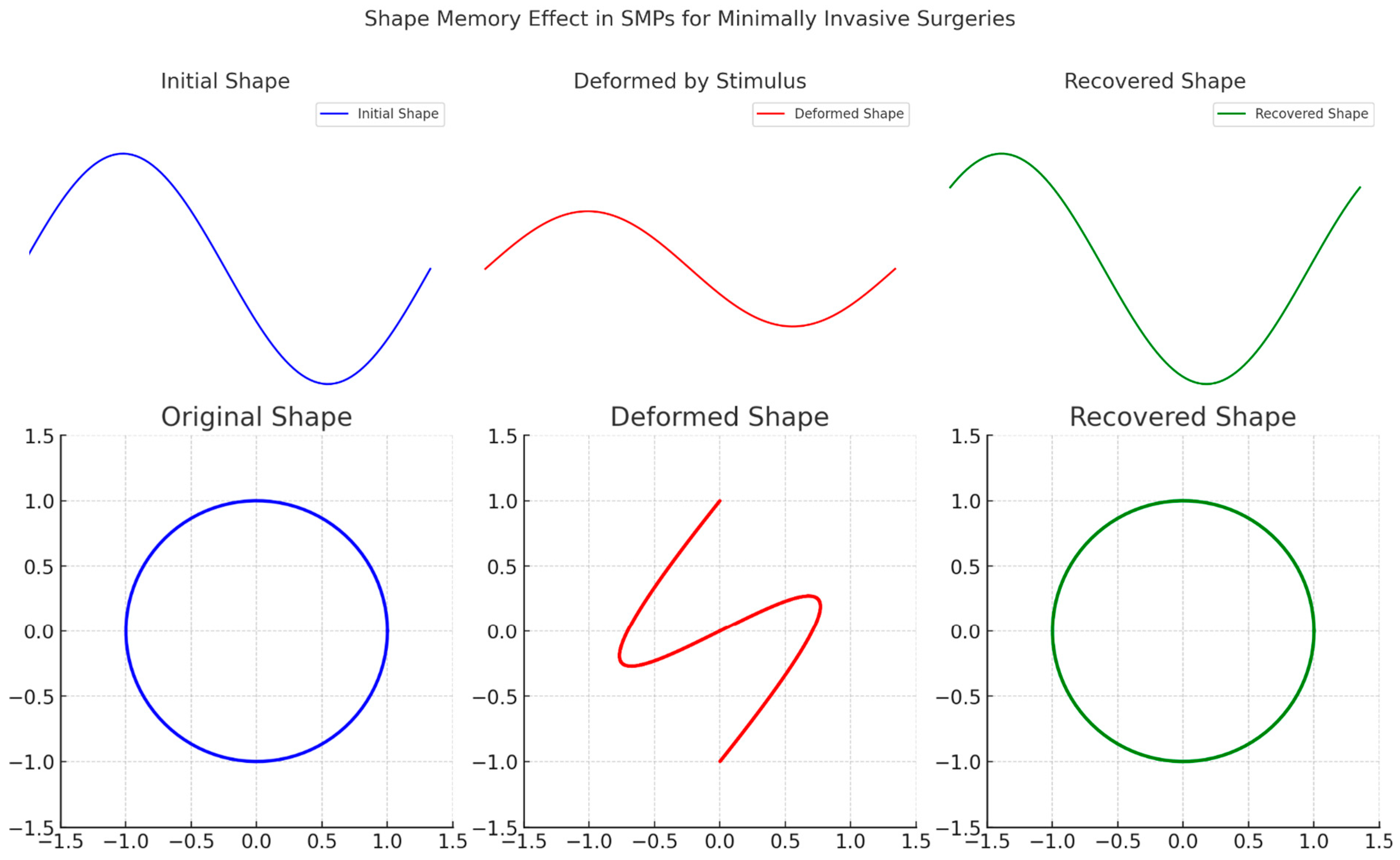
2. Sustainability Potential of Shape Memory Polymers
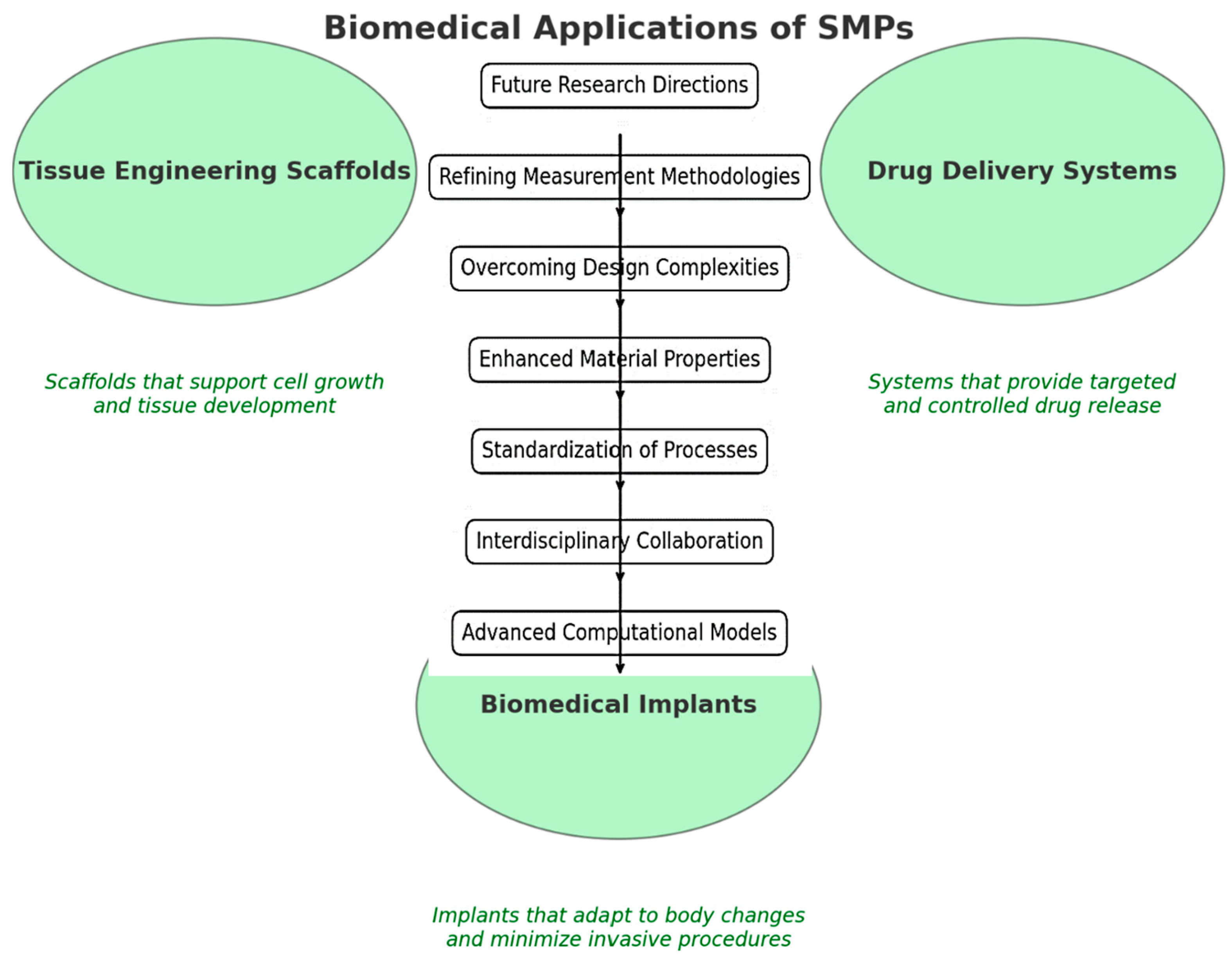
3. Biological Applications and Management Processes
3.1. Tissue Engineering
3.2. Drug Delivery Systems
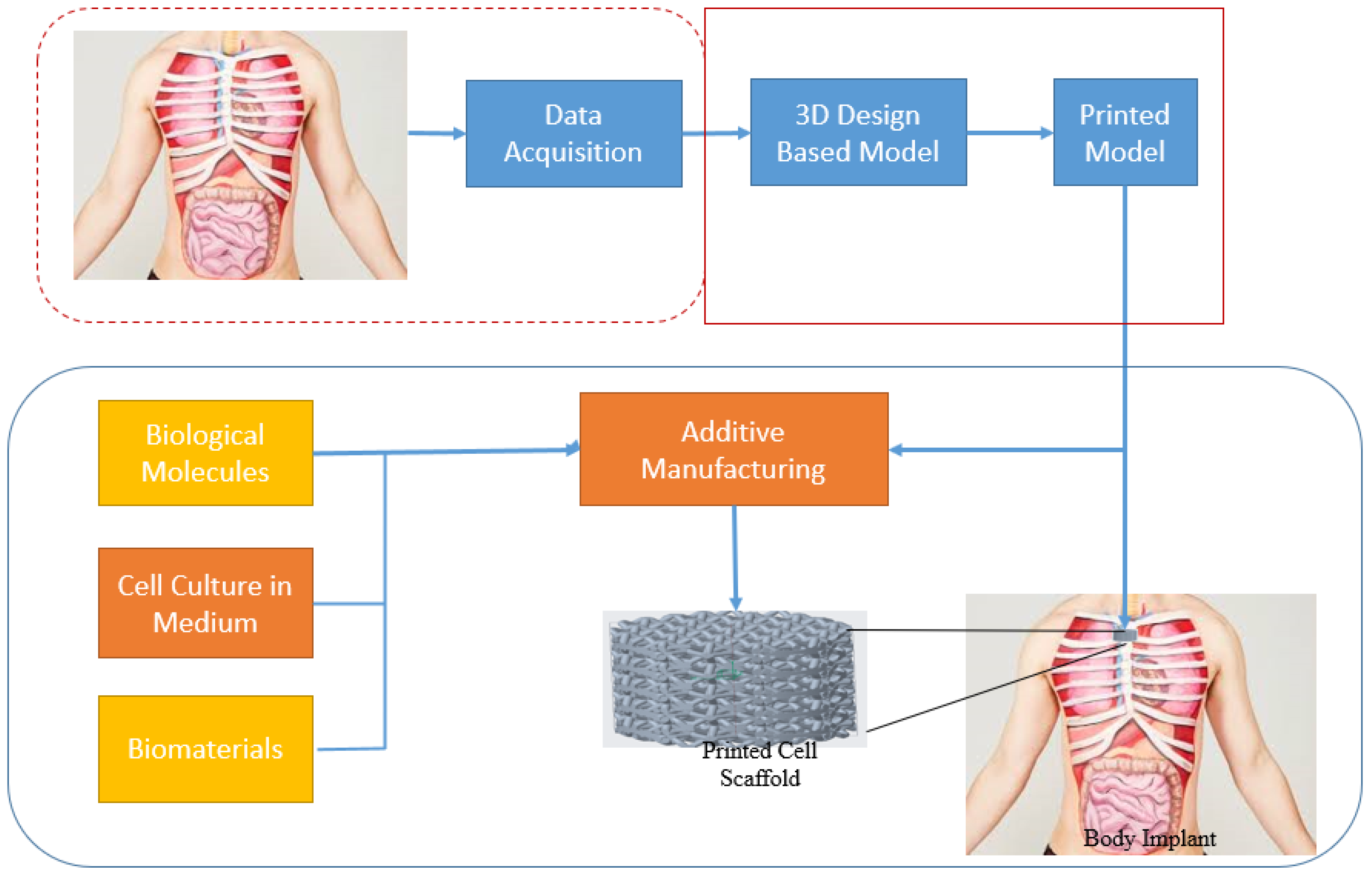
3.3. Biomedical Implants
3.4. Management Processes
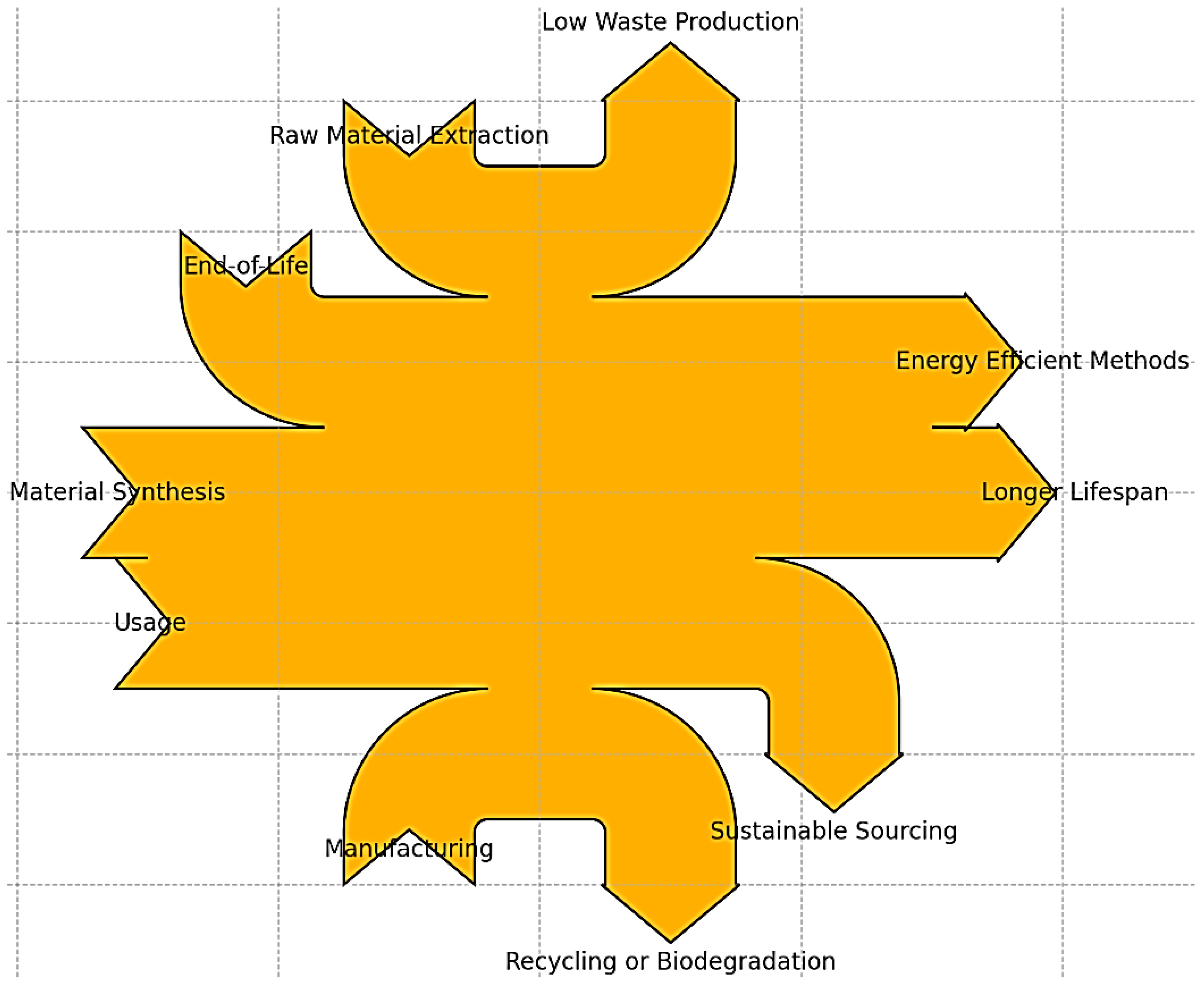
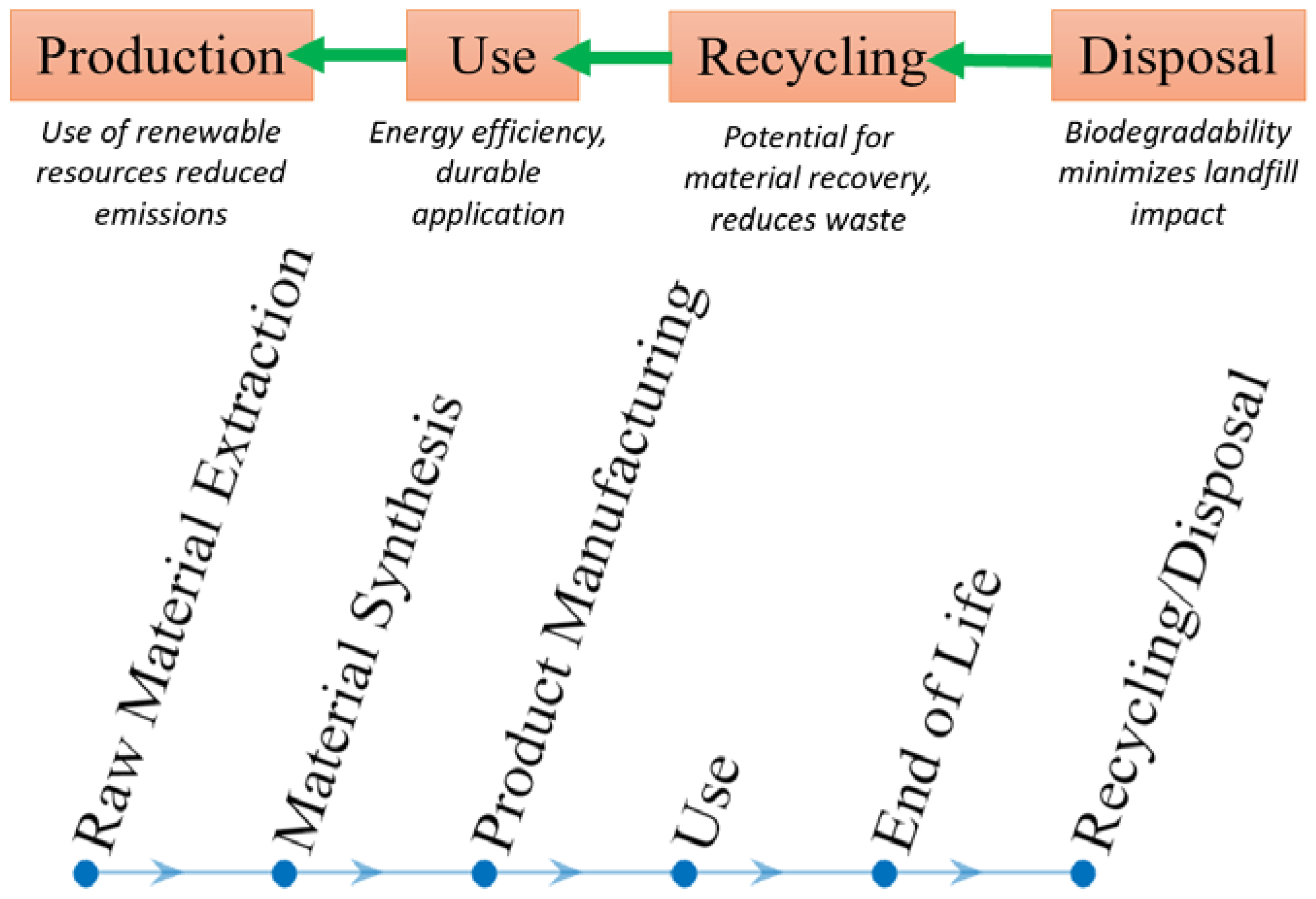
4. Environmental Impact Assessment
4.1. Production Stage
4.2. Life Cycle Assessment
5. Thermo-Reactive Polymer Hydrogels and Applications
5.1. Applications of Thermo-Reactive Polymer Hydrogels
5.1.1. Drug Delivery Systems
5.1.2. Tissue Engineering and Regenerative Medicine
5.2. Future Perspectives Polymer Hydrogels
6. Biomedical Considerations for Biomimetics
6.1. Bioprinting
6.2. Implantable Devices
6.3. Regulatory Considerations
6.4. Future Perspectives on Janus Nanoparticles in Shape Memory Applications
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ahn, S.; Byun, J.; Joo, H.; Jeong, J.; Lee, D.; Cho, K. 4D Printing of Continuous Shape Representation. Adv. Mater. Technol. 2021, 6, 2100133. [Google Scholar] [CrossRef]
- Zolfagharian, A.; Jarrah, H.R.; Bodaghi, M. 4D printing classroom in modern interactive learning environments. Bioprinting 2021, 24, e00169. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, X.; Liu, Y.; Ramakrishna, S. Review of mechanisms and deformation behaviors in 4D printing. Int. J. Adv. Manuf. Technol. 2019, 105, 4633–4649. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, W.; Zhang, W.; Paolo, D. A Novel Biopsy Capsule Robot Based on High-Speed Cutting Tissue. Cyborg Bionic Syst. 2022, 7, 9783517. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.B.; Bates, S.R.; Llewellyn-Jones, T.M.; Valori, L.P.; Dicker, M.P.; Trask, R.S. 4D printing with robust thermoplastic polyurethane hydrogel-elastomer trilayers. Mater. Des. 2019, 163, 107544. [Google Scholar] [CrossRef]
- Bajpai, A.; Baigent, A.; Raghav, S.; Brádaigh, C.Ó.; Koutsos, V.; Radacsi, N. 4D printing: Materials, technologies, and future applications in the biomedical field. Sustainability 2020, 12, 10628. [Google Scholar] [CrossRef]
- Li, X.; Jiang, H.; He, N.; Yuan, W.; Qian, Y.; Ouyang, Y. Graphdiyne-Related Materials in Biomedical Applications and Their Potential in Peripheral Nerve Tissue Engineering. Cyborg Bionic Syst. 2022, 2022, 9892526. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Sakhaei, A.H.; Lee, H.; Dunn, C.K.; Fang, N.X.; Dunn, M.L. Multimaterial 4D printing with tailorable shape memory polymers. Sci. Rep. 2016, 6, 31110. [Google Scholar] [CrossRef] [PubMed]
- Subash, A.; Kandasubramanian, B. 4D printing of shape memory polymers. Eur. Polym. J. 2020, 134, 109771. [Google Scholar] [CrossRef]
- Akbari, S.; Sakhaei, A.H.; Kowsari, K.; Yang, B.; Serjouei, A.; Zhang, Y.; Ge, Q. Enhanced multimaterial 4D printing with active hinges. Smart Mater. Struct. 2018, 27, 65027. [Google Scholar] [CrossRef]
- Merkel, A.; Chen, Y.; Villani, C.; George, A. GRP78 promotes the osteogenic and angiogenic response in periodontal ligament stem cells. Eur. Cells Mater. 2023, 45, 14–30. [Google Scholar] [CrossRef]
- Ahmed, J. Recent Advances of Novel Materials for 3D/4D Printing in Biomedical Applications. In 3D and 4D Printing in Biomedical Applications; Maniruzzaman, M., Ed.; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2019; pp. 239–271. [Google Scholar] [CrossRef]
- Bodaghi, M.; Damanpack, A.R.; Liao, W.H. Triple shape memory polymers by 4D printing. Smart Mater. Struct. 2018, 27, 65010. [Google Scholar] [CrossRef]
- Lin, W.-C.; Fan, F.-Y.; Cheng, H.-C.; Lin, Y.; Shen, Y.-K.; Lai, J.-S.; Wang, L.; Ruslin, M. Optimisation Shape-Memory Situations of a Stimulus Responsive Composite Material. Polymers 2021, 13, 697. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, F.; Liu, Y.; Leng, J. 4D printed shape memory polymers and their structures for biomedical applications. Sci. China Technol. Sci. 2020, 63, 545–560. [Google Scholar] [CrossRef]
- Adam, G.; Benouhiba, A.; Rabenorosoa, K.; Clévy, C.; Cappelleri, D.J. 4D printing: Enabling technology for microrobotics applications. Adv. Intell. Syst. 2021, 3, 2000216. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, F.; Zhai, W.; Cheng, S.; Li, J.; Wang, Y. Unraveling of Advances in 3D-Printed Polymer-Based Bone Scaffolds. Polymers 2022, 14, 566. [Google Scholar] [CrossRef]
- Bakarich, S.E.; Gorkin, R., III; Panhuis, M.I.H.; Spinks, G.M. 4D printing with mechanically robust, thermally actuating hydrogels. Macromol. Rapid Commun. 2015, 36, 1211–1217. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Zahedi, S.A. Improving bioactivity and strength of PEEK composite polymer for bone application. Mater. Chem. Phys. 2021, 266, 124485. [Google Scholar] [CrossRef]
- Andreu, A.; Su, P.C.; Kim, J.H.; Ng, C.S.; Kim, S.; Kim, I.; Lee, J.; Noh, J.; Subramanian, A.S.; Yoon, Y.J. 4D printing materials for Vat Photopolymerization. Addit. Manuf. 2021, 44, 102024. [Google Scholar] [CrossRef]
- Choong, Y.Y.C.; Maleksaeedi, S.; Eng, H.; Yu, S.; Wei, J.; Su, P.-C. High speed 4D printing of shape memory polymers with nanosilica. Appl. Mater. Today 2020, 18, 100515. [Google Scholar] [CrossRef]
- Xu, J.; Chang, L.; Chen, T.; Ren, T.; Zhang, Y.; Cai, Z. Study of the bending properties of variable stiffness chain mail fabrics. Compos. Struct. 2023, 322, 117369. [Google Scholar] [CrossRef]
- Ding, Z.; Yuan, C.; Peng, X.; Wang, T.; Qi, H.J.; Dunn, M.L. Direct 4D printing via active composite materials. Sci. Adv. 2017, 3, e1602890. [Google Scholar] [CrossRef] [PubMed]
- Demoly, F.; Dunn, M.L.; Wood, K.L.; Qi, H.J.; André, J.-C. The status, barriers, challenges, and future in design for 4D printing. Mater. Des. 2021, 212, 110193. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, L.; Liu, Y.; Liu, L.; Leng, J. 4D printing of personalised shape memory polymer vascular stents with negative Poisson’s ratio structure: A preliminary study. Sci. China Technol. Sci. 2020, 63, 578–588. [Google Scholar] [CrossRef]
- Leng, J.; Lu, H.; Liu, Y.; Huang, W.M.; Du, S. Shape-memory polymers—A class of novel smart materials. MRS Bull. 2009, 34, 848–855. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Zahedi, S.A.; Ismail, S.O.; Omigbodun, F.T.; Bowoto, O.K.; Olawumi, M.A.; Muhammad, M.A. 3D printing of PEEK–cHAp scaffold for medical bone implant. Bio-Des. Manuf. 2021, 4, 44–59. [Google Scholar] [CrossRef]
- Zheng, J.; Yue, R.; Yang, R.; Wu, Q.; Wu, Y.; Huang, M.; Liao, Y. Visualization of zika virus infection via a light-initiated bio-orthogonal cycloaddition labeling strategy. Front. Bioeng. Biotechnol. 2022, 10, 940511. [Google Scholar] [CrossRef]
- Xie, H.; Yang, K.-K.; Wang, Y.-Z. Photo-cross-linking: A powerful and versatile strategy to develop shape-memory polymers. Prog. Polym. Sci. 2019, 95, 32–64. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, L.; Zhang, F.; Leng, J.; Liu, Y. Shape memory polymers and their composites in biomedical applications. Mater. Sci. Eng. C 2019, 97, 864–883. [Google Scholar] [CrossRef]
- Ly, S.T.; Kim, J.Y. 4D printing–fused deposition modeling printing with thermal-responsive shape memory polymers. Int. J. Precis. Eng. Manuf.-Green Technol. 2017, 4, 267–272. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Zahedi, S.A.; Ismail, S.O.; Olawade, D.B. Recent advances in biopolymeric composite materials: Future sustainability of bone-implant. Renew. Sustain. Energy Rev. 2021, 150, 111505. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, B.H.; Li, Z. Biodegradable polyester shape memory polymers: Recent advances in design, material properties and applications. Mater. Sci. Eng. C 2018, 92, 1061–1074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yin, Z.; Ren, L.; Liu, Q.; Ren, L.; Yang, X.; Zhou, X. Advances in 4D Printed Shape Memory Polymers: From 3D Printing, Smart Excitation, and Response to Applications. Adv. Mater. Technol. 2022, 7, 2101568. [Google Scholar] [CrossRef]
- Zhang, B.; Li, H.; Cheng, J.; Ye, H.; Sakhaei, A.H.; Yuan, C.; Rao, P.; Zhang, Y.-F.; Chen, Z.; Wang, R.; et al. Shape-Memory Polymers: Mechanically Robust and UV-Curable Shape-Memory Polymers for Digital Light Processing Based 4D Printing (Adv. Mater. 27/2021). Adv. Mater. 2021, 33, 2170210. [Google Scholar] [CrossRef]
- Zarek, M.; Layani, M.; Eliazar, S.; Mansour, N.; Cooperstein, I.; Shukrun, E.; Szlar, A.; Cohn, D.; Magdassi, S. 4D printing shape memory polymers for dynamic jewellery and fashionwear. Virtual Phys. Prototyp. 2016, 11, 263–270. [Google Scholar] [CrossRef]
- Li, B.; Wang, W.; Zhao, L.; Li, M.; Yan, D.; Li, X.; Zhang, J.; Gao, Q.; Feng, Y.; Zheng, J.; et al. Aggregation-Induced Emission-Based Macrophage-Like Nanoparticles for Targeted Photothermal Therapy and Virus Transmission Blockage in Monkeypox. Adv. Mater. 2024, 36, 2305378. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.-H. Engineering and functionalisation of gelatin biomaterials: From cell culture to medical applications. Tissue Eng. Part B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Castro, N.J.; Meinert, C.; Levett, P.; Hutmacher, D.W. Current developments in multi-functional smart materials for 3D/4D bioprinting. Curr. Opin. Biomed. Eng. 2017, 2, 67–75. [Google Scholar] [CrossRef]
- Champeau, M.; Heinze, D.A.; Viana, T.N.; de Souza, E.R.; Chinellato, A.C.; Titotto, S. 4D Printing of Hydrogels: A Review. Adv. Funct. Mater. 2020, 30, 1910606. [Google Scholar] [CrossRef]
- Joyce, M.; Hodgkinson, T.; Lemoine, M.; González-Vázquez, A.; Kelly, D.J.; O’Brien, F.J. Development of a 3D-printed bioabsorbable composite scaffold with mechanical properties suitable for treating large, load-bearingarticular cartilage defects. Eur. Cells Mater. 2023, 45, 158–172. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Oshin, E.A.; Olawumi, A.M. Nanostructural computation of 4D printing carboxymethylcellulose (CMC) composite. Nano-Struct. Nano-Objects 2020, 21, 100423. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wei, Q.; Zhang, J. Light-responsive shape memory polymer composites. Eur. Polym. J. 2022, 173, 111314. [Google Scholar] [CrossRef]
- Pathan, N.; Shende, P. Strategic conceptualisation and potential of self-healing polymers in biomedical field. Mater. Sci. Eng. C 2021, 125, 112099. [Google Scholar] [CrossRef]
- Luan, S.; Yu, X.; Lei, S.; Ma, C.; Wang, X.; Xue, X.; Ding, Y.; Ma, T.; Zhu, B. Deep learning for fast super-resolution ultrasound microvessel imaging. Phys. Med. Biol. 2023, 68, 245023. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Mao, Z.; Chang, L.; Huang, X.; Cai, Z. Study of Impact Resistance Based on Porcupine Quills Bionic Thin-walled Structure. J. Bionic Eng. 2023, 20, 1942–1955. [Google Scholar] [CrossRef]
- Guan, J.; Jia, Y.; Zhang, B.; Zhao, G. Application of 4D bioprinting in tissue engineering. Chin. J. Tissue Eng. Res. 2022, 26, 446. [Google Scholar]
- Li, Y.-C.; Zhang, Y.S.; Akpek, A.; Shin, S.R.; Khademhosseini, A. 4D bioprinting: The next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication 2016, 9, 012001. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Ismail, S.O.; Afolalu, T.D.; Olawade, D.B.; Zahedi, M. Review on 3D printing: Fight against COVID-19. Mater. Chem. Phys. 2021, 258, 123943. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Han, X.; Zhao, C.; Wang, S.; Tang, X. Recent Advance in Biological Responsive Nanomaterials for Biosensing and Molecular Imaging Application. Int. J. Mol. Sci. 2022, 23, 1923. [Google Scholar] [CrossRef]
- Yu, X.; Luan, S.; Lei, S.; Huang, J.; Liu, Z.; Xue, X.; Ma, T.; Ding, Y.; Zhu, B. Deep learning for fast denoising filtering in ultrasound localization microscopy. Phys. Med. Biol. 2023, 68, 205002. [Google Scholar] [CrossRef]
- Holman, H.; Kavarana, M.N.; Rajab, T.K. Smart materials in cardiovascular implants: Shape memory alloys and shape memory polymers. Artif. Organs 2021, 45, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Damanpack, A.R.; Bodaghi, M.; Liao, W.H. Contact/impact modeling and analysis of 4D printed shape memory polymer beams. Smart Mater. Struct. 2020, 29, 085016. [Google Scholar] [CrossRef]
- Nam, S.; Pei, E. A taxonomy of shape-changing behavior for 4D printed parts using shape-memory polymers. Prog. Addit. Manuf. 2019, 4, 167–184. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Zahedi, S.A.; Adeoye, A.O.M. 3D printing of bone scaffolds with hybrid biomaterials. Compos. Part B Eng. 2019, 158, 428–436. [Google Scholar] [CrossRef]
- Zhao, C.; Tang, X.; Zhao, J.; Cao, J.; Jiang, Z.; Qin, J. MOF derived core-shell CuO/C with temperature-controlled oxygen-vacancy for real time analysis of glucose. J. Nanobiotechnol. 2022, 20, 507. [Google Scholar] [CrossRef]
- Abuzaid, W.; Alkhader, M.; Omari, M. Experimental analysis of heterogeneous shape recovery in 4d printed honeycomb structures. Polym. Test. 2018, 68, 100–109. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Zhang, F.; Lan, X.; Leng, J.; Liu, S.; Jia, X.; Cotton, C.; Sun, B.; Gu, B.; et al. Shape memory behavior and recovery force of 4D printed laminated Miura-origami structures subjected to compressive loading. Compos. Part B Eng. 2018, 153, 233–242. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, F.; Leng, J.; Wang, L.; Cotton, C.; Sun, B.; Chou, T.W. Synergistic effect enhanced shape recovery behavior of metal-4D printed shape memory polymer hybrid composites. Compos. Part B Eng. 2019, 179, 107536. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Song, Z.; Ren, L.; Liu, Q.; Ren, L. Programming Multistage Shape Memory and Variable Recovery Force with 4D Printing Parameters. Adv. Mater. Technol. 2019, 4, 1900535. [Google Scholar] [CrossRef]
- Zhang, B.; Li, H.; Cheng, J.; Ye, H.; Sakhaei, A.H.; Yuan, C.; Rao, P.; Zhang, Y.F.; Chen, Z.; Wang, R.; et al. Mechanically robust and UV-curable shape-memory polymers for digital light processing based 4D printing. Adv. Mater. 2021, 33, 2101298. [Google Scholar] [CrossRef]
- Liang, Y.; Gao, D.; Chen, B.; Zhao, J. Friction and Wear Study on Friction Pairs with a Biomimetic Non-smooth Surface of 316L Relative to CF/PEEK under a Seawater Lubricated Condition. Chin. J. Mech. Eng. 2019, 32, 66. [Google Scholar] [CrossRef]
- Liu, Y.; Zhai, X.; Deng, Y.; Wu, D. Tribological Property of Selective Laser Melting-Processed 316L Stainless Steel against Filled PEEK under Water Lubrication. Tribol. Trans. 2019, 62, 962–970. [Google Scholar] [CrossRef]
- Affatato, S.; De Mattia, J.S.; Bracco, P.; Pavoni, E.; Taddei, P. Does cyclic stress and accelerated ageing influence the wear behavior of highly crosslinked polyethylene? J. Mech. Behav. Biomed. Mater. 2016, 59, 418–429. [Google Scholar] [CrossRef]
- Barkarmo, S.; Longhorn, D.; Leer, K.; Johansson, C.B.; Stenport, V.; Franco-Tabares, S.; Kuehne, S.A.; Sammons, R. Biofilm formation on polyetheretherketone and titanium surfaces. Clin. Exp. Dent. Res. 2019, 5, 427–437. [Google Scholar] [CrossRef]
- Beltrán, A.M.; Civantos, A.; Dominguez-Trujillo, C.; Moriche, R.; Rodríguez-Ortiz, J.A.; García-Moreno, F.; Webster, T.J.; Kamm, P.H.; Restrepo, A.M.; Torres, Y. Porous Titanium Surfaces to Control Bacteria Growth: Mechanical Properties and Sulfonated Polyetheretherketone Coatings as Antibiofouling Approaches. Metals 2019, 9, 995. [Google Scholar] [CrossRef]
- Dalal, A.; Pawar, V.; McAllister, K.; Weaver, C.; Hallab, N.J. Orthopedic implant cobalt-alloy particles produce greater toxicity and inflammatory cytokines than titanium alloy and zirconium alloy-based particles in vitro, in human osteoblasts, fibroblasts, and macrophages. J. Biomed. Mater. Res. Part A 2012, 100A, 2147–2158. [Google Scholar] [CrossRef]
- Mahesh, V.; Joseph, A.S.; Mahesh, V.; Harursampath, D.; Vn, C. Investigation on the mechanical properties of additively manufactured PETG composites reinforced with OMMT nanoclay and carbon fibers. Polym. Compos. 2021, 42, 2380–2395. [Google Scholar] [CrossRef]
- Ou, K.; Wang, M.; Meng, C.; Guo, K.; Shariar Emon, N.; Li, J.; Qi, K.; Dai, Y.; Wang, B. Enhanced mechanical strength and stretchable ionic conductive hydrogel with double-network structure for wearable strain sensing and energy harvesting. Compos. Sci. Technol. 2024, 255, 110732. [Google Scholar] [CrossRef]
- Lin, C.; Liu, L.; Liu, Y.; Leng, J. 4D printing of shape memory polybutylene succinate/polylactic acid (PBS/PLA) and its potential applications. Compos. Struct. 2022, 279, 114729. [Google Scholar] [CrossRef]
- Tsai, P.I.; Wu, M.H.; Li, Y.Y.; Lin, T.H.; Tsai, J.S.; Huang, H.I.; Lai, H.J.; Lee, M.H.; Chen, C.Y. Additive-manufactured Ti-6Al-4 V/Polyetheretherketone composite porous cage for Interbody fusion: Bone growth and biocompatibility evaluation in a porcine model. BMC Musculoskelet Disord. 2021, 22, 171. [Google Scholar] [CrossRef]
- Zhuang, J.; Pan, H.; Feng, W. Ultrasensitive Photoelectric Immunoassay Platform Utilizing Biofunctional 2D Vertical SnS2/Ag2S Heterojunction. ACS Appl. Electron. Mater. 2024. [Google Scholar] [CrossRef]
- Camargo, J.C.; Machado, Á.R.; Almeida, E.C.; Silva, E.F.M.S. Mechanical properties of PLA-graphene filament for FDM 3D printing. Int. J. Adv. Manuf. Technol. 2019, 103, 2423–2443. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Daniyan, I.A.; Ikumapayi, O.M.; Malachi, O.B.; Malachi, I.O. Microanalysis of hybrid characterisation of PLA/cHA polymer scaffolds for bone regeneration. Polym. Test. 2020, 83, 106341. [Google Scholar] [CrossRef]
- Lu, J.; Liu, Y.; Huang, W.; Bi, K.; Zhu, Y.; Fan, Q. Robust Control Strategy of Gradient Magnetic Drive for Microrobots Based on Extended State Observer. Cyborg Bionic Syst. 2022, 6, 9835014. [Google Scholar] [CrossRef]
- Cheng, K.; Liu, Y.; Wang, R.; Zhang, J.; Jiang, X.; Dong, X.; Xu, X. Topological optimisation of 3D printed bone analog with PEKK for surgical mandibular reconstruction. J. Mech. Behav. Biomed. Mater. 2020, 107, 103758. [Google Scholar] [CrossRef]
- Hu, F.; Qiu, L.; Zhou, H. Medical device product innovation choices in Asia: An empirical analysis based on product space. Front. Public Health 2022, 10, 871575. [Google Scholar] [CrossRef]
- Hanemann, T.; Syperek, D.; Nötzel, D. 3D Printing of ABS Barium Ferrite Composites. Materials 2020, 13, 1481. [Google Scholar] [CrossRef]
- Rankouhi, B.; Javadpour, S.; Delfanian, F.; McTaggart, R.; Letcher, T. Experimental Investigation of Mechanical Performance and Printability of Gamma-Irradiated Additively Manufactured ABS. J. Mater. Eng. Perform. 2018, 27, 3643–3654. [Google Scholar] [CrossRef]
- Shi, Y.; Hou, X.; Na, Z.; Zhou, J.; Yu, N.; Liu, S.; Xin, L.; Gao, G.; Liu, Y. Bio-inspired Attachment Mechanism of Dynastes Hercules: Vertical Climbing for On-Orbit Assembly Legged Robots. J. Bionic Eng. 2023, 21, 137–148. [Google Scholar] [CrossRef]
- Zhang, T.; Li, C.; Li, W.; Wang, Z.; Gu, Z.; Li, J.; Yuan, J.; Ou-Yang, J.; Yang, X.; Zhu, B. A Self-Healing Optoacoustic Patch with High Damage Threshold and Conversion Efficiency for Biomedical Applications. Nano-Micro Lett. 2024, 16, 122. [Google Scholar] [CrossRef]
- Rahaman, M.; Xiao, W. Silicon nitride bioceramics in healthcare. Int. J. Appl. Ceram. Technol. 2018, 15, 861–872. [Google Scholar] [CrossRef]
- Fu, P.C.; Wang, J.Y.; Su, Y.; Liao, Y.Q.; Li, S.L.; Xu, G.L.; Huang, Y.J.; Hu, M.H.; Cao, L.M. Intravascular ultrasonography assisted carotid artery stenting for treatment of carotid stenosis: Two case reports. World J. Clin. Cases 2023, 11, 7127–7135. [Google Scholar] [CrossRef]
- Feng, P.; Jia, J.; Peng, S.; Yang, W.; Bin, S.; Shuai, C. Graphene oxide-driven interfacial coupling in laser 3D printed PEEK/PVA scaffolds for bone regeneration. Virtual Phys. Prototyp. 2020, 15, 211–226. [Google Scholar] [CrossRef]
- Wu, H.; Song, Y.; Li, J. Blockade of adrenergic β-receptor activation through local delivery of propranolol from a 3D collagen/polyvinyl alcohol/hydroxyapatite scaffold promotes bone repair in vivo. Cell Prolif. 2020, 53, e12725. [Google Scholar] [CrossRef]
- Boriani, S.; Pipola, V.; Cecchinato, R.; Ghermandi, R.; Tedesco, G.; Fiore, M.R.; Dionisi, F.; Gasbarrini, A. Composite PEEK/carbon fiber rods in the treatment for bone tumors of the cervical spine: A case series. Eur. Spine J. 2020, 29, 3229–3236. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, F.; Lan, X.; Leng, J.; Wu, A.S.; Bryson, T.M.; Cotton, C.; Gu, B.; Sun, B.; Chou, T.W. Shape memory behavior and recovery force of 4D printed textile functional composites. Compos. Sci. Technol. 2018, 160, 224–230. [Google Scholar] [CrossRef]
- Agrawal, N.; Arora, B. Self-Healing Polymers and Composites: Extrinsic Routes. Mini-Rev. Org. Chem. 2022, 19, 496–512. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Adebiyi, A.V.; Elemure, E.I. Microstructural 4D printing investigation of ultra-sonication biocomposite polymer. J. King Saud Univ.-Eng. Sci. 2021, 33, 54–60. [Google Scholar] [CrossRef]
- Invernizzi, M.; Turri, S.; Levi, M.; Suriano, R. 4D printed thermally activated self-healing and shape memory polycaprolactone-based polymers. Eur. Polym. J. 2018, 101, 169–176. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, W.; Zhang, Z.; Zhang, Y.-F.; Hingorani, H.; Liu, Z.; Liu, J.; Ge, Q. Self-Healing Four-Dimensional Printing with an Ultraviolet Curable Double-Network Shape Memory Polymer System. ACS Appl. Mater. Interfaces 2019, 11, 10328–10336. [Google Scholar] [CrossRef]
- Sharma, A.; Arya, S.K. Bio-Inspired Self-Healable Materials. In Self-Healing Smart Materials and Allied Applications; Wiley: Hoboken, NJ, USA, 2021; pp. 435–474. [Google Scholar]
- Ramesh, M.; Rajeshkumar, L.; Balaji, D.; Bhuvaneswari, V.; Sivalingam, S. Self-Healable Conductive Materials. In Self-Healing Smart Materials and Allied Applications; Wiley: Hoboken, NJ, USA, 2021; pp. 297–319. [Google Scholar]
- Ramesh, M.; Rajeshkumar, L.; Saravanakumar, R. Mechanically-Induced Self-Healable Materials. In Self-Healing Smart Materials and Allied Applications; Wiley: Hoboken, NJ, USA, 2021; pp. 379–403. [Google Scholar]
- Lee, H.-Y.; Cha, S.-H. Enhancement of self-healing property by introducing ethylene glycol group into thermally reversible Diels-Alder reaction based self-healable materials. Macromol. Res. 2017, 25, 640–647. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Adeoye, A.O.M.; Ismail, M. Analytical optimisation of a nanoparticle of microstructural fused deposition of resins for additive manufacturing. Compos. Part B Eng. 2018, 150, 248–254. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, Y.; Xie, B.; Jin, F.; Ma, L.; Zhang, H.; Li, Y.; Chen, X.; Hou, M.; Gao, J.; et al. Lightweight and drift-free magnetically actuated millirobots via asymmetric laser-induced graphene. Nat. Commun. 2024, 15, 4334. [Google Scholar] [CrossRef]
- Song, Z.; Ren, L.; Zhao, C.; Liu, H.; Yu, Z.; Liu, Q.; Ren, L. Biomimetic nonuniform, dual-stimuli self-morphing enabled by gradient four-dimensional printing. ACS Appl. Mater. Interfaces 2020, 12, 6351–6361. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, F.; Zhang, Z.; Cheng, Y.; Wang, Z.; Li, Y. Stimuli-responsive polydopamine-based smart materials. Chem. Soc. Rev. 2021, 50, 8319–8343. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Leng, J. Large-scale fabrication of superhydrophobic shape memory composite films for efficient anti-icing and de-icing. Sustain. Mater. Technol. 2023, 37, e00692. [Google Scholar] [CrossRef]
- Peng, B.; Yang, Y.; Cavicchi, K.A. Sequential shapeshifting 4D printing: Programming the pathway of multi-shape transformation by 3D printing stimuli-responsive polymers. Multi-Funct. Mater. 2020, 3, 042002. [Google Scholar] [CrossRef]
- Patdiya, J.; Kandasubramanian, B. Progress in 4D printing of stimuli responsive materials. Polym.-Plast. Technol. Mater. 2021, 60, 1845–1883. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Malachi, I.O.; Malachi, O.B.; Elemure, I.E.; Olawumi, A.M. Nano-structures of 4D morphology surface analysis of C1.7Mn0.6P0.1S0.07 (SAE 1045) tool wear. Nano-Struct. Nano-Objects 2020, 22, 100433. [Google Scholar] [CrossRef]
- Zhou, P.; Peng, R.; Xu, M.; Wu, V.; Navarro-Alarcon, D. Path Planning With Automatic Seam Extraction Over Point Cloud Models for Robotic Arc Welding. IEEE Robot. Autom. Lett. 2021, 6, 5002–5009. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, Y.; Wang, M.; Liang, Y.; Ren, L.; Ren, L. Recent progress in 4D printing of stimuli-responsive polymeric materials. Sci. China Technol. Sci. 2020, 63, 532–544. [Google Scholar] [CrossRef]
- Idaszek, J.; Costantini, M.; Karlsen, T.A.; Jaroszewicz, J.; Colosi, C.; Testa, S.; Fornetti, E.; Bernardini, S.; Seta, M.; Kasarełło, K.; et al. 3D bioprinting of hydrogel constructs with cell and material gradients for the regeneration of full-thickness chondral defect using a microfluidic printing head. Biofabrication 2019, 11, 044101. [Google Scholar] [CrossRef] [PubMed]
- Ramiah, P.; Du Toit, L.C.; Choonara, Y.E.; Kondiah, P.P.; Pillay, V. Hydrogel-based bioinks for 3D bioprinting in tissue regeneration. Front. Mater. 2020, 7, 76. [Google Scholar] [CrossRef]
- Zheng, Z.; Eglin, D.; Alini, M.; Richards, G.R.; Qin, L.; Lai, Y. Visible Light-Induced 3D Bioprinting Technologies and Corresponding Bioink Materials for Tissue Engineering: A Review. Engineering 2021, 7, 966–978. [Google Scholar] [CrossRef]
- You, F.; Chen, X.; Cooper, D.M.L.; Chang, T.; Eames, B.F. Homogeneous hydroxyapatite/alginate composite hydrogel promotes calcified cartilage matrix deposition with potential for three-dimensional bioprinting. Biofabrication 2018, 11, 015015. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, X.; Guan, T.; Chen, Y.; Qi, C. 3D bioprinting of tissue engineering scaffold for cell culture. Rapid Prototyp. J. 2020, 26, 835–840. [Google Scholar] [CrossRef]
- Thrivikraman, G.; França, C.M.; Athirasala, A.; Tahayeri, A.; Bertassoni, L.E. Nanomaterials in 3D bioprinting: Current approaches and future possibilities. Nanotechnol. Prev. Regen. Med. Emerg. Big Pict. 2017, 47, 349. [Google Scholar]
- Tan, B.; Kuang, S.; Li, X.; Cheng, X.; Duan, W.; Zhang, J.; Liu, W.; Fan, Y. Stereotactic technology for 3D bioprinting: From the perspective of robot mechanism. Biofabrication 2021, 13, 043001. [Google Scholar] [CrossRef] [PubMed]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef]
- Khorsandi, D.; Fahimipour, A.; Abasian, P.; Saber, S.S.; Seyedi, M.; Ghanavati, S.; Ahmad, A.; De Stephanis, A.A.; Taghavinezhaddilami, F.; Leonova, A.; et al. 3D and 4D printing in dentistry and maxillofacial surgery: Printing techniques, materials, and applications. Acta Biomater. 2021, 122, 26–49. [Google Scholar] [CrossRef]
- Mishra, A.; Srivastava, V. Biomaterials and 3D printing techniques used in the medical field. J. Med. Eng. Technol. 2021, 45, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Weems, A.C.; Pérez-Madrigal, M.M.; Arno, M.C.; Dove, A.P. 3D printing for the clinic: Examining contemporary polymeric biomaterials and their clinical utility. Biomacromolecules 2020, 21, 1037–1059. [Google Scholar] [CrossRef]
- Alam, F.; Verma, P.; Mohammad, W.J.; Teo, J.; Varadarajan, K.M.; Kumar, S. Architected poly(lactic acid)/poly(ε-caprolactone)/halloysite nanotube composite scaffolds enabled by 3D printing for biomedical applications. Mater. Sci. 2021, 56, 14070–14083. [Google Scholar] [CrossRef]
- Deshmukh, K.; Houkan, M.T.; AlMaadeed, M.A.; Sadasivuni, K.K. Introduction to 3D and 4D printing technology: State of the art and recent trends. In 3D and 4D Printing of Polymer Nanocomposite Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–24. [Google Scholar]
- Espera, A.H.; Valino, A.D.; Palaganas, J.O.; Souza, L.; Chen, Q. 3D Printing of a Robust Polyamide-12-Carbon Black Composite via Selective Laser Sintering: Thermal and Electrical Conductivity. Macromol. Mater. Eng. 2019, 304, 1800718. [Google Scholar] [CrossRef]
- Falahati, M.; Ahmadvand, P.; Safaee, S.; Chang, Y.C.; Lyu, Z.; Chen, R.; Li, L.; Lin, Y. Smart polymers and nanocomposites for 3D and 4D printing. Mater. Today 2020, 40, 215–245. [Google Scholar] [CrossRef]
- Pisani, S.; Genta, I.; Modena, T.; Dorati, R.; Benazzo, M.; Conti, B. Shape-Memory Polymers Hallmarks and Their Biomedical Applications in the Form of Nanofibers. Int. J. Mol. Sci. 2022, 23, 1290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Le Duigou, A.; Correa, D.; Ueda, M.; Matsuzaki, R.; Castro, M. A review of 3D and 4D printing of natural fibre biocomposites. Mater. Des. 2020, 194, 108911. [Google Scholar] [CrossRef]
- Kladko, D.V.; Falchevskaya, A.S.; Serov, N.S.; Prilepskii, A.Y. Nanomaterial Shape Influence on Cell Behavior. Int. J. Mol. Sci. 2021, 22, 5266. [Google Scholar] [CrossRef]
- Tan, K.X.; Danquah, M.K.; Jeevanandam, J.; Barhoum, A. Development of Janus Particles as Potential Drug Delivery Systems for Diabetes Treatment and Antimicrobial Applications. Pharmaceutics 2023, 15, 423. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vlăsceanu, G.M.; Iovu, H.; Ioniţă, M. Graphene inks for the 3D printing of cell culture scaffolds and related molecular arrays. Compos. Part B Eng. 2019, 162, 712–723. [Google Scholar] [CrossRef]
- Yang, F.; Guo, X.; Zeng, Z.; Xiao, J.; Li, H.; Luo, Y.; Guan, L.; Zheng, W.; Zhou, W.; Dong, X. Sr2MgSi2O7:Eu2+, Dy3+ phosphor-reinforced wood plastic composites with photoluminescence properties for 3D printing. Polym. Compos. 2021, 42, 3125–3136. [Google Scholar] [CrossRef]
- Wang, K.; Li, S.; Wu, Y.; Rao, Y.; Peng, Y. Simultaneous reinforcement of both rigidity and energy absorption of polyamide-based composites with hybrid continuous fibers by 3D printing. Compos. Struct. 2021, 267, 113854. [Google Scholar] [CrossRef]
- Liu, Z.; McClements, D.J.; Shi, A.; Zhi, L.; Tian, Y.; Jiao, B.; Liu, H.; Wang, Q. Janus particles: A review of their applications in food and medicine. Crit. Rev. Food Sci. Nutr. 2023, 63, 10093–10104. [Google Scholar] [CrossRef] [PubMed]
- Le, T.C.; Zhai, J.; Chiu, W.H.; Tran, P.A.; Tran, N. Janus particles: Recent advances in the biomedical applications. Int. J. Nanomed. 2019, 14, 6749–6777. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eslami, H.; Müller-Plathe, F. Self-Assembly Pathways of Triblock Janus Particles into 3D Open Lattices. Small 2024, 20, e2306337. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.T.; Lesieur, S.; Faivre, V. Janus nanoparticles: Materials, preparation and recent advances in drug delivery. Expert Opin. Drug Deliv. 2014, 11, 1061–1074. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, L.; Ni, S.; Li, D.; Liu, J.; Chu, H.Y.; Zhang, N.; Sun, M.; Li, N.; Ren, Q.; et al. Targeting loop3 of sclerostin preserves its cardiovascular protective action and promotes bone formation. Nat. Commun. 2022, 13, 4241. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Zaman, A.; Sayed, E.; Evans, D.; Morgan, S.; Samwell, C.; Hall, J.; Arshad, M.S.; Singh, N.; Qutachi, O.; et al. Electrohydrodynamic atomisation driven design and engineering of opportunistic particulate systems for applications in drug delivery, therapeutics and pharmaceutics. Adv. Drug Deliv. Rev. 2021, 176, 113788. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Tan, K.X.; Rodrigues, J.; Danquah, M.K. Target-Specific Delivery and Bioavailability of Pharmaceuticals via Janus and Dendrimer Particles. Pharmaceutics 2023, 15, 1614. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
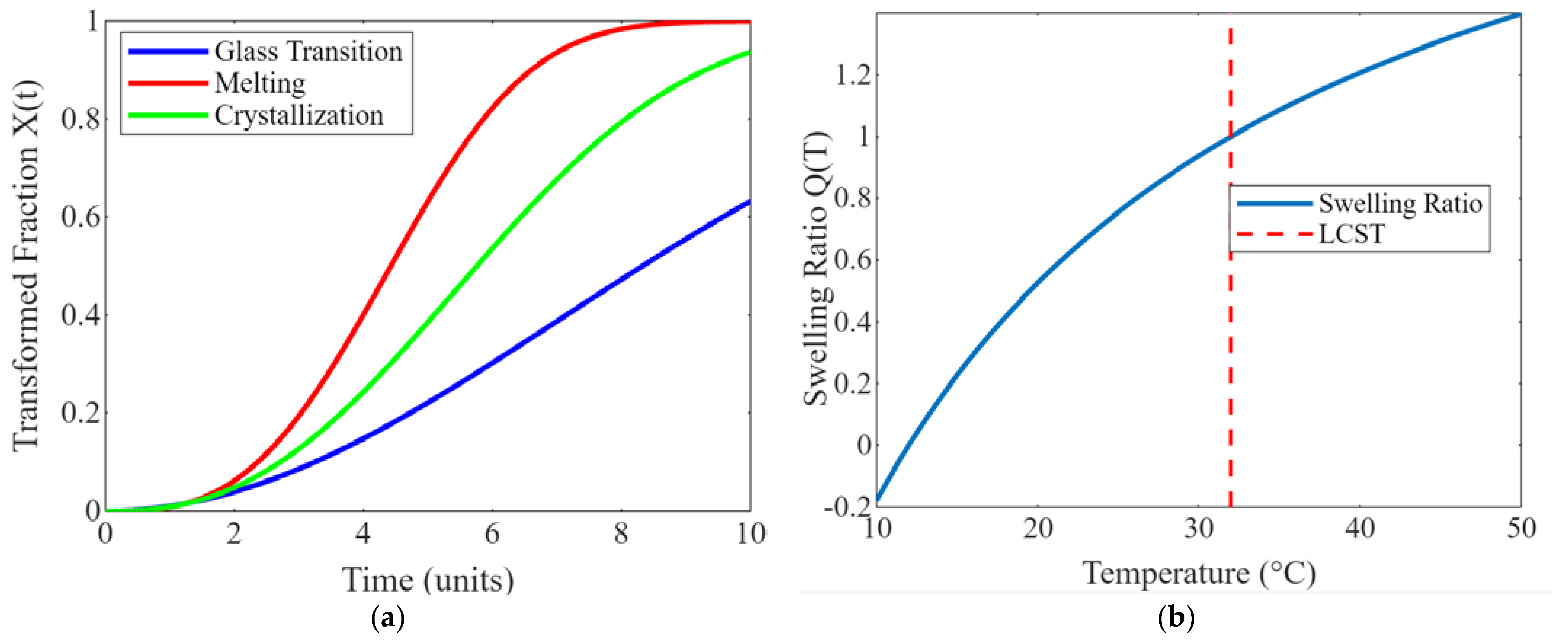
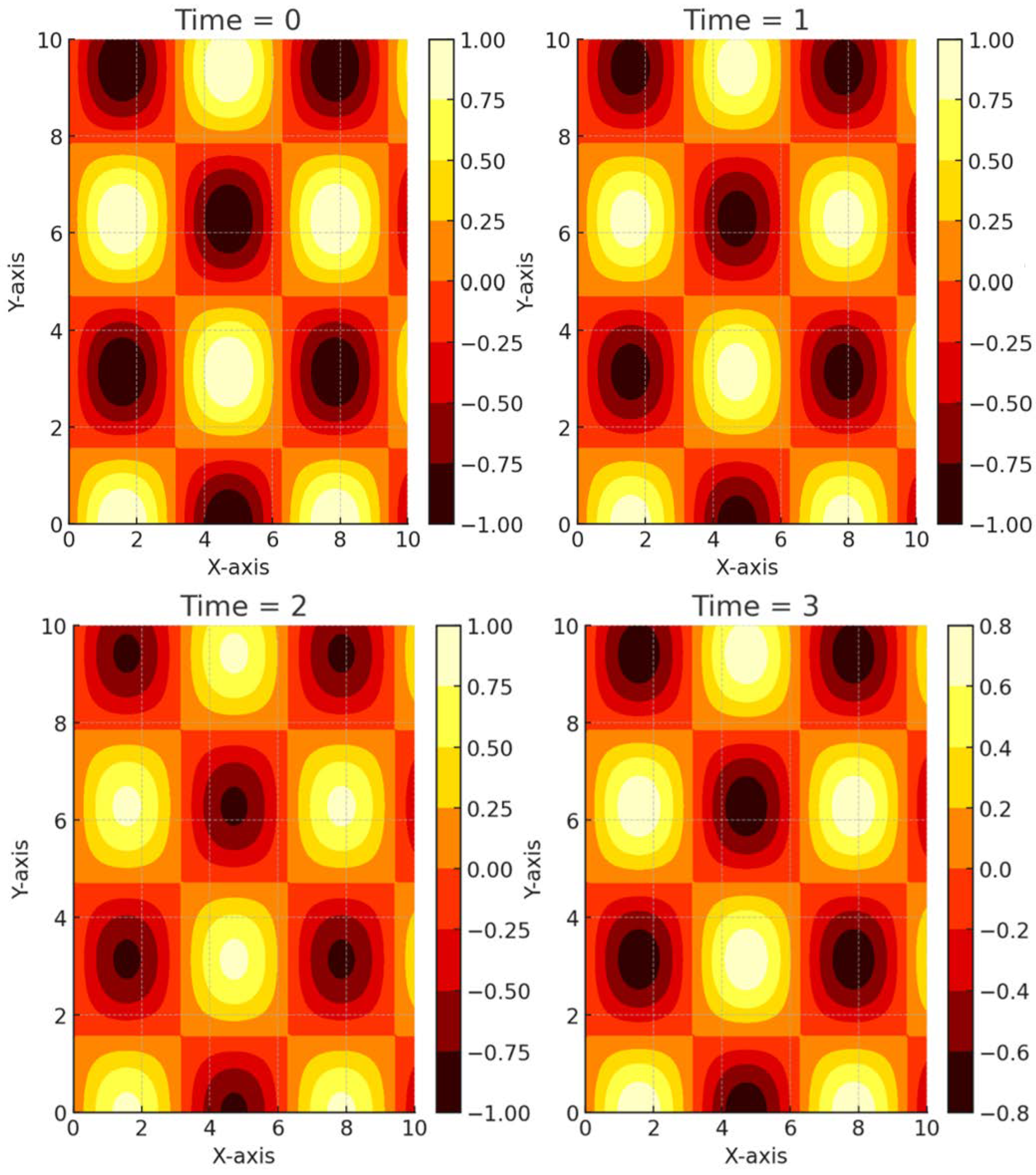
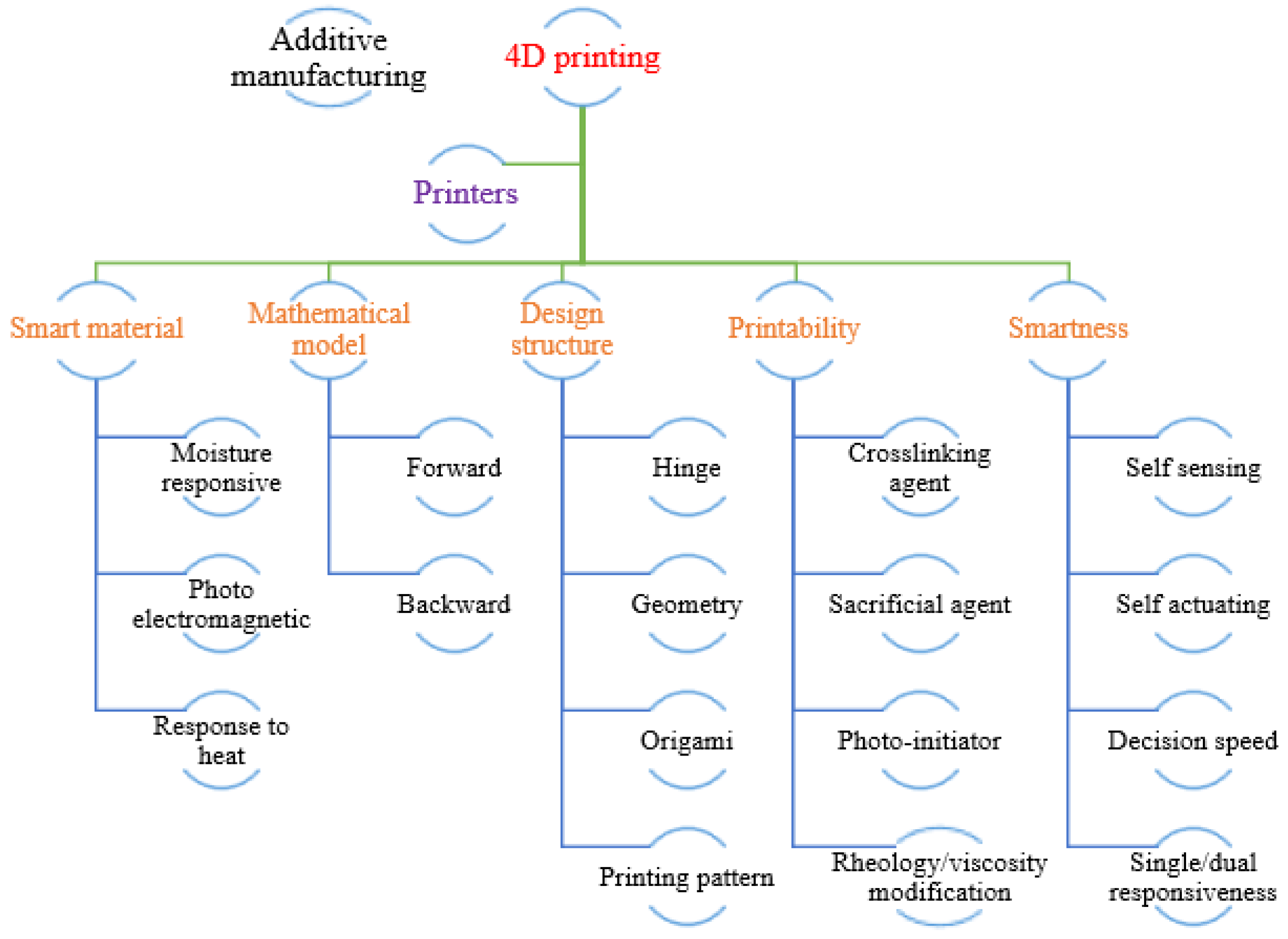
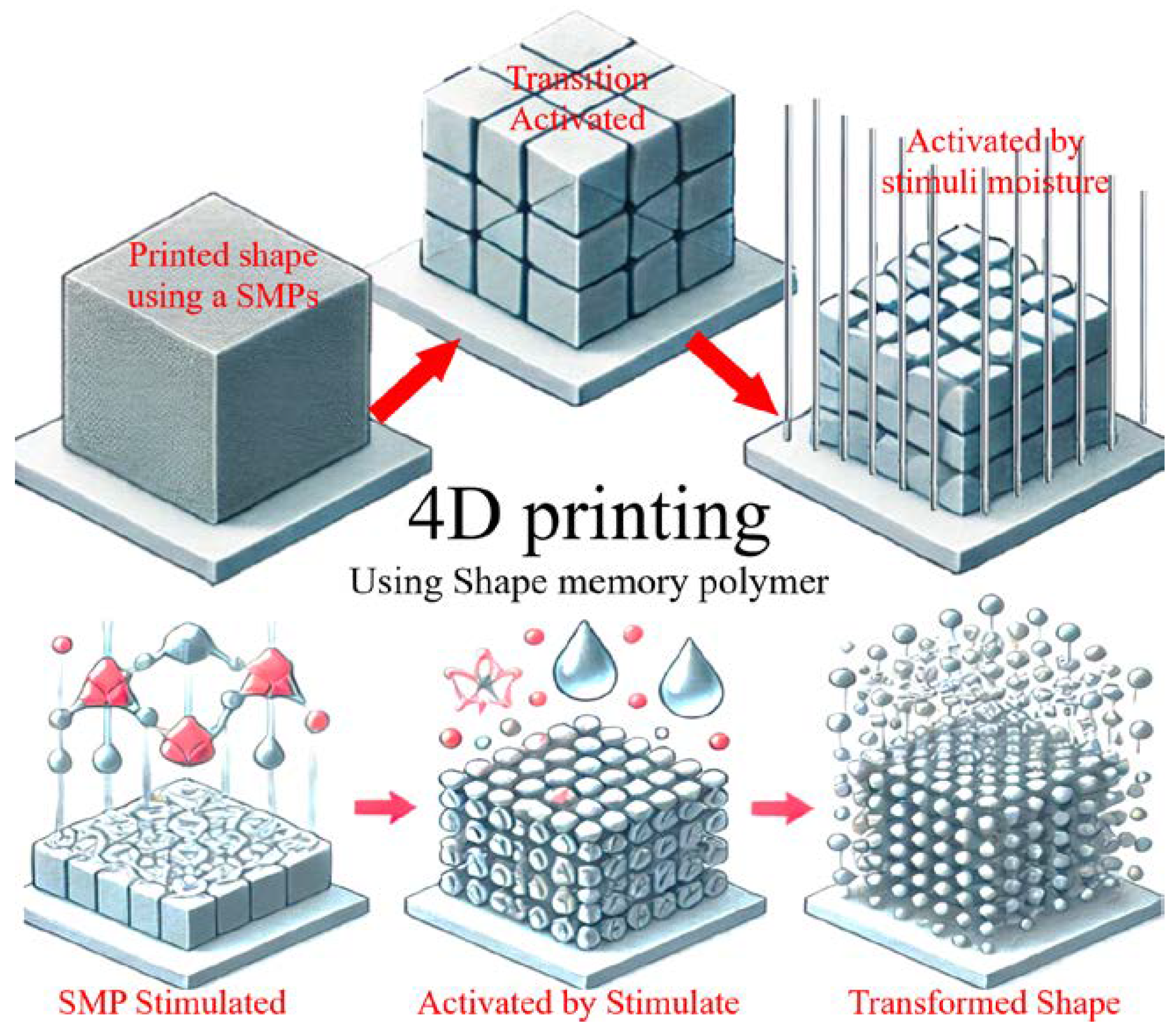


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olawumi, M.A.; Oladapo, B.I.; Olugbade, T.O.; Omigbodun, F.T.; Olawade, D.B. AI-Driven Data Analysis of Quantifying Environmental Impact and Efficiency of Shape Memory Polymers. Biomimetics 2024, 9, 490. https://doi.org/10.3390/biomimetics9080490
Olawumi MA, Oladapo BI, Olugbade TO, Omigbodun FT, Olawade DB. AI-Driven Data Analysis of Quantifying Environmental Impact and Efficiency of Shape Memory Polymers. Biomimetics. 2024; 9(8):490. https://doi.org/10.3390/biomimetics9080490
Chicago/Turabian StyleOlawumi, Mattew A., Bankole I. Oladapo, Temitope Olumide Olugbade, Francis T. Omigbodun, and David B. Olawade. 2024. "AI-Driven Data Analysis of Quantifying Environmental Impact and Efficiency of Shape Memory Polymers" Biomimetics 9, no. 8: 490. https://doi.org/10.3390/biomimetics9080490






