Abstract
Introduction. Interstitial Cells of Cajal (ICCs) play a critical role in the regulation of gastrointestinal motility and have been implicated in various functional gastrointestinal disorders. Recent research indicates a possible association between ICCs and the tumor risk of Gastrointestinal Stromal Tumors (GISTs). This research aims to examine the clinical, histopathological, and biomolecular characteristics of ICCs and their relevance in assessing GIST risk. Materials and Methods. This study examined fourteen GIST patients who underwent surgical intervention at the Surgery Department of Carol Davila Nephrology Hospital in Bucharest. Parameters including age, gender, tumor location/ dimensions were scrutinized. Immunohistochemistry employing markers CD117, DOG-1, and CD34 was employed to ascertain the presence of ICCs and GISTs. Results. The GIST risk stratification revealed distribution with 35.71% very low-risk, 21.42% low-risk, 14.28% intermediate-risk, and 28.57% high-risk categories. Predominantly, 57.14% of cases fell within the very low-risk and low-risk categories. Positive immunoreactivity for CD117 and DOG-1 was noted in 92.86% of patients, while CD34 exhibited positivity in 85.71% of cases. Gastric GISTs manifested heightened marker expression. Notably, immunohistochemistry unveiled robust positivity for CD117, DOG-1, and CD34, illustrating a positive correlation between elevated ICC levels and high-risk GISTs. Conclusions. The findings propose an association between ICC levels and high-risk GISTs, accentuating the diagnostic utility of CD117, DOG-1, and CD34 markers in GIST assessment.
Introduction
ICCs were initially identified and characterized by Santiago Ramón y Cajal in the 19th century and their presence has since been confirmed in various regions of the gastrointestinal tract [1]. Over the past 100 years, extensive research has illuminated the diverse distribution of ICCs [2]. Functionally, ICCs serve as vital components of the gastrointestinal system, playing a crucial role in the regulation of gastrointestinal motility. Acting as intrinsic pacemakers, ICCs generate electrical impulses that propagate through the smooth muscle layers, coordinating peristaltic contractions and facilitating the movement of food along the digestive tract. Moreover, ICCs are involved in the mediation and modulation of gastrointestinal neurotransmitters, contributing to the regulation of intestinal contractions, relaxation of smooth muscles, and coordination of various physiological processes [3]. The characterization of ICCs was initially based on morphological criteria until the groundbreaking discovery that ICCs express CD117 which binds to the ligand steel factor stem cell factor (SCF) [4]. This revelation marked a significant advancement in ICC research. However, it's important to note that not all ICCs express c-Kit, particularly those found in the deep muscular plexus of the human small intestine [5]. Additionally, other cell types besides ICCs, such as mast cells, melanocytes, neurons, and glia, also express c-Kit [6]. ICCs also express CD34 [7] and stain positively for proteins like Wilm's tumor gene protein 1 and calretinin [6]. Selective markers like Na+/K+/2Cl– co-transporter (NKCC1), neurokinin-1 receptor, and CD44 have been identified on certain sub-types of ICCs, but further research is needed to confirm their utility as ICC markers [8,9,10].
GISTs were initially described 40 years ago, as a subset of mesenchymal tumors that primarily arise in the gastrointestinal system displaying distinctive features such as submucosal growth [11,12]. Among gastrointestinal tumors, GISTs are relatively common, comprising 0.1 - 3% of cases, with nearly 30% exhibiting malignant behaviour [13,14]. Typically, asymptomatic until they reach a certain size, GISTs are often discovered serendipitously during imaging studies or surgical interventions, posing a diagnostic challenge due to the absence of specific clinical symptoms, although gastrointestinal bleeding is frequently observed as a common clinical finding [15,16]. GISTs are infrequent in children or young adults, typically emerging only in association with specific conditions such as neurofibromatosis, specifically neurofibromatosis-1, or Carney's triad. The rarity of these manifestations in younger individuals underscores their unique association with these specific medical conditions, emphasizing the need for careful considerations and evaluation when encountered in such age groups [17].
Recent research explores the role of ICCs in GIST development, suggesting a potential link between ICCs and GISTs. ICCs and GISTs share similar markers, indicating a common origin. ICCs, undergoing genetic alterations similar to GISTs, may act as precursors or provide a supportive microenvironment for GIST growth [18]. The uncontrolled growth of ICCs acts as the catalyst for the onset of GISTs, closely associated with the proto-oncogene on chromosome 4q 11-12, which transcribes the transmembrane KIT [19].
In a landmark study conducted by Hirota and colleagues in 1998, a critical breakthrough was achieved in understanding the pathogenesis of GISTs. This study uncovered a gain-of-function mutation within the c-KIT gene, particularly affecting Exon 11, which encodes a portion of the protein's transmembrane domain. Remarkably, this mutation was identified in approximately 90% of GIST cases, highlighting its central role in the disease's development [20,21]. The c-KIT protein is a type of tyrosine kinase receptor that, under normal conditions, plays a pivotal role in cellular growth and differentiation processes. However, the mutations identified by Hirota et al. lead to an aberrant activation of this receptor, bypassing the normal regulatory mechanisms. This uncontrolled activation is a key event in the pathogenesis of GISTs, as it triggers hyperplasia of the interstitial cells of Cajal (ICCs). These cells, known for their role as the pacemakers of the gastrointestinal tract, facilitating coordinated muscle contractions, begin to proliferate excessively in response to the mutated c-KIT signaling. This hyperplasia of ICCs is considered a precursor stage in the development of GISTs. Further investigations into the molecular dynamics of GISTs have revealed that a significant majority, approximately 75%, exhibit continuous activation of tyrosine kinase, a consequence directly attributable to mutations in the KIT gene. This sustained activation is a hallmark of the disease, driving the growth and survival of tumor cells. The discovery of these mutations not only provided profound insights into the molecular underpinnings of GISTs but also paved the way for the development of targeted therapies, revolutionizing the treatment landscape for this once poorly understood disease [22].
GISTs are profoundly influenced by activating mutations in PDGFRA and KIT genes, shaping their genetic landscape and clinical behaviour [23]. Immunohistochemistry proves indispensable for distinguishing GISTs from other tumors, demonstrating variable marker expression. CD34, CD117, and DOG-1 play crucial roles in enhancing diagnostic precision [18,22]. Furthermore, a crucial need arises to differentiate GISTs from alternative spindle cell variations or different tumor types; GISTs commonly exhibit non-reactivity to desmin and S100 protein, displaying diverse degrees of positivity for smooth muscle actin [24,25]. Understanding the genetic and molecular mechanisms, including KIT and PDGFRA mutations, not only aids in diagnosis but also holds promise for targeted therapies, emphasizing the complex interplay of genetics, immunophenotypic markers, and diagnostic tools in managing GISTs [26,27]. Numerous classification systems have been proposed, each leveraging distinct parameters such as tumor size, location, and mitotic rate. These comprehensive frameworks are designed to intricately assess the diverse aspects of GISTs, aiming to provide a nuanced understanding of their aggressiveness and prognosis. By incorporating diverse markers, these classifications contribute to a more holistic characterization of GISTs, facilitating refined stratification and offering valuable insights into the complex clinical behaviours and potential outcomes associated with this category of tumors [28,29,30]. The research was designed to investigate the histological and molecular characteristics of ICCs and GISTs, with a particular emphasis on evaluating the role of ICCs in the tumor risk of GISTs.
Materials and Methods
The study included all patients diagnosed with GISTs who underwent surgical procedures at the Department of General Surgery, Carol Davila Nephrology Hospital Bucharest (n=14), during the period from January 2016 to June 2022. The Department of Pathology at Carol Davila Nephrology Hospital Bucharest conducted histological examinations of the specimens.
We aimed to explore a range of variables, such as age, gender, tumor site, size, following the acquisition of written consent from the participants. All individuals included in this study underwent surgical procedures, during which paraffin-embedded specimens were collected. Hematoxylin- eosin staining was conducted on GIST specimens embedded in paraffin, accompanied by diaminobenzidine staining, to identify pathological changes within the tumor tissue. GIST samples underwent fixation using a 4% paraformaldehyde solution and were subsequently encased in paraffin. The paraffin-embedded tumor specimens were sectioned into 5- μm-thick slices and affixed to glass slides.
Notably, none of the enrolled patients underwent any form of radiation therapy or chemotherapy before undergoing surgery. Moreover, individuals who underwent surgical procedures were subsequently re-engaged to facilitate a comprehensive follow-up assessment, examining the ongoing trajectory of the disease. The follow-up duration extended over a span of 2 to 60 months, with an average period of 31 months, allowing for a thorough evaluation of the long-term outcomes and progression of the condition.
For this study, the staging and risk stratification processes were executed in accordance with the criteria of a modified version of the NIH risk assessment, as advocated by Joensuu and outlined in Table 1. It's worth noting that the Joensuu classification, currently widely utilized, incorporates parameters such as tumor size, mitotic index, primary tumor site, and tumor rupture. Notably, individuals falling into the high-risk category confront a 15%-20% likelihood of experiencing recurrent disease [29].

Table 1.
Joensuu criteria GIST risk assessment [29].
Results
Clinicopathological Characteristics
In alignment with the Joensuu modified National Institutes of Health (NIH) classification criteria, our comprehensive analysis of 14 Gastrointestinal Stromal Tumor cases unveiled a diverse risk distribution. A substantial portion of the cases, comprising 35.71% (5 cases) fell within the very low-risk category, suggesting a comparatively favorable prognosis. In contrast, 28.57% of the GIST cases (4 cases) were classified as high-risk, indicating a potentially more aggressive disease course (Table 2).

Table 2.
Joensuu Modified NIH criteria.
In the studied patient collective, the gender distribution was almost balanced, with 6 women (42.85%) and 8 men (57.14%). This nearly equal representation resulted in a male-to-female ratio of approximately 1.33:1.
The age distribution spanned from 31 to 82 years, with a median age of 56.5 years. Notably, there was a notable concentration observed in the demographic between 53 and 82 years. A significant majority of the patients, accounting for 78.57%, were aged 50 years or above, while a minority, comprising 21.42%, were below the age of 50.
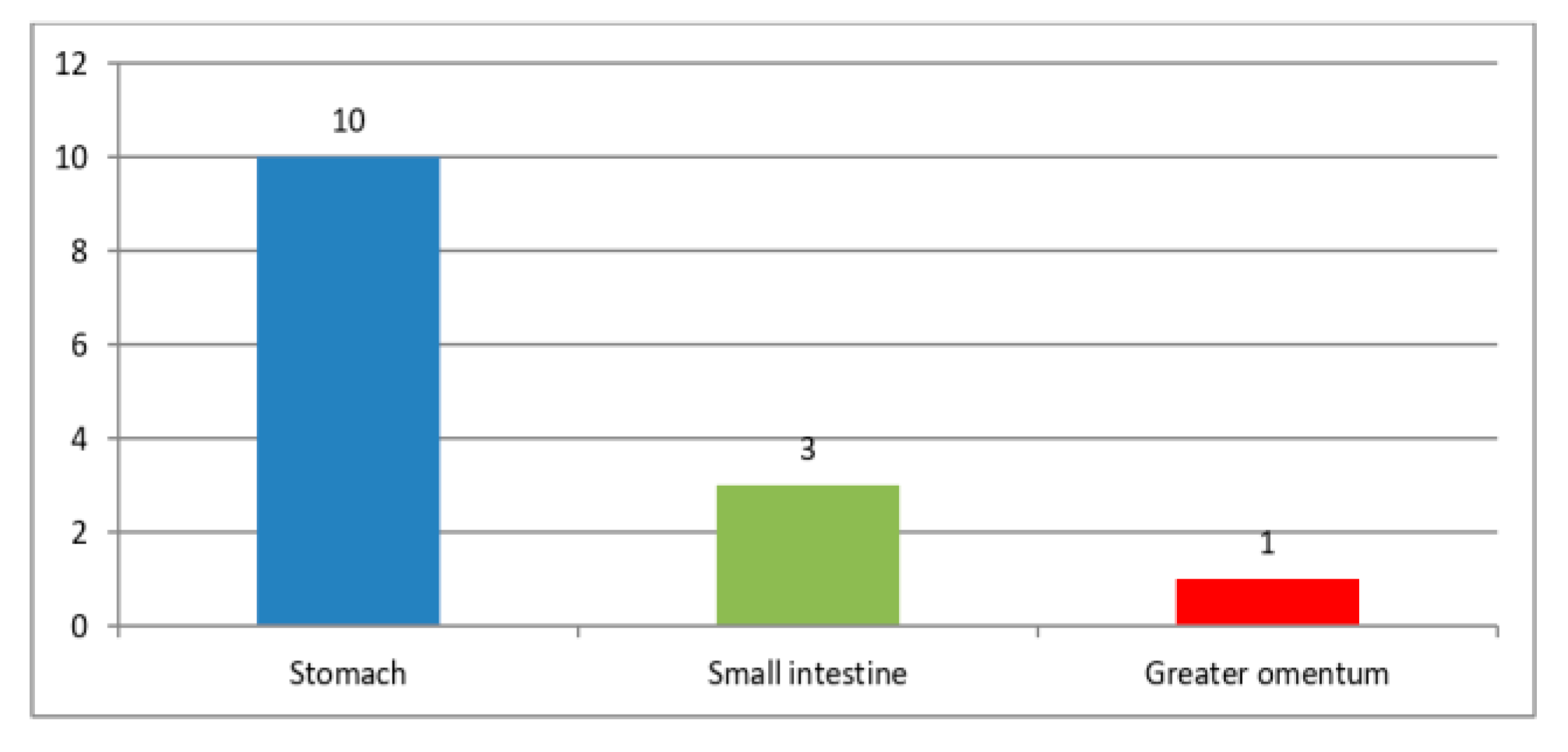
The majority of GISTs were primarily located in the stomach, comprising 71.4% of cases (10 cases), followed by the small intestine, which accounted for 21.4% (3 cases); additionally, a smaller proportion of Tumors, specifically 7.2% (1 case), were identified in the greater omentum (Figure 1). Among the 14 patients, 2 individuals, constituting 14.3%, were found to have liver metastases. The Tumors exhibited a significant size range, varying from 4 to 20 cm in diameter, highlighting their diverse dimensions (Table 3). This underscores the significant diversity in their overall size.
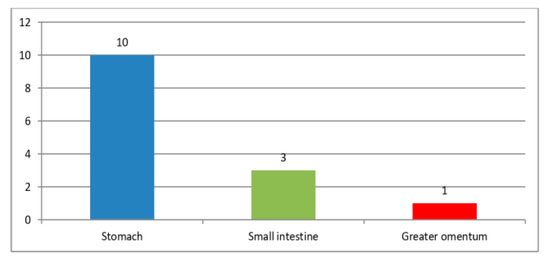
Figure 1.
GISTs location.

Table 3.
GISTs size variation.
The clinical presentation of the patients under study primarily centered a dyspeptic syndrome, observed in 7 individuals, constituting 50% of the cases. The second most prevalent clinical sign was bleeding, which manifested in 5 patients, accounting for 35.7% of the total. Among these cases, one patient presented with hematemesis, representing 7.14% of the cohort, while 4 patients exhibited melena, contributing to 28.5% of the total cases. Other notable signs and symptoms included early satiety, observed in 2 patients (14.28%), and fatigue with pallor, presenting in 5 patients (35.7%). Furthermore, 3 patients (21.4%) experienced significant weight loss, and a palpable abdominal mass was identified in 3 patients (21.4%). This clinical profile provides insight into the diverse ways in which patients presented with gastrointestinal stromal Tumors (GISTs), emphasizing the variability in symptoms and highlighting the need for a thorough clinical evaluation (Table 4).

Table 4.
Clinical presentation.
Immunohistochemistry markers
In the course of our investigation, we employed specific biomarkers, namely CD117, DOG-1, and CD34, to discern the presence of ICCs and GISTs. Notably, 13 cases from the study demonstrated positivity for both CD117 and DOG-1, constituting 92.86% of the total cases assessed. Furthermore, CD34 positivity was observed in 12 cases, accounting for 85.71% of the study cohort. Among these, 9 cases, equivalent to 64.29%, displayed positivity for all three markers - CD117, DOG-1, and CD34.
Moreover, a noteworthy observation emerged regarding the correlation between Tumor location and the expression of CD117, CD34 and DOG-1. The stomach exhibits a notably higher prevalence of positive cells for all three markers compared to GISTs located in the small bowel and omentum.
Specifically, 64.29% of stomach-based Tumors display positive expression for CD117, DOG-1, and CD34, while the corresponding percentages for small bowel Tumors are 21.42%, and for omentum Tumors are 7.14%. This observation may underscore a potential association between Tumor location and the differential expression of these immuno-markers, shedding light on the intricate variations in marker positivity based on the anatomical site of the Tumor (Table 5).

Table 5.
CD117, DOG-1, CD 34 positivity.
CD 117
In the subset of GISTs classified as high-risk, a noteworthy majority—constituting 75% of cases (3 patients)—exhibited a pronounced presence of ICCs accompanied by heightened CD117 expression. Interestingly, one patient within this high-risk category presented a unique profile, showcasing reduced expression of both ICCs and CD117. Furthermore, from the very low- risk group, 40% of patients (2 individuals) displayed unexpectedly high levels of both CD117 and ICCs.
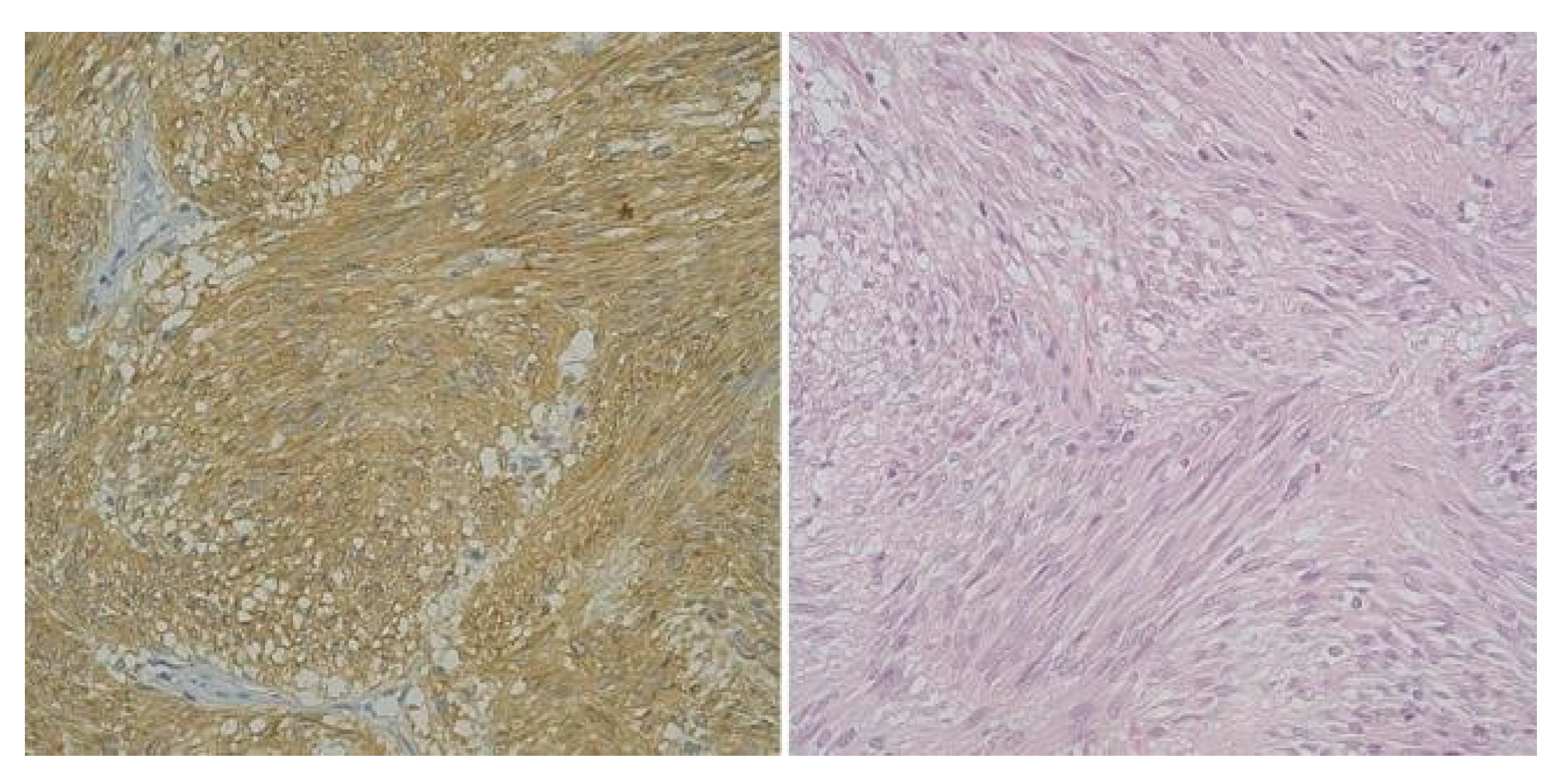
A distinctive observation emerged, shedding light on the diversity within the high-risk and very low-risk groups. The remaining cases, categorized as very low-risk and low- risk (6 patients), consistently demonstrated diminished levels of both ICCs and CD117 (Figure 2).
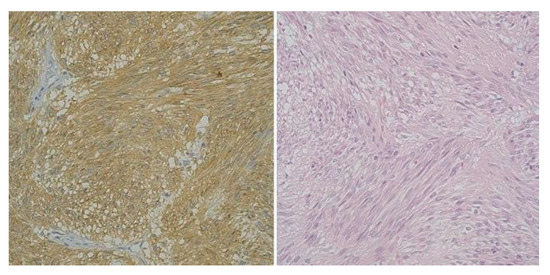
Figure 2.
High-risk gastric GIST, Fusiform Pattern (CD 117 x 40) and High-risk gastric GIST -Fusiform Pattern (H and E x 40).
These nuanced findings suggest a potential correlation between the abundance of ICCs and CD117 expression and the overall risk classification of GISTs. This underscores the intricate interplay of these immunohistochemical markers in delineating the tumor's risk profile.
CD 34
In tandem with CD117, the expression of CD34 also appears to exert influence on the risk profile GISTs. Notably, the majority of high-risk GIST cases (75%, encompassing 3 cases) exhibited significantly elevated levels of both ICCs and CD34 and only one high-risk GIST case presented with lower levels of ICCs and reduced CD34 expression.
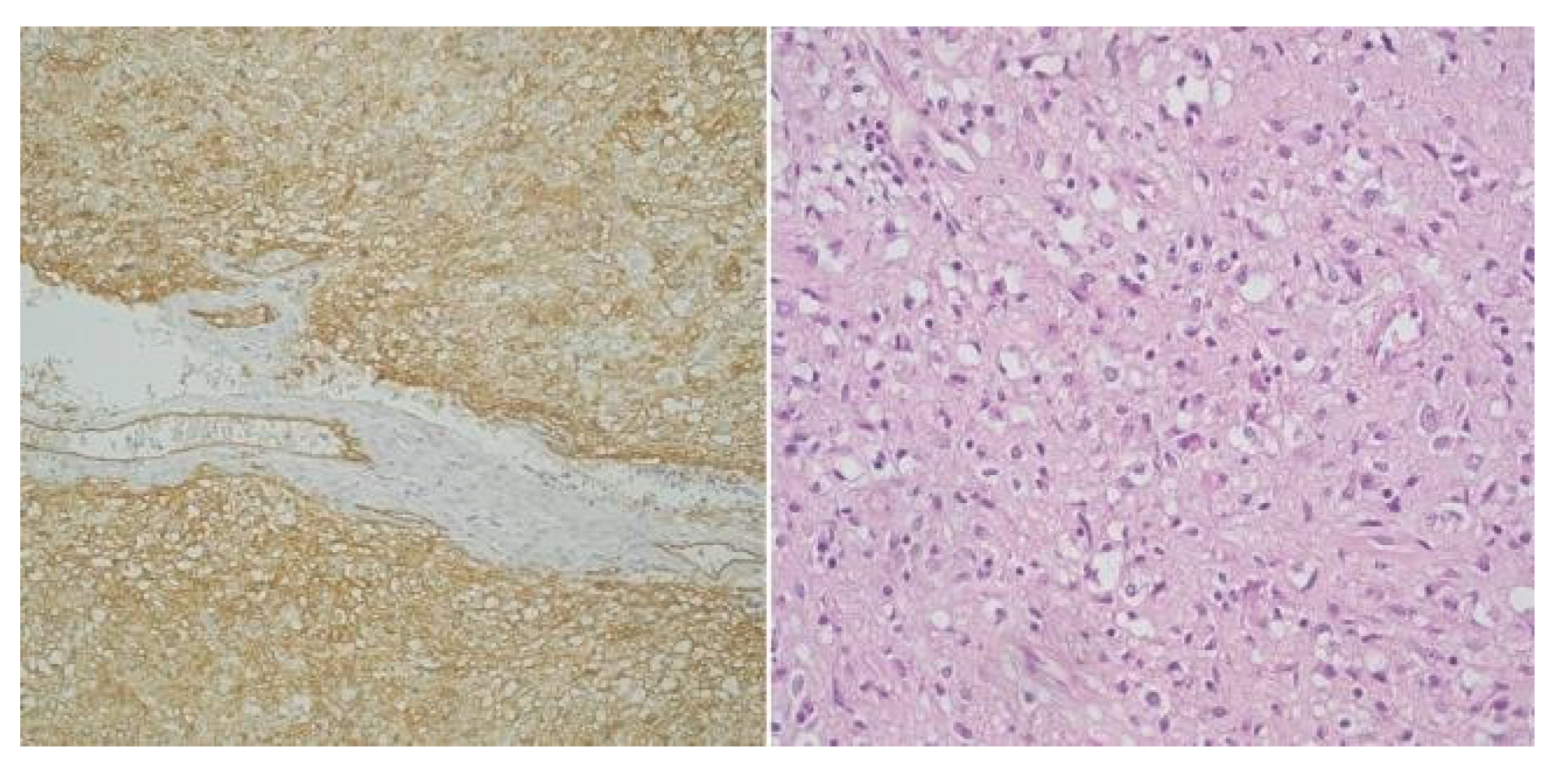
Contrastingly, only 40% of cases classified as very low- risk (2 cases) and one case as low-risk displayed heightened levels of both ICCs and CD34. The remaining cases within the very low-risk and low-risk GIST categories consistently demonstrated diminished levels of both ICCs and CD34 (Figure 3). This pattern, mirroring that seen with CD117, further emphasizes the nuanced relationship between CD34 and the risk stratification of GISTs.
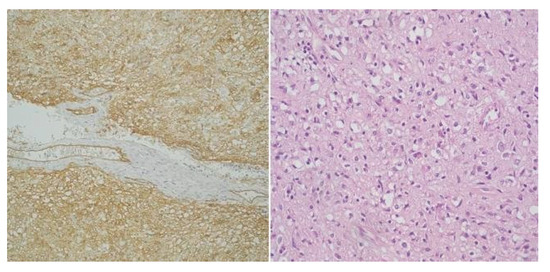
Figure 3.
High-risk gastric GIST - Mixt pattern, fusiform and epitheliod (CD34 x 20) and High-risk gastric GIST - Mixt pattern, fusiform and epitheliod (H and E x 20).
DOG-1
DOG-1 expression was observed to be significantly prevalent across the study, with a notable 92.86% (13 out of 14 patients) of the GIST cases exhibiting positive DOG- 1 staining. This widespread expression underscores the relevance of DOG-1 as a critical marker in the context of GISTs. Among the high-risk GIST patient subgroup, a distinctive pattern emerged: all four cases within this category showed pronounced DOG-1 expression, paralleled by an increased presence of ICCs. This correlation suggests a potential link between elevated DOG-1 expression, ICC density, and the heightened risk classification of GISTs, indicating that DOG-1 could serve as a biomarker for more aggressive Tumor behaviour.
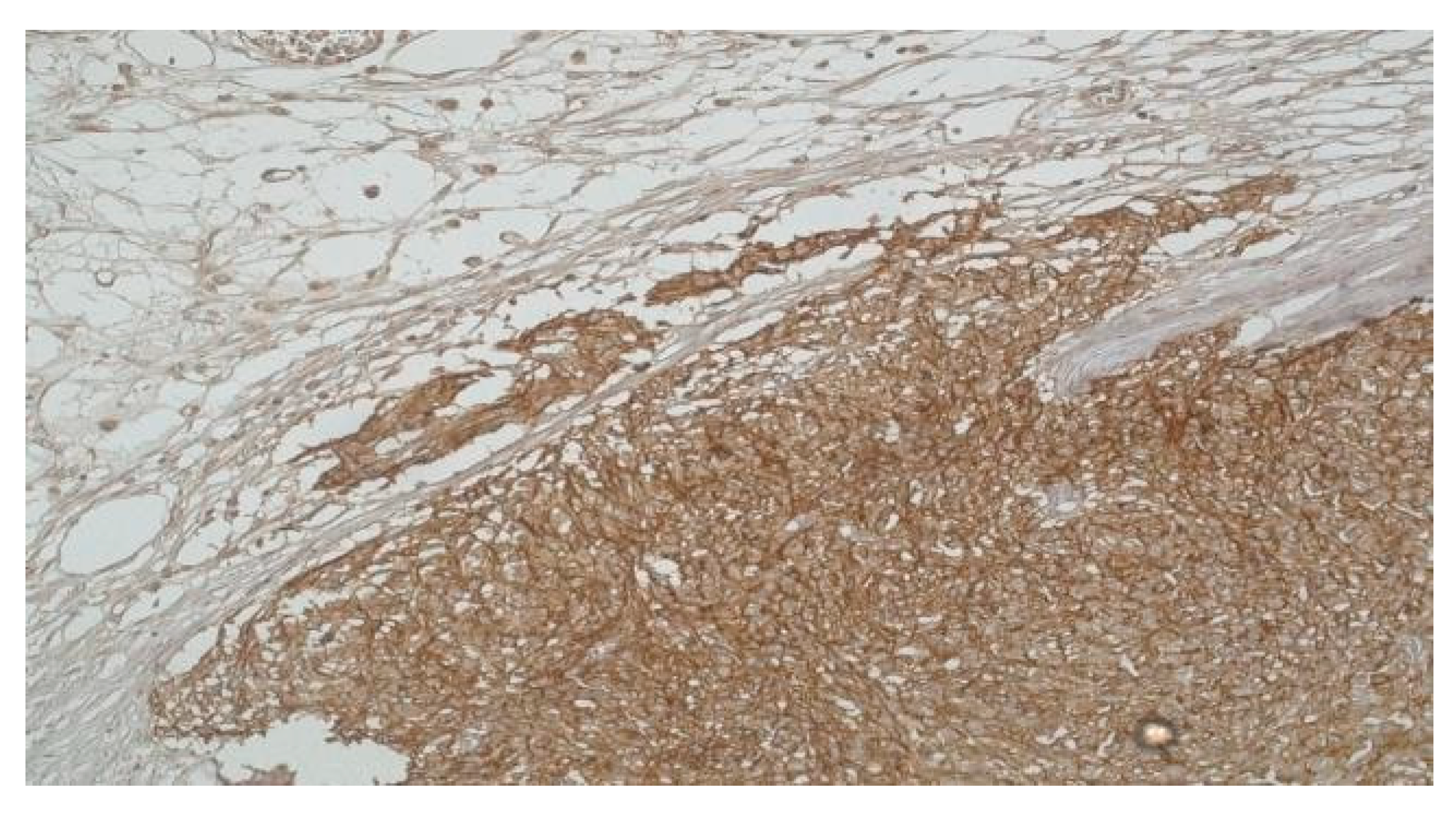
Conversely, within the very low-risk category, a smaller fraction, specifically 40% (2 out of 5 cases), demonstrated a similar elevation in both ICCs and DOG-1 levels. This observation introduces an intriguing contrast to the predominant trend in high-risk cases, indicating that while elevated DOG-1 and ICC levels can be associated with higher risk, their presence does not exclusively define it. Moreover, the study revealed that the remaining cases classified as very low-risk and low-risk—a total of 6 patients—consistently exhibited reduced levels of both ICCs and DOG-1. This uniform decrease across the majority of lower-risk GISTs further affirms the potential utility of DOG-1 and ICC quantification in stratifying GIST risk (Figure 4).
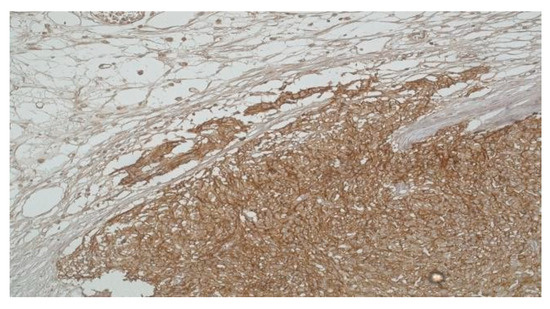
Figure 4.
High-risk Gastric GIST with infiltration into the subserosal adipose tissue (DOG-1 x 20).
Discussions
GISTs represent unique mesenchymal neoplasms that have the potential to affect any segment of the gastrointestinal tract. Recent epidemiological data, suggests the hat the yearly occurrence of GIST is roughly 1/100,000, underscore the significance of these Tumors in clinical contexts [22,31]. Many GIST cases manifest without typical clinical symptoms, with some patients presenting gastrointestinal bleeding as the initial sign the majority of them are benign, with only almost 30% of cases exhibiting malignancy [22,32].
GISTs have their roots in ICCs, characterized by the expression of stemness-related markers like CD117 (c-kit) and CD34 [1,33,34]. ICCs, serving as key orchestrators of gastrointestinal motility, intricately govern the rhythmic movements within the digestive system [35]. Disruptions, injuries, or dysfunction affecting ICCs in the gastrointestinal tract can lead to significant disturbances in motility [2,36]. The natural apoptotic process of ICCs over time underscores the imperative need to sustain ICC networks for effective tissue regeneration. The tyrosine kinase receptor c-kit (CD117) and its ligand stem cell factor (SCF), assume a pivotal role in fostering the development, maturation, and continual phenotypic upkeep of ICCs [25,34,37].
Recent research indicates that approximately 95% of GISTs exhibit positivity for CD117, also known as c-kit, highlighting a predominant molecular marker in the majority of cases. Furthermore, a noteworthy percentage, ranging from 40% to 70%, demonstrates positivity for CD34. These findings underscore the significance of CD117 as a prevalent marker and emphasize the variable but substantial expression of CD34 in GISTs [25,34,38-40]. In alignment with data from previous studies, our current investigation unveiled robust immunopositivity for CD117 and CD34 in GISTs. Specifically, out of the total cases examined (n=14), 13 cases (92.86%) demonstrated positive expression for CD117, and 13 cases (85.71%) exhibited CD34 positivity. Furthermore, our findings revealed a compelling correlation between a higher count of ICCs and an elevated GIST risk. This correlation suggests that an increased GIST risk may be intricately linked to a more pronounced presence of ICCs. In the high-risk subset, constituting 75% of cases (n=4), a significant majority exhibited heightened CD117 expression.
Notably, one high-risk patient presented a unique profile characterized by reduced expression of both ICCs and CD117. The parallel exploration of CD34, particularly within high-risk cases, revealed a substantial majority with elevated levels of both ICCs and CD34. Adding complexity, only one high-risk GIST case presented with lower counts of ICCs and reduced CD34 expression. Other findings surfaced in lower- risk categories such as: in the very low-risk group, 40% of patients (2 cases) displayed unexpectedly high levels of both CD117 and ICCs. Additionally, among very low-risk cases, 40% (2 cases), and among low-risk cases, 20% (1 case) exhibited elevated CD34 expression, underscoring the nuanced relationship between CD34 expression and risk stratification even in lower-risk categories. These insights contribute to a comprehensive understanding of GIST pathology, emphasizing the potential diagnostic and prognostic significance of concurrent CD117 and CD34 expression, as well as the association with ICC abundance in GIST development.
DOG-1 is a novel protein that plays a pivotal role in the diagnosis and understanding of GISTs, particularly in relation to ICCs. DOG-1 serves as a sensitive and specific immunohistochemical marker for GISTs, demonstrating increased sensitivity in identifying GISTs with PDGFRA mutations and those that are KIT-negative. ICCs express DOG-1, and the protein is considered a specific marker for these cells. The expression pattern of DOG-1 in ICCs mirrors its presence in GISTs, suggesting a close relationship between DOG-1, ICCs, and GISTs. This association underscores the utility of DOG-1 in accurately diagnosing GISTs, even in cases where traditional markers like KIT may be negative [25,34,41-43]. Our study revealed a prevalent DOG-1 expression in a significant proportion of the study group, counting for a prevalence of 92.86% (13 patients). Notably, within the subset of high-risk GIST patients, all four cases demonstrated robust DOG-1 expression in conjunction with high levels of ICCs. This observation underscores the potential significance of DOG- 1 as a marker associated with a more aggressive phenotype within the high-risk category. Conversely, in cases classified as very low-risk, only 40% (2 cases) exhibited heightened levels of both ICCs and DOG-1 while the remaining cases within both the very low-risk and low-risk GIST categories consistently demonstrated diminished levels of both ICCs and DOG-1. Intriguingly, the remaining cases within both the very low-risk and low-risk GIST categories consistently demonstrated diminished levels of both ICCs and DOG-1. This pattern further emphasizes the potential correlation between DOG-1 expression, ICC abundance, and the overall risk classification of GISTs.
Within our study, an evaluation based on the modified NIH risk classification for primary GIST revealed that a substantial majority, precisely 57.14% of GIST cases, were categorized as very low-risk and low-risk. This classification system provides a nuanced understanding of the varying levels of risk associated with GIST. Notably, our findings align with previous research, which reported a high-risk prevalence of 27.05% in patient populations [26,40].
Contrary to our findings, existing literature has put forth varying perspectives on the incidence of GISTs concerning gender distribution. While other reports have posited the similarity in GIST incidence between males and females, some have proposed an elevated predisposition in males [44,45]. This inconclusive aspect emphasizes the complexity of GIST epidemiology and underscores the need for continued comprehensive research to discern any potential gender-related nuances in GIST occurrence.
While GISTs are infrequently identified in young adults, their occurrence is exceedingly rare in children [17]. Our study uncovered several instances (3 cases) where GIST was diagnosed in young adults, emphasizing the importance of closely monitoring the potential incidence of GIST in this age group. The preeminent anatomical site for GISTs was notably the stomach, constituting a significant majority at 71.4% of cases (10 cases), followed by the small intestine as the second most prevalent location, accounting for 21.4% (3 cases). A smaller fraction, specifically 7.2% (1 case), was identified in the greater omentum - an alignment with existing literature [15,22,30]. Notably, among the 14 patients examined, 14.3% (2 individuals) were diagnosed with liver metastases, reinforcing the potential for GISTs to exhibit systemic involvement. The Tumors displayed considerable variability in size, ranging from 4 to 20 cm in diameter, underscoring the marked diversity in their overall dimensions.
These findings provide a comprehensive insight into the anatomical distribution, metastatic tendencies, and size heterogeneity of GISTs in our study.
The objective of this study was to investigate the potential association between the prevalence ICCs and the risk associated GISTs. However, the study encountered several notable constraints, including its limited scope due to being conducted at a single center and the small size of the sample group, which hindered the statistical analysis, specifically the calculation of a P-value for significance. Despite these limitations, it's noteworthy that the results align with the broader body of existing research on the topic. The findings from this study reaffirm the role of CD117/c-kit, CD34, and DOG-1 as critical markers for identifying both GISTs and ICCs. While the initial data suggest a potential correlation between the levels of ICCs and high risk GISTs, it is critical to acknowledge the necessity for more extensive and thorough research to verify this hypothesis definitively. Future studies, ideally conducted across various centers to ensure a more diverse and substantial dataset, would allow for a more detailed and definitive exploration of how ICC density may influence the risk of GIST development. Undertaking such comprehensive research efforts would greatly enhance our understanding of the mechanisms driving GIST pathogenesis and could significantly influence the development of more effective diagnostic and treatment modalities in the future.
Conclusions
The study reinforces the significance of CD117/c-kit, CD34, and DOG-1 as essential biomarkers for accurately identifying GISTs. These markers not only facilitate the distinction of GISTs from other Tumor types but also suggest a shared origin with ICCs, underlining their importance in the diagnostic process. The high prevalence of these markers among the GIST cases studied underscores their utility in clinical diagnostics.
There appears to be a possible correlation between the density of ICCs and the risk categorization of GISTs. Cases classified as high-risk tend to show a greater abundance of ICCs alongside elevated expression levels of CD117, CD34, and DOG-1. This relationship suggests that ICC density could potentially serve as an indicator of disease aggressiveness and risk.
While the findings offer valuable insights into GIST pathology and the potential link between ICCs and GIST risk, the limitations of the study, including its single-center nature and small sample size, highlight the necessity for broader, multi-center studies. Such research would provide a more diverse dataset, enabling a deeper and more statistically robust exploration of the relationship between ICCs, biomarker expression, and GIST risk.
The study also illuminates the variability in GIST clinical presentations, locations, and sizes, as well as the presence of metastases in certain cases. This diversity underscores the complexity of GISTs and the need for individualized diagnostic and treatment approaches. It emphasizes the importance of utilizing a comprehensive set of diagnostic tools, including immunohistochemistry and molecular testing, to accurately characterize each GIST and tailor treatment strategies accordingly.
Author Contributions
Conceptualization: PR and MZ; Methodology: PR and VP; Software: MB and VG; Validation: PR, FP and VS1; Formal analysis: DG and SP; Investigation: AT and VP; Resources: VS2 and ISC; Data curation and writing original draft preparation: VS1; Writing, review and editing: all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Compliance with Ethical Standards
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethical Committee at University of Medicine and Farmacy “Carol Davila” Bucharest, Romania (protocol code PO-35- F-03b, date of approval 19.12.2023).
Conflicts of Interest
There are no known conflicts of interest in the publication of this article. The manuscript was read and approved by all authors.
References
- Min, K.W.; Leabu, M. Interstitial cells of Cajal (ICC) and gastrointestinal stromal tumor (GIST): Facts, speculations, and myths. J Cell Mol Med. 2006, 10, 995–1013. [Google Scholar] [CrossRef] [PubMed]
- Foong, D.; Zhou, J.; Zarrouk, A.; Ho, V.; O'Connor, M.D. Understanding the Biology of Human Interstitial Cells of Cajal in Gastrointestinal Motility. Int J Mol Sci. 2020, 21, 4540. [Google Scholar] [CrossRef] [PubMed]
- Vanner, S.; Greenwood-Van Meerveld, B.; Mawe, G.; et al. Fundamentals of Neurogastroenterology: Basic Science. Gastroenterology 2016. [Google Scholar] [CrossRef]
- Maeda, H.; Yamagata, A.; Nishikawa, S.; et al. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992, 116, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Torihashi, S.; Horisawa, M.; Watanabe, Y. c-Kit immunoreactive interstitial cells in the human gastrointestinal tract. J Auton Nerv Syst. 1999, 75, 38–50. [Google Scholar] [CrossRef]
- Al-Shboul, O.A. The importance of interstitial cells of cajal in the gastrointestinal tract. Saudi J Gastroenterol. 2013, 19, 3–15. [Google Scholar] [CrossRef]
- Robinson, T.L.; Sircar, K.; Hewlett, B.R.; Chorneyko, K.; Riddell, R.H.; Huizinga, J.D. Gastrointestinal stromal tumors may originate from a subset of CD34-positive interstitial cells of Cajal. Am J Pathol. 2000, 156, 1157–1163. [Google Scholar] [CrossRef]
- Lorincz, A.; Redelman, D.; Horváth, V.J.; et al. Progenitors of interstitial cells of cajal in the postnatal murine stomach. Gastroenterology. 2008, 134, 1083–1093. [Google Scholar] [CrossRef]
- Chen, H.; Redelman, D.; Ro, S.; Ward, S.M.; Ordög, T.; Sanders, K.M. Selective labeling and isolation of functional classes of interstitial cells of Cajal of human and murine small intestine. Am J Physiol Cell Physiol. 2007, 292, C497–C507. [Google Scholar] [CrossRef]
- Wouters, M.; De Laet, A.; Donck, L.V.; et al. Subtractive hybridization unravels a role for the ion cotransporter NKCC1 in the murine intestinal pacemaker. Am J Physiol Gastrointest Liver Physiol. 2006, 290, G1219–G1227. [Google Scholar] [CrossRef]
- Mazur, M.T.; Clark, H.B. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol. 1983, 7, 507–519. [Google Scholar] [CrossRef]
- Nishida, T.; Hirota, S. Biological and clinical review of stromal tumors in the gastrointestinal tract. Histol Histopathol. 2000, 15, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, M.; Predescu, D.; Iosif, C.; et al. Clinical and therapeutic considerations of GIST. J Med Life. 2014, 7, 139–149. [Google Scholar]
- Miettinen, M.; Lasota, J. Gastrointestinal stromal tumors. Gastroenterol Clin North Am. 2013, 42, 399–415. [Google Scholar] [CrossRef]
- Rammohan, A.; Sathyanesan, J.; Rajendran, K.; et al. A gist of gastrointestinal stromal tumors: A review. World J Gastrointest Oncol. 2013, 5, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, H.; Hohenberger, P. Management of small gastrointestinal stromal tumours—Authors' reply. Lancet. 2013, 382, 1701–1702. [Google Scholar] [PubMed]
- Benesch, M.; Wardelmann, E.; Ferrari, A.; Brennan, B.; Verschuur, A. Gastrointestinal stromal tumors (GIST) in children and adolescents: A comprehensive review of the current literature. Pediatr Blood Cancer. 2009, 53, 1171–1179. [Google Scholar] [CrossRef]
- Wu, C.E.; Tzen, C.Y.; Wang, S.Y.; Yeh, C.N. Clinical Diagnosis of Gastrointestinal Stromal Tumor (GIST): From the Molecular Genetic Point of View. Cancers 2019, 11, 679. [Google Scholar] [CrossRef]
- Hirota, S.; Isozaki, K. Pathology of gastrointestinal stromal tumors. Pathol Int. 2006, 56, 1–9. [Google Scholar] [CrossRef]
- Hirota, S.; Isozaki, K.; Moriyama, Y.; et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998, 279, 577–580. [Google Scholar] [CrossRef]
- Lasota, J.; Miettinen, M. Clinical significance of oncogenic KIT and PDGFRA mutations in gastrointestinal stromal tumours. Histopathology. 2008, 53, 245–266. [Google Scholar] [CrossRef]
- Ahmed, M. Recent advances in the management of gastrointestinal stromal tumor. World J Clin Cases. 2020, 8, 3142–3155. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, Y.; Sasaki, M.; Kouyama, M.; Tazaki, T.; Takahashi, S.; Nakamitsu, A. Current treatment strategies and future perspectives for gastrointestinal stromal tumors. World J Gastrointest Pathophysiol. 2022, 13, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Hirota, S. Differential diagnosis of gastrointestinal stromal tumor by histopathology and immunohistochemistry. Transl Gastroenterol Hepatol. 2018, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Mantese, G. Gastrointestinal stromal tumor: Epidemiology, diagnosis, and treatment. Curr Opin Gastroenterol. 2019, 35, 555–559. [Google Scholar] [CrossRef]
- Schaefer, I.M.; Mariño-Enríquez, A.; Fletcher, J.A. What is New in Gastrointestinal Stromal Tumor? Adv Anat Pathol. 2017, 24, 259–267. [Google Scholar] [CrossRef]
- Badalamenti, G.; Rodolico, V.; Fulfaro, F.; et al. Gastrointestinal stromal tumors (GISTs): Focus on histopathological diagnosis and biomolecular features. Ann Oncol. 2007, 18 (Suppl 6), vi136–vi140. [Google Scholar] [CrossRef]
- Fletcher, C.D.; Berman, J.J.; Corless, C.; et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002, 33, 459–465. [Google Scholar] [CrossRef]
- Joensuu, H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008, 39, 1411–1419. [Google Scholar] [CrossRef]
- Miettinen, M.; Lasota, J. Gastrointestinal stromal tumors: Review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006, 130, 1466–1478. [Google Scholar] [CrossRef]
- Casali, P.G.; Abecassis, N.; Aro, H.T.; et al. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018, 29 (Suppl. 4), iv68–iv78. [Google Scholar] [CrossRef]
- Kersting, S.; Janot-Matuschek, M.S.; Schnitzler, C.; Chourio Barboza, D.E.; Uhl, W.; Mittelkötter, U. GIST: Correlation of risk classifications and outcome. J Med Life. 2022, 15, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Liu, X.; Xu, J.; Qiu, C.; Wang, R.; Zheng, J. Expression profiles of stemness genes in gastrointestinal stromal tumor. Hum Pathol. 2018, 76, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Parab, T.M.; DeRogatis, M.J.; Boaz, A.M.; et al. Gastrointestinal stromal tumors: A comprehensive review. J Gastrointest Oncol. 2019, 10, 144–154. [Google Scholar] [CrossRef]
- Wei, R.; Parsons, S.P.; Huizinga, J.D. Network properties of interstitial cells of Cajal affect intestinal pacemaker activity and motor patterns, according to a mathematical model of weakly coupled oscillators. Exp Physiol. 2017, 102, 329–346. [Google Scholar] [CrossRef] [PubMed]
- Faussone-Pellegrini, M.S. Relationships between neurokinin receptor- expressing interstitial cells of Cajal and tachykininergic nerves in the gut. J Cell Mol Med. 2006, 10, 20–32. [Google Scholar] [CrossRef]
- Rich, A.; Miller, S.M.; Gibbons, S.J.; et al. Local presentation of Steel factor increases expression of c-kit immunoreactive interstitial cells of Cajal in culture. Am J Physiol Gastrointest Liver Physiol. 2003, 284, G313–G320. [Google Scholar] [CrossRef]
- Nishida, T.; Blay, J.Y.; Hirota, S.; Kitagawa, Y.; Kang, Y.K. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 2016, 19, 3–14. [Google Scholar] [CrossRef]
- Bure, I.; Braun, A.; Kayser, C.; et al. The expression of hematopoietic progenitor cell antigen CD34 is regulated by DNA methylation in a site-dependent manner in gastrointestinal stromal tumours. Int J Cancer. 2017, 141, 2296–2304. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, Z.P.; Zhu, Y.; Fu, F.; Tian, L. Contribution of Interstitial Cells of Cajal to Gastrointestinal Stromal Tumor Risk. Med Sci Monit. 2021, 27, e929575. [Google Scholar] [CrossRef]
- Sözütek, D.; Yanık, S.; Akkoca, A.N.; et al. Diagnostic and prognostic roles of DOG1 and Ki-67, in GIST patients with localized or advanced/metastatic disease. Int J Clin Exp Med. 2014, 7, 1914–1922. [Google Scholar]
- Barbu, L.A.; Mărgăritescu, N.D.; Ghiluşi, M.C.; et al. Severe upper gastrointestinal bleeding from gastrointestinal stromal tumor of the stomach. Rom J Morphol Embryol. 2016, 57, 1397–1401. [Google Scholar] [PubMed]
- Güler, B.; Özyılmaz, F.; Tokuç, B.; Can, N.; Taştekin, E. Histopathological Features of Gastrointestinal Stromal Tumors and the Contribution of DOG1 Expression to the Diagnosis. Balkan Med J. 2015, 32, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Hamzaoui, L.; Medhioub, M.; Bouassida, M.; et al. Gastrointestinal stromal tumours (GISTs): A descriptive study on 29 cases. Arab J Gastroenterol. 2016, 17, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Kramer, K.; Knippschild, U.; Mayer, B.; et al. Impact of age and gender on tumor related prognosis in gastrointestinal stromal tumors (GIST). BMC Cancer. 2015, 15, 57. [Google Scholar] [CrossRef]
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).