Neonatal Screening for Sickle Cell Disease in Belgium for More than 20 Years: An Experience for Comprehensive Care Improvement
Abstract
:1. Introduction
2. Material and Methods
2.1. Neonatal Screening for Sickle Cell Disease
2.2. Belgian Network of Health Care Providers and Registry for Sickle Cell Patients
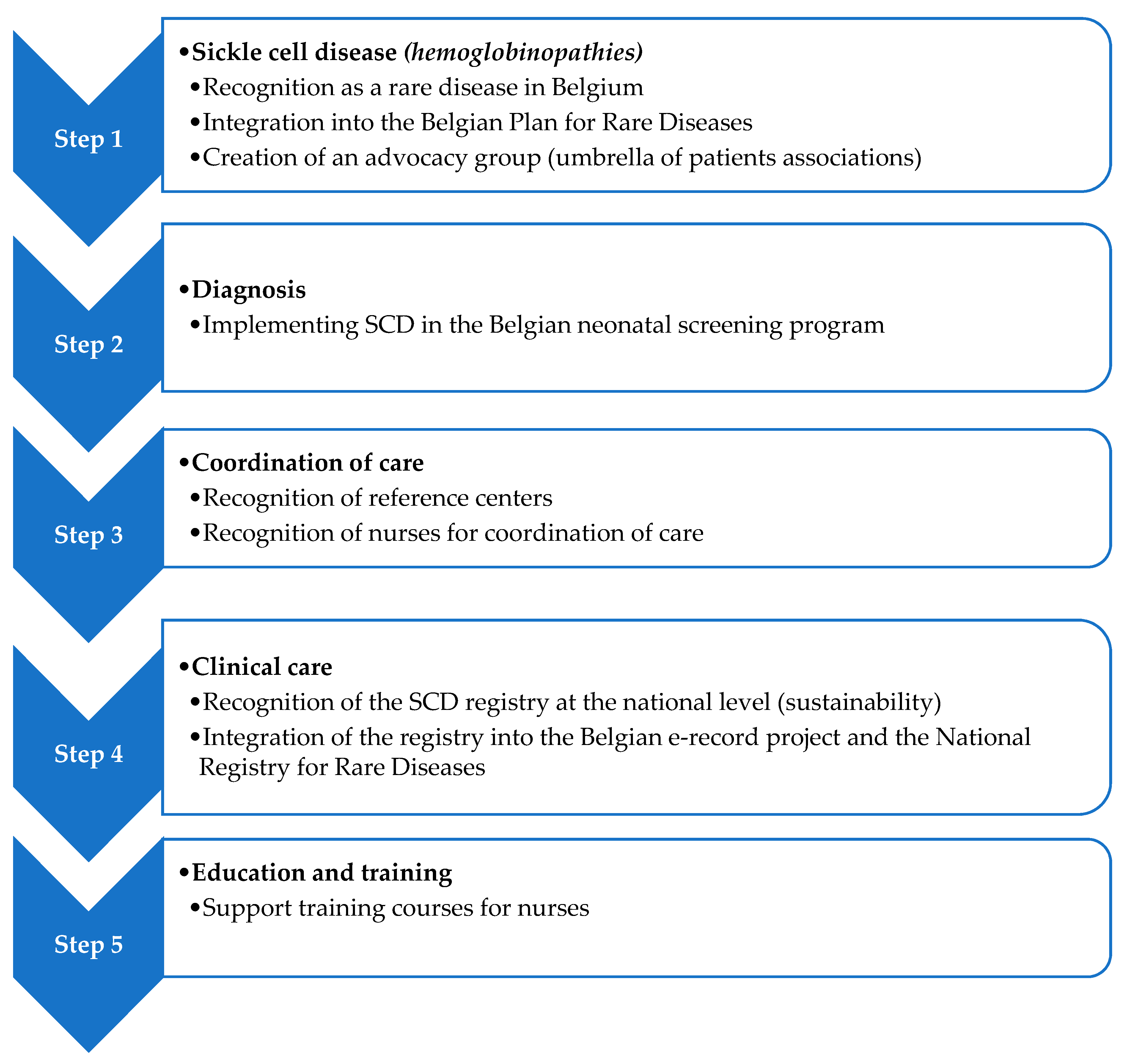
2.3. Comprehensive Care Improvement Plan
3. Results
3.1. Neonatal Screening for Sickle Cell Disease
3.2. Belgian Network of Health Care Providers and Registry for Sickle Cell Patients
3.3. Comprehensive Care Improvement Plan
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sickle cell anemia. Available online: http://apps.who.int/gb/archive/pdf_files/wha59/a59_9-en.pdf (accessed on 11 November 2018).
- Aguilar Martinez, P.; Angastiniotis, M.; Eleftheriou, A.; Gulbis, B.; Mañú Pereira Mdel, M.; Petrova-Benedict, R.; Corrons, J.L. Haemoglobinopathies in Europe: Health & migration policy perspectives. Orphanet J. Rare Dis. 2014, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Lê, P.Q.; Gulbis, B.; Dedeken, L.; Dupont, S.; Vanderfaeillie, A.; Heijmans, C.; Huybrechts, S.; Devalck, C.; Efira, A.; Dresse, M.F.; et al. Survival among children and adults with sickle cell disease in belgium: Benefit from hydroxyurea treatment. Pediatr. Blood Cancer 2015, 62, 1956–1961. [Google Scholar] [CrossRef] [PubMed]
- Gaston, M.H.; Verter, J.I.; Woods, G.; Pegelow, C.; Kelleher, J.; Presbury, G.; Zarkowsky, H.; Vichinsky, E.; Iyer, R.; Lobel, J.S.; et al. For the Prophylactic Penicillin Study Group. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. N. Engl. J. Med. 1986, 314, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Castilla-Rodríguez, I.; Cela, E.; Vallejo-Torres, L.; Valcárcel-Nazco, C.; Dulín, E.; Espada, M.; Rausell, D.; Mar, J.; Serrano-Aguilar, P. Cost-effectiveness analysis of newborn screening for sickle-cell disease in Spain. Exp. Opin. Orphan Drugs 2016, 4, 567–575. [Google Scholar] [CrossRef]
- Ketelslegers, O.; Eyskens, F.; Boemer, F.; Bours, V.; Minon, J.M.; Gulbis, B. Epidemiological data on sickle cell disease in Belgium. Belg. J. Hematol. 2015, 6, 135–141. [Google Scholar]
- Wolff, F.; Cotton, F.; Gulbis, B. Screening for haemoglobinopathies on cord blood: Laboratory and clinical experience. J. Med. Screen. 2012, 19, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Gulbis, B.; Cotton, F.; Ferster, A.; Ketelslegers, O.; Dresse, M.F.; Rongé-Collard, E.; Minon, J.M.; Lê, P.Q.; Vertongen, F. Neonatal haemoglobinopathy screening in Belgium. J. Clin. Pathol. 2009, 62, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Boemer, F.; Cornet, Y.; Libioulle, C.; Segers, A.; Bours, V.; Schoos, R. 3-Years experience review of neonatal screening for hemoglobin disorders using tandem mass spectrometry. Clin. Chim. Acta 2011, 412, 1476–1479. [Google Scholar] [CrossRef] [PubMed]
- Weatherall, D.J. The inherited diseases of hemoglobin are an emerging global health burden. Blood 2010, 115, 4331–4436. [Google Scholar] [CrossRef] [PubMed]
- Piel, F.B.; Tatem, A.J.; Huang, Z.; Gupta, S.; Williams, T.N.; Weatherall, D.J. Global migration and the changing distribution of sickle haemoglobin: A quantitative study of temporal trends between 1960 and 2000. Lancet Glob. Health 2014, 2, 80–89. [Google Scholar] [CrossRef]
- Migrations en Belgique: Données statistiques. Available online: www.myria.be/files/Migration-rapport-2015-C2.pdf (accessed on 7 October 2018).
- Vichinsky, E.; Hurst, D.; Earles, A.; Kleman, K.; Lubin, B. Newborn screening for sickle cell disease: Effect on mortality. Pediatrics 1988, 81, 749–755. [Google Scholar] [PubMed]
- King, L.; Fraser, R.; Forbes, M.; Grindley, M.; Ali, S.; Reid, M. Newborn sickle cell disease screening: The Jamaican experience (1995–2006). J. Med. Screen. 2007, 14, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Sabarense, A.P.; Lima, G.O.; Silva, L.M.; Viana, M.B. Survival of children with sickle cell disease in the comprehensive newborn screening programme in Minas Gerais, Brazil. Paediatr. Int. Child Health 2015, 35, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Quinn, C.T.; Rogers, Z.R.; Buchanan, G.R. Survival of children with sickle cell disease. Blood 2004, 103, 4023–4027. [Google Scholar] [CrossRef] [PubMed]
- Quinn, C.T.; Rogers, Z.R.; McCavit, T.L.; Buchanan, G.R. Improved survival of children and adolescents with sickle cell disease. Blood 2010, 115, 3447–3452. [Google Scholar] [CrossRef] [PubMed]
- Lê, P.Q.; Ferster, A.; Dedeken, L.; Vermylen, C.; Vanderfaeillie, A.; Rozen, L.; Heijmans, C.; Huybrechts, S.; Devalck, C.; Efira, A.; et al. Neonatal screening improves sickle cell disease clinical outcome in Belgium. J. Med. Screen 2018, 25, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Couque, N.; Girard, D.; Ducrocq, R.; Boizeau, P.; Haouari, Z.; Misud, F.; Holvoet, L.; Ithier, G.; Belloy, M.; Odièvre, M.H.; et al. Improvement of medical care in a cohort of newborns with sickle-cell disease in North Paris: Impact of national guidelines. Br. J. Haematol. 2016, 173, 927–937. [Google Scholar] [CrossRef] [PubMed]
| Screening Centre | Sample | First Screening Test | Confirmation Test (Same Sample) | Report of Results | Ref. |
|---|---|---|---|---|---|
| Brussels Region | Umbilical cord blood liquid | IEF (<2008) CZE (≥2008) | HPLC | Any hemoglobinopathy detected | [7] |
| CHR Citadelle | Umbilical cord blood liquid | CZE | HPLC | Any hemoglobinopathy detected | [8] |
| Liège Region | Heel prick/filter paper | TMS | DNA | Any hemoglobinopathy detected | [9] |
| Year | Neonates Screened | SCD | SCD | FAS | FAS | FAS | FAS |
|---|---|---|---|---|---|---|---|
| All Regions | Both Regions | Both Regions | Brussels Region | Brussels Region | Liège Region | Liège Region | |
| (n) | (n) | Birth Prevalence | (n) | Birth Prevalence | (n) | Birth Prevalence | |
| 2009 | 40,026 | 25 | 1:1601 | 421 | 1:54 | 159 | 1:109 |
| 2010 | 40,579 | 25 | 1:1561 | 449 | 1:52 | 150 | 1:115 |
| 2011 | 40,262 | 36 | 1:1088 | 458 | 1:50 | 112 | 1:154 |
| 2012 | 40,675 | 24 | 1:1768 | 460 | 1:51 | 175 | 1:98 |
| 2013 | 40,241 | 22 | 1:1829 | 520 | 1:45 | 161 | 1:104 |
| 2014 | 40,144 | 28 | 1:1487 | 447 | 1:52 | 192 | 1:87 |
| 2015 | 39,748 | 27 | 1:1529 | 449 | 1:52 | 174 | 1:94 |
| 2016 | 39,292 | 25 | 1:1572 | 504 | 1:46 | 200 | 1:82 |
| 2017 | 37,364 | 35 | 1:1068 | 503 | 1:42 | 193 | 1:83 |
| Total | 358,331 | 251 | 1:1427 | 4211 | 1:49 | 1516 | 1:100 |
| Type | Birth Prevalence | Birth Prevalence |
|---|---|---|
| Brussels Region | Liège Region | |
| n = 206,984 | n = 151,347 | |
| FS | 1:1522 | 1:2481 |
| FSC | 1:7666 | 1:16,816 |
| FSX | 1:68,995 | - |
| FE | - | 1:151,347 |
| FC | 1:34,497 | 1:21,621 |
| F- | 1:103,492 | 1:21,621 |
| FAS | 1:49 | 1:100 |
| FAC | 1:394 | 1:655 |
| FAE | 1:2275 | 1:2259 |
| FAD * | 1:2587 | 1:7567 |
| FAO * | 1:4600 | 1:75,674 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulbis, B.; Lê, P.-Q.; Ketelslegers, O.; Dresse, M.-F.; Adam, A.-S.; Cotton, F.; Boemer, F.; Bours, V.; Minon, J.-M.; Ferster, A. Neonatal Screening for Sickle Cell Disease in Belgium for More than 20 Years: An Experience for Comprehensive Care Improvement. Int. J. Neonatal Screen. 2018, 4, 37. https://doi.org/10.3390/ijns4040037
Gulbis B, Lê P-Q, Ketelslegers O, Dresse M-F, Adam A-S, Cotton F, Boemer F, Bours V, Minon J-M, Ferster A. Neonatal Screening for Sickle Cell Disease in Belgium for More than 20 Years: An Experience for Comprehensive Care Improvement. International Journal of Neonatal Screening. 2018; 4(4):37. https://doi.org/10.3390/ijns4040037
Chicago/Turabian StyleGulbis, Béatrice, Phu-Quoc Lê, Olivier Ketelslegers, Marie-Françoise Dresse, Anne-Sophie Adam, Frédéric Cotton, François Boemer, Vincent Bours, Jean-Marc Minon, and Alina Ferster. 2018. "Neonatal Screening for Sickle Cell Disease in Belgium for More than 20 Years: An Experience for Comprehensive Care Improvement" International Journal of Neonatal Screening 4, no. 4: 37. https://doi.org/10.3390/ijns4040037
APA StyleGulbis, B., Lê, P.-Q., Ketelslegers, O., Dresse, M.-F., Adam, A.-S., Cotton, F., Boemer, F., Bours, V., Minon, J.-M., & Ferster, A. (2018). Neonatal Screening for Sickle Cell Disease in Belgium for More than 20 Years: An Experience for Comprehensive Care Improvement. International Journal of Neonatal Screening, 4(4), 37. https://doi.org/10.3390/ijns4040037