Evaluation of the Newborn Screening Pilot for Sickle Cell Disease in Suriname Using the Non-Adoption, Abandonment, Scale-Up, Spread, and Sustainability (NASSS) Framework
Abstract
:1. Introduction
2. Materials and Methods
2.1. Outline of the NSP Pilot for SCD
2.2. Ethical Considerations
2.3. Evaluation
3. Results
3.1. NSP Pilot for SCD
3.2. The Systematic Evaluation of Challenges of Implementing NSP for SCD Using the NASSS Framework
3.2.1. Domain 1: The Illness
3.2.2. Domain 2: The Technology
3.2.3. Domain 3: The Value Proposition
3.2.4. Domain 4: The Adopter System
3.2.5. Domain 5: The Organisation
3.2.6. Domain 6: The Wider Context
4. Discussion
- Strengths and weaknesses
- Implications
- Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ranque, B.; Kitenge, R.; Ndiaye, D.D.; Ba, M.D.; Adjoumani, L.; Traore, H.; Coulibaly, C.; Guindo, A.; Boidy, K.; Mbuyi, D.; et al. Estimating the risk of child mortality attributable to sickle cell anaemia in sub-Saharan Africa: A retrospective, multicentre, case-control study. Lancet Haematol. 2022, 9, e208–e216. [Google Scholar] [CrossRef] [PubMed]
- Vichinsky, E.; Hurst, D.; Earles, A.; Kleman, K.; Lubin, B. Newborn screening for sickle cell disease: Effect on mortality. Pediatrics 1988, 81, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Thomas, P.; Cupidore, L.; Serjeant, B.; Serjeant, G. Improved survival in homozygous sickle cell disease: Lessons from a cohort study. BMJ 1995, 311, 1600–1602. [Google Scholar] [CrossRef] [PubMed]
- Telfer, P.; Coen, P.; Chakravorty, S.; Wilkey, O.; Evans, J.; Newell, H.; Smalling, B.; Amos, R.; Stephens, A.; Rogers, D.; et al. Clinical outcomes in children with sickle cell disease living in England: A neonatal cohort in East London. Haematologica 2007, 92, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Quinn, C.T.; Rogers, Z.R.; McCavit, T.L.; Buchanan, G.R. Improved survival of children and adolescents with sickle cell disease. Blood 2010, 115, 3447–3452. [Google Scholar] [CrossRef] [PubMed]
- Knight-Madden, J.; Lee, K.; Elana, G.; Elenga, N.; Marcheco-Teruel, B.; Keshi, N.; Etienne-Julan, M.; King, L.; Asnani, M.; Romana, M.; et al. Newborn Screening for Sickle Cell Disease in the Caribbean: An Update of the Present Situation and of the Disease Prevalence. Int. J. Neonatal. Screen 2019, 5, 5. [Google Scholar] [CrossRef]
- Algemeen Bureau Voor de Statistiek. Suriname Census 2012 Volume I Demografische en Sociale Karakteristieken en Migratie. Available online: https://statistics-suriname.org/wp-content/uploads/2019/05/Publicatie-Census-8-Volume-1-Demografische-en-Sociale-Karakteristieken-en-Migratie.pdf (accessed on 21 February 2024).
- Diemer, F.S.; Haan, Y.C.; Nannan Panday, R.V.; van Montfrans, G.A.; Oehlers, G.P.; Brewster, L.M. Health literacy in Suriname. Soc. Work Health Care 2017, 56, 283–293. [Google Scholar] [CrossRef]
- Inter-American Development Bank. Suriname Health Sector Assessment. Available online: https://publications.iadb.org/publications/english/viewer/Suriname-Health-Sector-Assessment.pdf (accessed on 18 April 2023).
- Greenhalgh, T.; Wherton, J.; Papoutsi, C.; Lynch, J.; Hughes, G.; A’Court, C.; Hinder, S.; Fahy, N.; Procter, R.; Shaw, S. Beyond Adoption: A New Framework for Theorizing and Evaluating Nonadoption, Abandonment, and Challenges to the Scale-Up, Spread, and Sustainability of Health and Care Technologies. J. Med. Internet Res. 2017, 19, e367. [Google Scholar] [CrossRef] [PubMed]
- Brousse, V.; Buffet, P.; Rees, D. The spleen and sickle cell disease: The sick(led) spleen. Br. J. Haematol. 2014, 166, 165–176. [Google Scholar] [CrossRef]
- Booth, C.; Inusa, B.; Obaro, S.K. Infection in sickle cell disease: A review. Int. J. Infect. Dis. 2010, 14, e2–e12. [Google Scholar] [CrossRef]
- Leikin, S.L.; Gallagher, D.; Kinney, T.R.; Sloane, D.; Klug, P.; Rida, W. Mortality in children and adolescents with sickle cell disease. Cooperative Study of Sickle Cell Disease. Pediatric 1989, 84, 500–508. [Google Scholar] [CrossRef]
- Gaston, M.H.; Verter, J.I.; Woods, G.; Pegelow, C.; Kelleher, J.; Presbury, G.; Zarkowsky, H.; Vichinsky, E.; Iyer, R.; Lobel, J.S.; et al. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. N. Engl. J. Med. 1986, 314, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- National Health Service. Treatment, Sickle Cell Disease. Available online: https://www.nhs.uk/conditions/sickle-cell-disease/treatment/ (accessed on 12 March 2023).
- National Heart, Lung and Blood Institute. Evidence-Based Management of Sickle Cell Disease: Expert Panel Report. Available online: https://www.nhlbi.nih.gov/sites/default/files/media/docs/sickle-cell-disease-report%20020816_0.pdf (accessed on 13 March 2023).
- Murray, C.; Hall, S.K.; Griffiths, P. An evaluation of the Sebia capillarys Neonat Haemoglobin FAST™ system for routine newborn screening for sickle cell disease. Int. J. Lab. Hematol. 2011, 33, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Frömmel, C. Newborn Screening for Sickle Cell Disease and Other Hemoglobinopathies: A Short Review on Classical Laboratory Methods-Isoelectric Focusing, HPLC, and Capillary Electrophoresis. Int. J. Neonatal. Screen 2018, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Arishi, W.A.; Alhadrami, H.A.; Zourob, M. Techniques for the Detection of Sickle Cell Disease: A Review. Micromachines 2021, 12, 519. [Google Scholar] [CrossRef] [PubMed]
- Kuznik, A.; Habib, A.G.; Munube, D.; Lamorde, M. Newborn screening and prophylactic interventions for sickle cell disease in 47 countries in sub-Saharan Africa: A cost-effectiveness analysis. BMC Health Serv. Res. 2016, 16, 304. [Google Scholar] [CrossRef] [PubMed]
- Nederlandse Organisatie Voor Toegepast-Natuurwetenschappelijk Onderzoek. De Neonatale Hielprikscreening Monitor 2016. Available online: https://www.rivm.nl/sites/default/files/2018-11/Monitor%20NHS%202016%20definitief%20-%20online.pdf (accessed on 23 December 2019).
- NHS Sickle Cell and Thalassaemia Screening Programme Data Report 2016 to 2017: Trends and Performance Analysis. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/713120/SCT_data_report_2016_to_2017.pdf (accessed on 23 December 2019).
- Büyükgebiz, A. Newborn screening for congenital hypothyroidism. J. Clin. Res. Pediatr. Endocrinol. 2013, 5 (Suppl. 1), 8–12. [Google Scholar] [CrossRef] [PubMed]
- Lak, R.; Yazdizadeh, B.; Davari, M.; Nouhi, M.; Kelishadi, R. Newborn screening for galactosaemia. Cochrane Database Syst. Rev. 2017, 12, CD012272. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Sickle-Cell Anaemia: Report by the Secretariat. Available online: https://apps.who.int/iris/handle/10665/20890 (accessed on 23 December 2023).
- Kafando, E.; Sawadogo, M.; Cotton, F.; Vertongen, F.; Gulbis, B. Neonatal screening for sickle cell disorders in Ouagadougou, Burkina Faso: A pilot study. J. Med. Screen 2005, 12, 112–114. [Google Scholar] [CrossRef] [PubMed]
- McGann, P.T.; Ferris, M.G.; Ramamurthy, U.; Santos, B.; de Oliveira, V.; Bernardino, L.; Ware, R.E. A prospective newborn screening and treatment program for sickle cell anemia in Luanda, Angola. Am. J. Hematol. 2013, 88, 984–989. [Google Scholar] [CrossRef]
- World Health Organization. Suriname: WHO and UNICEF Estimates of Immunization Coverage: 2018 Revision. Available online: https://www.who.int/immunization/monitoring_surveillance/data/sur.pdf (accessed on 23 December 2019).
- Steele, C.; Sinski, A.; Asibey, J.; Hardy-Dessources, M.D.; Elana, G.; Brennan, C.; Odame, I.; Hoppe, C.; Geisberg, M.; Serrao, E.; et al. Point-of-care screening for sickle cell disease in low-resource settings: A multi-centre evaluation of HemoTypeSC, a novel rapid test. Am. J. Hematol. 2019, 94, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, O.A.; Hustace, T.; Voltaire, M.; Mantero, A.; Liberus, U.; Saint Fleur, R. Newborn Screening for Sickle Cell Disease Using Point-of-Care Testing in Low-Income Setting. Pediatrics 2019, 144, e20184105. [Google Scholar] [CrossRef] [PubMed]
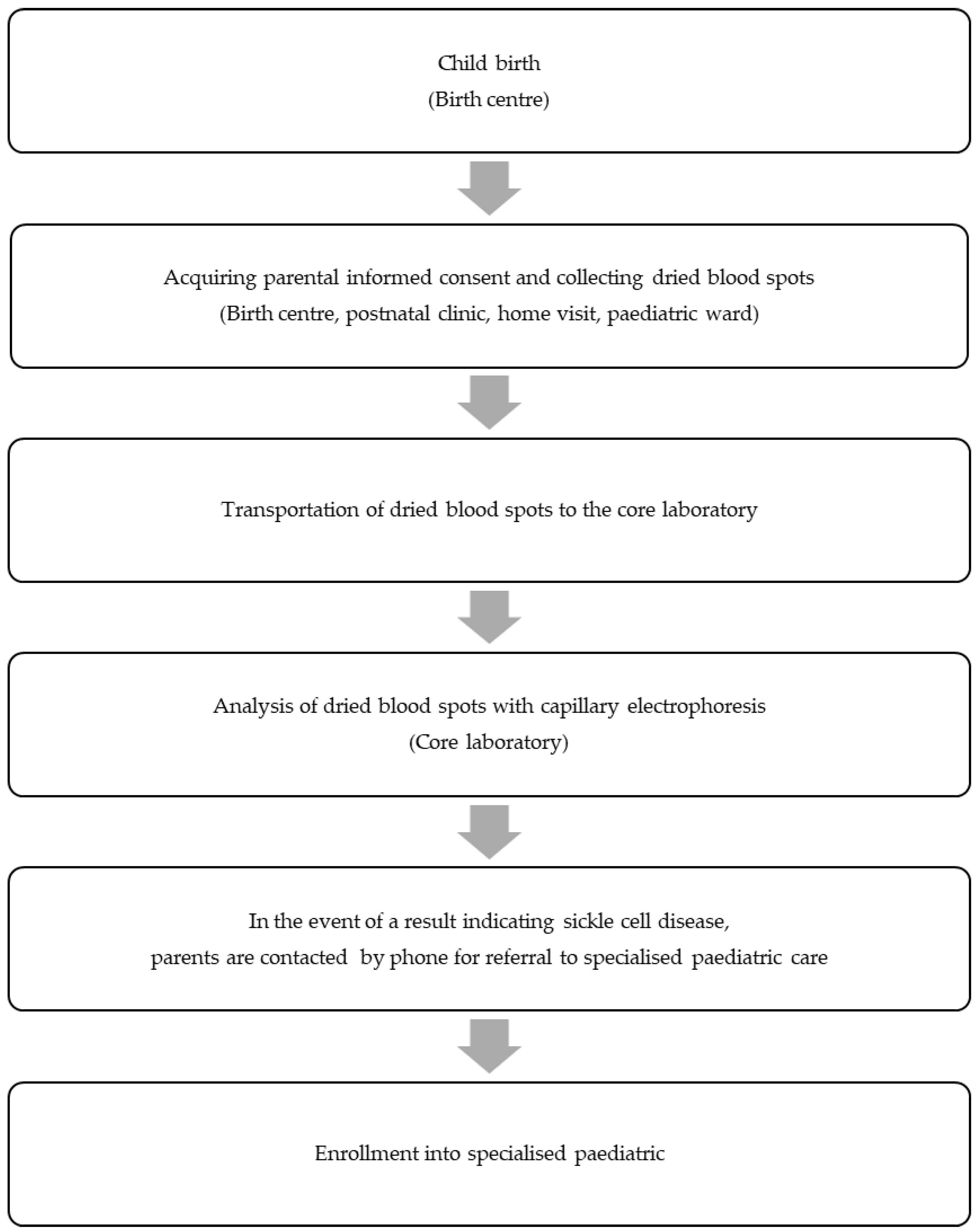
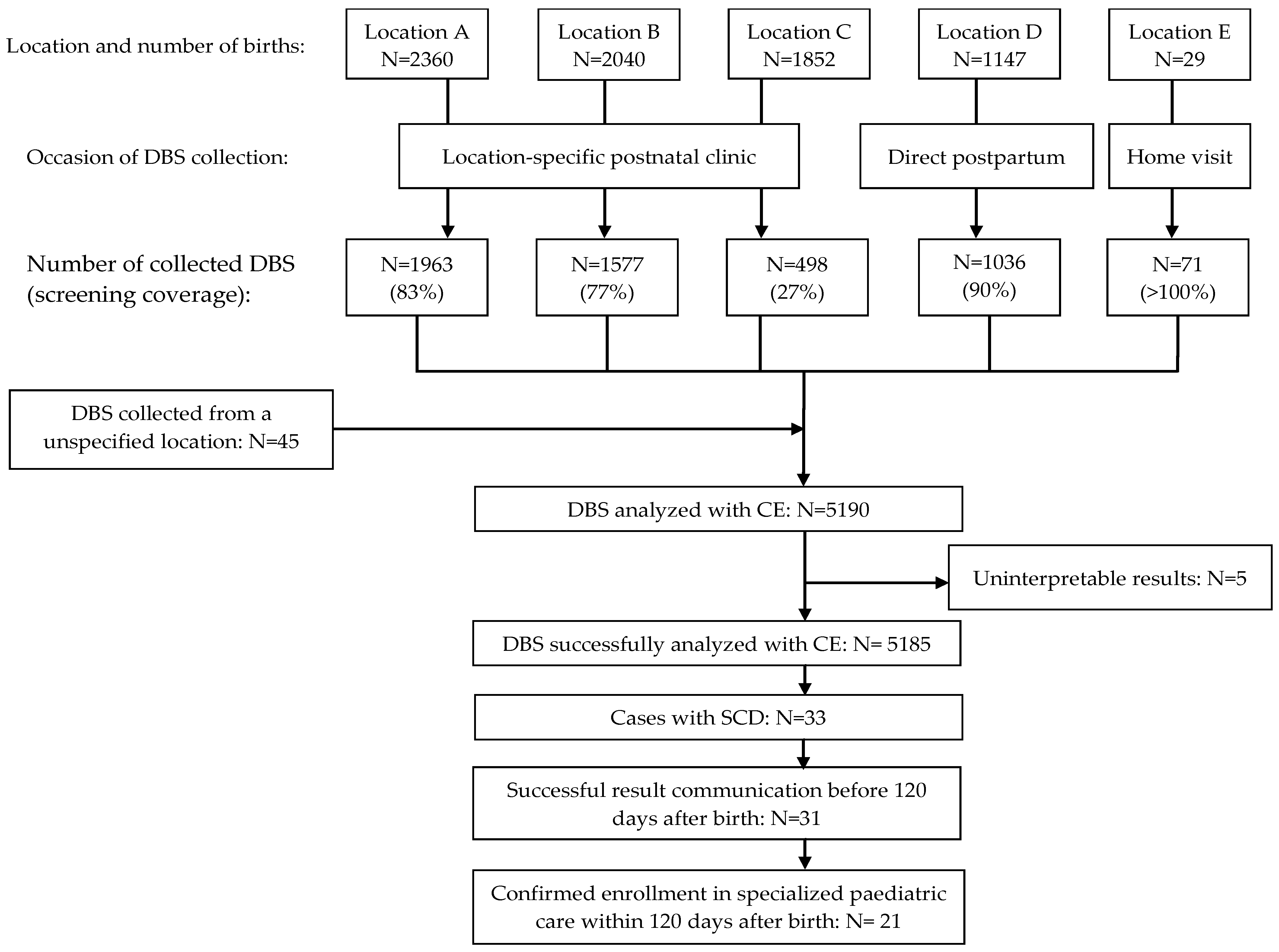
| Location | Timing of DBS Collection | Environment | Motivation |
|---|---|---|---|
| Birth centres A, B, C | 7–14 days after birth | Postnatal clinic | Postpartum discharge was usually ≤24 h after birth |
| Birth centre D | Directly postpartum | Obstetric ward | Absence of standard postnatal clinic |
| Birth centre E | 7 days after birth | Home visit | Discharge was usually ≤24 h after birth |
| Paediatric ward of locations A, B, C, D | 24 h after birth | Paediatric ward | Need for newborn monitoring |
| Domain and Questions | Adapted Questions |
|---|---|
| Domain 1: The illness | |
| 1A. What is the nature of the condition or illness? | Does newborn screening for sickle cell disease result in health gain? |
| 1B. What are the relevant sociocultural factors and comorbidities? | What are potential sociocultural factors that could interfere with participation in the newborn screening programme? |
| Domain 2: The technology | |
| 2A. What are the key features of the technology? | No changes |
| 2B. What kind of knowledge does the technology bring into play? | No changes |
| 2C. What knowledge and/or support is required to use the technology? | No changes |
| 2D. What is the technology supply model? | No changes |
| Domain 3: The value proposition | |
| 3A. What is the developer’s business case for the technology (supply side value)? | Not applicable |
| 3B. What is its desirability, efficacy, safety, and cost-effectiveness (demand-side value)? | No changes |
| Domain 4: The adopter system | |
| 4A. What changes in staff roles, practices, and identities are implied? | No changes |
| 4B. What is expected of the patient (and/or immediate caregiver)? Is this achievable by and acceptable to them? | What is expected of the parents of the newborn—is this achievable by them? |
| 4C. What is assumed about the extended network of lay caregivers? | Not applicable |
| Domain 5: The organisation | |
| 5A. What is the organisation’s capacity to innovate? | No changes |
| 5B. How ready is the organisation for this technology-supported change? | No changes |
| 5C. How easy will the adoption and funding decision be? | No changes |
| 5D. What changes will be needed in team interactions and routines? | No changes |
| 5E. What work is involved in implementation and who will do it? | No changes |
| Domain 6: The wider context | |
| 6A. What is the political, economic, regulatory, professional (e.g., medicolegal), and sociocultural context for programme rollout? | What is the political, economic, and regulatory context for programme rollout? |
| Domain 7: The time dimension | |
| 7A. How much scope is there for adapting and coevolving the technology and the service over time? | Not included |
| 7B. How resilient is the organisation to handling critical events and adapting to unforeseen eventualities? | Not included |
| All Screened Newborns (N = 5190) | Newborns with Sickle Cell Disease (N = 33) | ||
|---|---|---|---|
| Gender | Female | 2533 (48.7%) | 23 (69.7%) |
| Male | 2583 (49.8%) | 10 (30,3%) | |
| Missing | 74 (1.4%) | 0 | |
| Ethnicity | Maroons | 1333 (25.7%) | 12 (36.4%) |
| Creoles | 1171 (22.6%) | 10 (30.3%) | |
| Mixed ethnic background | 982 (18.9%) | 8 (24.2%) | |
| Indian–Surinamese | 766 (14.8%) | 0 | |
| Javanese–Surinamese | 463 (8.9%) | 0 | |
| Chinese–Surinamese | 134 (2.6%) | 0 | |
| Indigenous | 138 (2.7%) | 2 (6.1%) | |
| Missing | 203 (3.9%) | 1 (3.0%) |
| Domain and Questions | Rating | Comment |
|---|---|---|
| Domain 1: The illness | ||
| 1A. Does newborn screening of sickle cell disease result in health gain? | 😊 | Newborn screening of sickle cell disease and enrolment in a comprehensive treatment programme has been shown to reduce mortality in affected children. |
| 1B. What are potential sociocultural factors that could interfere with participation in the newborn screening programme? | 😐 | Social and cultural factors, including health literacy, could impact the traceability and healthcare-seeking behaviour of parents with newborns who are affected. The potential demand for parental financial contribution could be a barrier to participating in the newborn screening programme. |
| Domain 2: The technology | ||
| 2A. What are the key features of the technology? | 😐 | Capillary electrophoresis was carried out in the core laboratory. This caused logistical challenges. |
| 2B. What kind of knowledge does the technology bring into play? | 😊 | Interpretation of the electropherogram was straightforward. |
| 2C. What knowledge and/or support is required to use the technology? | 😊 | The knowledge to obtain the dried bloodspot and to perform the laboratory analysis was successfully transferred to the local medical staff. |
| 2D. What is the technology supply model? | 😢 | Capillary electrophoresis technology is vulnerable to supplier withdrawal. |
| Domain 3: The value proposition | ||
| 3A. Not applicable | ||
| 3B. What is its desirability, efficacy, safety, and cost-effectiveness (demand-side value)? | 😐 | From the parent’s point of view, newborn screening for sickle cell disease is a desirable form of preventive healthcare. Potential safety risks include uninterpretable results and failure to contact parents timely for results communication. |
| Domain 4: The adopter system | ||
| 4A. What changes in staff roles, practices, and identities are implied? | 😊 | Small changes in the staff roles and practices were needed, all appropriate to the existing professional identities. |
| 4B. What is expected of the parents of the newborn? Is this achievable by them? | 😢 | In birth centre locations A, B, and C, parents needed to bring their newborn to the postnatal clinic to participate in the newborn screening programme. Parents of affected newborns were expected to be traceable by phone. Both expectations were not always met by parents. |
| 4C. Not applicable | ||
| Domain 5: The organisation | ||
| 5A. What is the organisation’s capacity to innovate? | 😐 | The organizations were enthusiastic about innovation; however, as in most middle-income countries, due to structural severe resource pressures, innovation is not a top priority. |
| 5B. How ready is the organisation for this technology-supported change? | 😐 | |
| 5C. How easy will the adoption and funding decision be? | 😢 | A new strategy for funding needs to be developed when needed when the continuation or upscaling of the NSP will be taking place. |
| 5D. What changes will be needed in team interactions and routines? | 😊 | Small changes in the team routines were implemented. |
| 5E. What work is involved in implementation and who will do it? | 😐 | A small dedicated team will be required to manage the newborn screening programme. |
| Domain 6: The wider context | ||
| 6A. What is the political, economic, and regulatory context for programme rollout? | 😐 | Investment in preventive healthcare, such as newborn screening, corresponds to the health sector development plan of the Ministry of Health of Suriname. However, during economically uncertain times, the available resources will most likely be allocated for acute and curative healthcare goals. The current medical referral system in Suriname is not optimised to support the timely enrolment of the affected newborns into specialised paediatric care. |
| Domain 7: The time dimension | ||
| 7A. Not applicable | ||
| 7B. Not applicable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, M.-J.; Roosblad, J.; Codrington, J.; Peters, M.; Toekoen, A.; van Rheenen, P.F.; Juliana, A. Evaluation of the Newborn Screening Pilot for Sickle Cell Disease in Suriname Using the Non-Adoption, Abandonment, Scale-Up, Spread, and Sustainability (NASSS) Framework. Int. J. Neonatal Screen. 2024, 10, 46. https://doi.org/10.3390/ijns10030046
Tang M-J, Roosblad J, Codrington J, Peters M, Toekoen A, van Rheenen PF, Juliana A. Evaluation of the Newborn Screening Pilot for Sickle Cell Disease in Suriname Using the Non-Adoption, Abandonment, Scale-Up, Spread, and Sustainability (NASSS) Framework. International Journal of Neonatal Screening. 2024; 10(3):46. https://doi.org/10.3390/ijns10030046
Chicago/Turabian StyleTang, Ming-Jan, Jimmy Roosblad, John Codrington, Marjolein Peters, Aartie Toekoen, Patrick F. van Rheenen, and Amadu Juliana. 2024. "Evaluation of the Newborn Screening Pilot for Sickle Cell Disease in Suriname Using the Non-Adoption, Abandonment, Scale-Up, Spread, and Sustainability (NASSS) Framework" International Journal of Neonatal Screening 10, no. 3: 46. https://doi.org/10.3390/ijns10030046







