Deficiency of the Tmem232 Gene Causes Male Infertility with Morphological Abnormalities of the Sperm Flagellum in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Plasmids
2.3. Antibodies
2.4. Immunoprecipitation
2.5. Immunoblotting
2.6. Immunofluorescence in Testes
2.7. Immunofluorescence in Single Spermatozoa
2.8. Real-Time PCR
2.9. Tissue Collection and Histological Analysis
2.10. Mouse Sperm Collection
2.11. Sperm Motility Assessment Using CASA
2.12. Mouse Fertility Testing
2.13. Transmission Electron Microscopy
2.14. Scanning Electron Microscopy (SEM)
2.15. Statistical Analysis
3. Results
3.1. TMEM232 Is an Evolutionarily Conserved Testis-Enriched Protein
3.2. Tmem232 Knockout Male Mice Are Infertile
3.3. Tmem232 Knockout Leads to Abnormal Sperm Morphology and Causes Low Sperm Motility
3.4. Knockdown of Tmem232 Leads to Failed Cytoplasmic Clearance
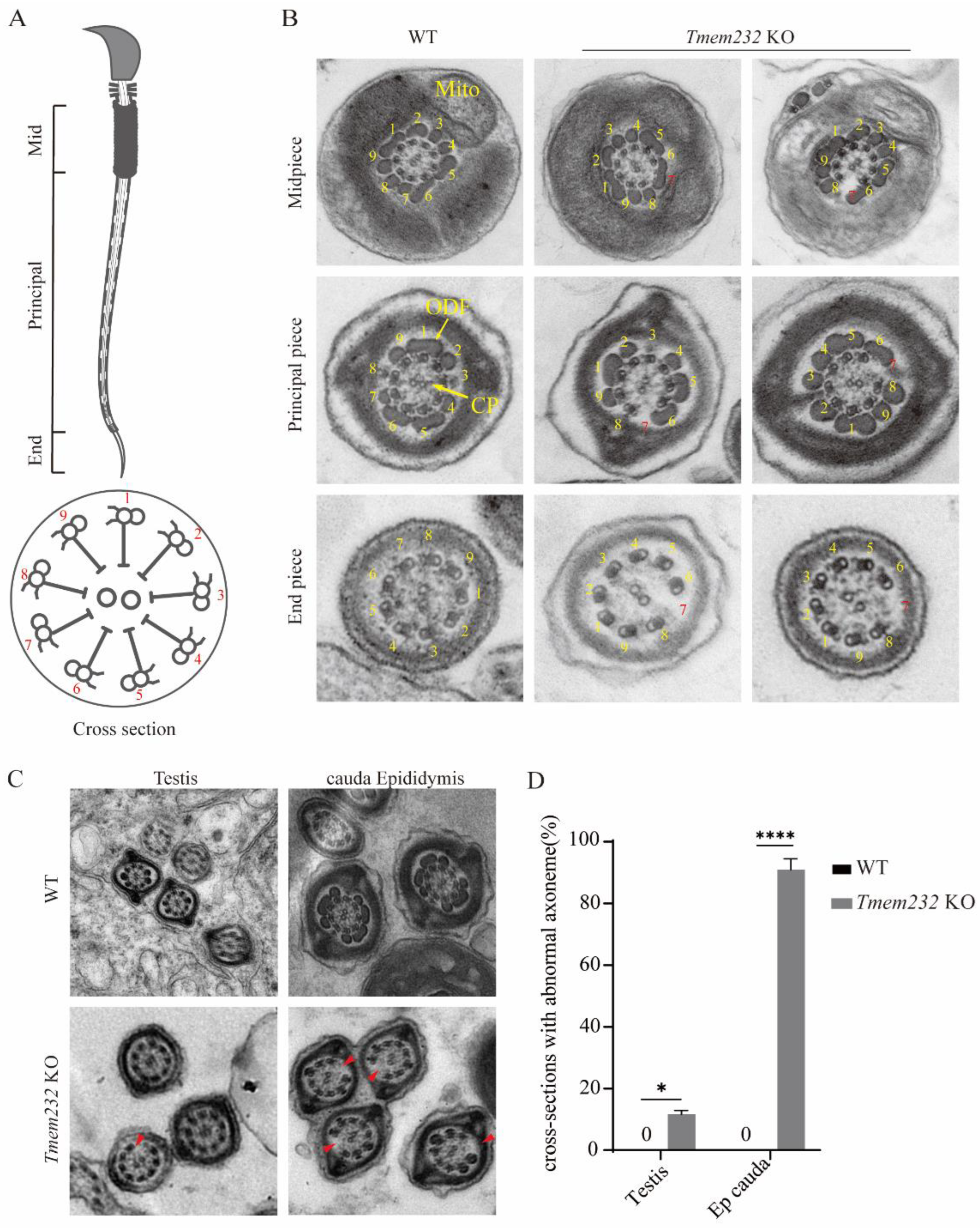
3.5. Tmem232 Knockout Spermatozoa Flagella Lack Microtubule Doublet 7
3.6. TMEM232 Interacts with ODF1 and Is Involved in the Formation of ODFs Structure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Kretser, D.M.; Loveland, K.L.; Meinhardt, A.; Simorangkir, D.; Wreford, N. Spermatogenesis. Hum. Reprod. 1998, 13 (Suppl. S1), 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Stratton, C.J.; Morozumi, K.; Jin, J.; Yanagimachi, R.; Yan, W. Lack of Spem1 causes aberrant cytoplasm removal, sperm deformation, and male infertility. Proc. Natl. Acad. Sci. USA 2007, 104, 6852–6857. [Google Scholar] [CrossRef] [Green Version]
- Lehti, M.S.; Sironen, A. Formation and function of sperm tail structures in association with sperm motility defects. Biol. Reprod. 2017, 97, 522–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyata, H.; Morohoshi, A.; Ikawa, M. Analysis of the sperm flagellar axoneme using gene-modified mice. Exp. Anim. 2020, 69, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Eddy, E.M.; Toshimori, K.; O’Brien, D.A. Fibrous sheath of mammalian spermatozoa. Microsc. Res. Tech. 2003, 61, 103–115. [Google Scholar] [CrossRef]
- Oyama, Y.; Miyata, H.; Shimada, K.; Larasati, T.; Fujihara, Y.; Ikawa, M. TULP2 deletion mice exhibit abnormal outer dense fiber structure and male infertility. Reprod. Med. Biol. 2022, 21, e12467. [Google Scholar] [CrossRef]
- Toure, A.; Martinez, G.; Kherraf, Z.E.; Cazin, C.; Beurois, J.; Arnoult, C.; Ray, P.F.; Coutton, C. The genetic architecture of morphological abnormalities of the sperm tail. Hum. Genet. 2021, 140, 21–42. [Google Scholar] [CrossRef]
- Zhao, W.; Li, Z.; Ping, P.; Wang, G.; Yuan, X.; Sun, F. Outer dense fibers stabilize the axoneme to maintain sperm motility. J. Cell. Mol. Med. 2018, 22, 1755–1768. [Google Scholar] [CrossRef]
- Gyobu, S.; Miyata, H.; Ikawa, M.; Yamazaki, D.; Takeshima, H.; Suzuki, J.; Nagata, S. A Role of TMEM16E Carrying a Scrambling Domain in Sperm Motility. Mol. Cell. Biol. 2016, 36, 645–659. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, H.; Gupta, S.; Myles, D.G.; Primakoff, P. Characterization of mouse sperm TMEM190, a small transmembrane protein with the trefoil domain: Evidence for co-localization with IZUMO1 and complex formation with other sperm proteins. Reproduction 2011, 141, 437–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noda, T.; Lu, Y.; Fujihara, Y.; Oura, S.; Koyano, T.; Kobayashi, S.; Matzuk, M.M.; Ikawa, M. Sperm proteins SOF1, TMEM95, and SPACA6 are required for sperm-oocyte fusion in mice. Proc. Natl. Acad. Sci. USA 2020, 117, 11493–11502. [Google Scholar] [CrossRef]
- Wu, B.; Yu, X.; Liu, C.; Wang, L.; Huang, T.; Lu, G.; Chen, Z.J.; Li, W.; Liu, H. Essential Role of CFAP53 in Sperm Flagellum Biogenesis. Front. Cell Dev. Biol. 2021, 9, 676910. [Google Scholar] [CrossRef]
- Huang, T.; Yin, Y.; Liu, C.; Li, M.; Yu, X.; Wang, X.; Zhang, H.; Muhammad, T.; Gao, F.; Li, W.; et al. Absence of murine CFAP61 causes male infertility due to multiple morphological abnormalities of the flagella. Sci. Bull. 2020, 65, 854–864. [Google Scholar] [CrossRef]
- Xu, K.; Su, X.; Fang, K.; Lv, Y.; Huang, T.; Li, M.; Wang, Z.; Yin, Y.; Muhammad, T.; Liu, S.; et al. The Slingshot phosphatase 2 is required for acrosome biogenesis during spermatogenesis in mice. eLife 2023, 12, e83129. [Google Scholar] [CrossRef]
- Bezerra, J.A.; da Silva, A.M.; Peixoto, G.C.; da Silva Mde, A.; Franco de Oliveira, M.; Silva, A.R. Influence of recovery method and centrifugation on epididymal sperm from collared peccaries (Pecari tajacu Linnaeus, 1758). Zool. Sci. 2014, 31, 338–342. [Google Scholar] [CrossRef]
- Handelsman, D.J.; Walters, K.A.; Ly, L.P. Simplified Method to Measure Mouse Fertility. Endocrinology 2020, 161, bqaa114. [Google Scholar] [CrossRef]
- Yin, Y.; Mu, W.; Yu, X.; Wang, Z.; Xu, K.; Wu, X.; Cai, Y.; Zhang, M.; Lu, G.; Chan, W.Y.; et al. LRRC46 Accumulates at the Midpiece of Sperm Flagella and Is Essential for Spermiogenesis and Male Fertility in Mouse. Int. J. Mol. Sci. 2022, 23, 8525. [Google Scholar] [CrossRef]
- Horowitz, E.; Zhang, Z.; Jones, B.H.; Moss, S.B.; Ho, C.; Wood, J.R.; Wang, X.; Sammel, M.D.; Strauss, J.F., 3rd. Patterns of expression of sperm flagellar genes: Early expression of genes encoding axonemal proteins during the spermatogenic cycle and shared features of promoters of genes encoding central apparatus proteins. Mol. Hum. Reprod. 2005, 11, 307–317. [Google Scholar] [CrossRef]
- Hermo, L.; Pelletier, R.M.; Cyr, D.G.; Smith, C.E. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 3: Developmental changes in spermatid flagellum and cytoplasmic droplet and interaction of sperm with the zona pellucida and egg plasma membrane. Microsc. Res. Tech. 2010, 73, 320–363. [Google Scholar] [CrossRef]
- Sun, Q.Y.; Liu, K.; Kikuchi, K. Oocyte-specific knockout: A novel in vivo approach for studying gene functions during folliculogenesis, oocyte maturation, fertilization, and embryogenesis. Biol. Reprod. 2008, 79, 1014–1020. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Chen, X.; Zhang, H.; Sha, Y.; Meng, R.; Shao, T.; Yang, X.; Jin, P.; Zhuang, Y.; Min, W.; et al. TBC1D21 is an essential factor for sperm mitochondrial sheath assembly and male fertilitydouble dagger. Biol. Reprod. 2022, 107, 619–634. [Google Scholar] [CrossRef]
- Oura, S.; Kazi, S.; Savolainen, A.; Nozawa, K.; Castaneda, J.; Yu, Z.; Miyata, H.; Matzuk, R.M.; Hansen, J.N.; Wachten, D.; et al. Cfap97d1 is important for flagellar axoneme maintenance and male mouse fertility. PLoS Genet. 2020, 16, e1008954. [Google Scholar] [CrossRef]
- Olson, G.E.; Sammons, D.W. Structural chemistry of outer dense fibers of rat sperm. Biol. Reprod. 1980, 22, 319–332. [Google Scholar] [CrossRef]
- Haidl, G.; Becker, A.; Henkel, R. Poor development of outer dense fibers as a major cause of tail abnormalities in the spermatozoa of asthenoteratozoospermic men. Hum. Reprod. 1991, 6, 1431–1438. [Google Scholar] [CrossRef]
- Wang, H.; Wan, H.; Li, X.; Liu, W.; Chen, Q.; Wang, Y.; Yang, L.; Tang, H.; Zhang, X.; Duan, E.; et al. Atg7 is required for acrosome biogenesis during spermatogenesis in mice. Cell Res. 2014, 24, 852–869. [Google Scholar] [CrossRef] [Green Version]
- Shang, Y.; Wang, H.; Jia, P.; Zhao, H.; Liu, C.; Liu, W.; Song, Z.; Xu, Z.; Yang, L.; Wang, Y. Autophagy regulates spermatid differentiation via degradation of PDLIM1. Autophagy 2016, 12, 1575–1592. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.Y.; Mruk, D.D. The biology of spermatogenesis: The past, present and future. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 1459–1463. [Google Scholar] [CrossRef] [Green Version]
- Han, F.; Dong, M.Z.; Lei, W.L.; Xu, Z.L.; Gao, F.; Schatten, H.; Wang, Z.B.; Sun, X.F.; Sun, Q.Y. Oligoasthenoteratospermia and sperm tail bending in PPP4C-deficient mice. Mol. Hum. Reprod. 2021, 27, gaaa083. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, W.; Zhang, Y.; Zhang, L.; Teves, M.E.; Liu, H.; Strauss, J.F., 3rd; Pazour, G.J.; Foster, J.A.; Hess, R.A.; et al. Intraflagellar transport protein IFT20 is essential for male fertility and spermiogenesis in mice. Mol. Biol. Cell 2016, 27, 3705–3716. [Google Scholar] [CrossRef]
- Schalles, U.; Shao, X.; van der Hoorn, F.A.; Oko, R. Developmental Expression of the 84-kDa ODF Sperm Protein: Localization to both the Cortex and Medulla of Outer Dense Fibers and to the Connecting Piece. Dev. Biol. 1998, 199, 250–260. [Google Scholar] [CrossRef] [Green Version]
- Shao, X.; Tarnasky, H.A.; Lee, J.P.; Oko, R.; van der Hoorn, F.A. Spag4, a novel sperm protein, binds outer dense-fiber protein Odf1 and localizes to microtubules of manchette and axoneme. Dev. Biol. 1999, 211, 109–123. [Google Scholar] [CrossRef] [Green Version]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Mu, W.; Wang, Z.; Xu, K.; Yin, Y.; Lu, G.; Chan, W.-Y.; Liu, H.; Lv, Y.; Liu, S. Deficiency of the Tmem232 Gene Causes Male Infertility with Morphological Abnormalities of the Sperm Flagellum in Mice. Cells 2023, 12, 1614. https://doi.org/10.3390/cells12121614
He X, Mu W, Wang Z, Xu K, Yin Y, Lu G, Chan W-Y, Liu H, Lv Y, Liu S. Deficiency of the Tmem232 Gene Causes Male Infertility with Morphological Abnormalities of the Sperm Flagellum in Mice. Cells. 2023; 12(12):1614. https://doi.org/10.3390/cells12121614
Chicago/Turabian StyleHe, Xiuqing, Wenyu Mu, Ziqi Wang, Ke Xu, Yingying Yin, Gang Lu, Wai-Yee Chan, Hongbin Liu, Yue Lv, and Shangming Liu. 2023. "Deficiency of the Tmem232 Gene Causes Male Infertility with Morphological Abnormalities of the Sperm Flagellum in Mice" Cells 12, no. 12: 1614. https://doi.org/10.3390/cells12121614
APA StyleHe, X., Mu, W., Wang, Z., Xu, K., Yin, Y., Lu, G., Chan, W.-Y., Liu, H., Lv, Y., & Liu, S. (2023). Deficiency of the Tmem232 Gene Causes Male Infertility with Morphological Abnormalities of the Sperm Flagellum in Mice. Cells, 12(12), 1614. https://doi.org/10.3390/cells12121614





