Long-Term Neurological Outcomes of Adult Patients with Phenylketonuria before and after Newborn Screening in Japan
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NBS | newborn screening |
| PKU | phenylketonuria |
| PAH | phenylalanine hydroxylase |
| Phe | phenylalanine |
| BH4 | tetrahydrobiopterin |
| HPA | hyperphenylalaninemia; |
| IQ | intelligence quotient |
References
- Mitchell, J.J.; Trakadis, Y.J.; Scriver, C.R. Phenylalanine hydroxylase deficiency. Genet. Med. 2011, 13, 697–707. [Google Scholar] [CrossRef]
- Ozalp, I.; Coşkun, T.; Tokatli, A.; Kalkanoğlu, H.S.; Dursun, A.; Tokol, S.; Köksal, G.; Ozgüc, M.; Köse, R. Newborn PKU screening in Turkey: At present and organization for future. Turk. J. Pediatr. 2001, 43, 97–101. [Google Scholar] [PubMed]
- Vockley, J.; Andersson, H.C.; Antshel, K.M.; Braverman, N.E.; Burton, B.K.; Frazier, D.M.; Mitchell, J.; Smith, W.E.; Thompson, B.H.; Berry, S.A. Phenylalanine hydroxylase deficiency: Diagnosis and management guideline. Genet. Med. 2014, 16, 188–200. [Google Scholar] [PubMed] [Green Version]
- Shibata, N.; Hasegawa, Y.; Yamada, K.; Kobayashi, H.; Purevsuren, J.; Yang, Y.; Dung, V.C.; Khanh, N.N.; Verma, I.C.; Bijarnia-Mahay, S.; et al. Diversity in the incidence and spectrum of organic acidemias, fatty acid oxidation disorders, and amino acid disorders in Asian countries: Selective screening vs. expanded newborn screening. Mol. Genet. Metab. Rep. 2018, 16, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K. Long term follow-up of patients with inborn errors of metabolism detected by the newborn screening program in Japan. Southeast Asian J. Trop. Med. Public Health 2003, 34 (Suppl. S3), 19–23. [Google Scholar] [PubMed]
- Lord, J.; Thomason, M.J.; Littlejohns, P.; Chalmers, R.A.; Bain, M.D.; Addison, G.M.; Wilcox, A.H.; Seymour, C.A. Secondary analysis of economic data: A review of cost-benefit studies of neonatal screening for phenylketonuria. J. Epidemiol. Community Health 1999, 53, 179–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhondt, J.L.; Farriaux, J.P.; Sailly, J.C.; Lebrun, T. Economic evaluation of cost-benefit ratio of neonatal screening procedure for phenylketonuria and hypothyroidism. J. Inherit. Metab. Dis. 1991, 14, 633–639. [Google Scholar] [CrossRef]
- Koch, R.; Burton, B.; Hoganson, G.; Peterson, R.; Rhead, W.; Rouse, B.; Scott, R.; Wolff, J.; Stern, A.M.; Guttler, F.; et al. Phenylketonuria in adulthood: A collaborative study. J. Inherit. Metab. Dis. 2002, 25, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Moyle, J.J.; Fox, A.M.; Arthur, M.; Bynevelt, M.; Burnett, J.R. Meta-analysis of neuropsychological symptoms of adolescents and adults with PKU. Neuropsychol. Rev. 2007, 17, 91–101. [Google Scholar] [CrossRef]
- Waisbren, S.E.; Noel, K.; Fahrbach, K.; Cella, C.; Frame, D.; Dorenbaum, A.; Levy, H. Phenylalanine blood levels and clinical outcomes in phenylketonuria: A systematic literature review and meta-analysis. Mol. Genet. Metab. 2007, 92, 63–70. [Google Scholar] [CrossRef]
- Burton, B.K.; Leviton, L.; Vespa, H.; Coon, H.; Longo, N.; Lundy, B.D.; Johnson, M.; Angelino, A.; Hamosh, A.; Bilder, D. A diversified approach for PKU treatment: Routine screening yields high incidence of psychiatric distress in phenylketonuria clinics. Mol. Genet. Metab. 2013, 108, 8–12. [Google Scholar] [CrossRef]
- Antshel, K.M. ADHD, learning, and academic performance in phenylketonuria. Mol. Genet. Metab. 2010, 99 (Suppl. S1), S52–S58. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Ohwada, M.; Kitagawa, T. Long-term follow-up study of patients with phenylketonuria detected by the newborn screening programme in Japan. J. Inherit. Metab. Dis. 2007, 30, 608. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Wada, Y. Outcome of the patients detected by newborn screening in Japan. Acta Paediatr. Jpn. 1988, 30, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Trefz, F.; Maillot, F.; Motzfeldt, K.; Schwarz, M. Adult phenylketonuria outcome and management. Mol. Genet. Metab. 2011, 104, S26–S30. [Google Scholar] [CrossRef] [PubMed]
- Burlina, A.P.; Lachmann, R.H.; Manara, R.; Cazzorla, C.; Celato, A.; van Spronsen, F.J.; Burlina, A. The neurological and psychological phenotype of adult patients with early-treated phenylketonuria: A systematic review. J. Inherit. Metab. Dis. 2019, 42, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Nardecchia, F.; Manti, F.; Chiarotti, F.; Carducci, C.; Carducci, C.; Leuzzi, V. Neurocognitive and neuroimaging outcome of early treated young adult PKU patients: A longitudinal study. Mol. Genet. Metab. 2015, 115, 84–90. [Google Scholar] [CrossRef]
- Van Wegberg, A.M.J.; MacDonald, A.; Ahring, K.; Bélanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Giżewska, M.; et al. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet J. Rare Dis. 2017, 12, 162. [Google Scholar] [CrossRef] [Green Version]
- Hofman, D.L.; Champ, C.L.; Lawton, C.L.; Henderson, M.; Dye, L. A systematic review of cognitive functioning in early treated adults with phenylketonuria. Orphanet J. Rare Dis. 2018, 13, 150. [Google Scholar] [CrossRef]
- Owada, M.; Aoki, K.; Kitagawa, T. Taste preferences and feeding behaviour in children with phenylketonuria on a semisynthetic diet. Eur. J. Pediatr. 2000, 159, 846–850. [Google Scholar] [CrossRef]
- Bilder, D.A.; Kobori, J.A.; Cohen-Pfeffer, J.L.; Johnson, E.M.; Jurecki, E.R.; Grant, M.L. Neuropsychiatric comorbidities in adults with phenylketonuria: A retrospective cohort study. Mol. Genet. Metab. 2017, 121, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.J.; Leuzzi, V. White matter pathology in phenylketonuria. Mol. Genet. Metab. 2010, 99 (Suppl. S1), S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Ilgaz, F.; Pinto, A.; Gökmen-Özel, H.; Rocha, J.C.; van Dam, E.; Ahring, K.; Bélanger-Quintana, A.; Dokoupil, K.; Karabulut, E.; MacDonald, A. Long-Term Growth in Phenylketonuria: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 2070. [Google Scholar] [CrossRef] [Green Version]
- Demirkol, M.; Giżewska, M.; Giovannini, M.; Walter, J. Follow up of phenylketonuria patients. Mol. Genet. Metab. 2011, 104, S31–S39. [Google Scholar] [CrossRef] [PubMed]
- Mazzucato, M.; Visonà Dalla Pozza, L.; Minichiello, C.; Manea, S.; Barbieri, S.; Toto, E.; Vianello, A.; Facchin, P. The Epidemiology of Transition into Adulthood of Rare Diseases Patients: Results from a Population-Based Registry. Int. J. Environ. Res. Public Health 2018, 15, 2212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mütze, U.; Roth, A.; Weigel, J.F.; Beblo, S.; Baerwald, C.G.; Bührdel, P.; Kiess, W. Transition of young adults with phenylketonuria from pediatric to adult care. J. Inherit. Metab. Dis. 2011, 34, 701–709. [Google Scholar] [CrossRef]
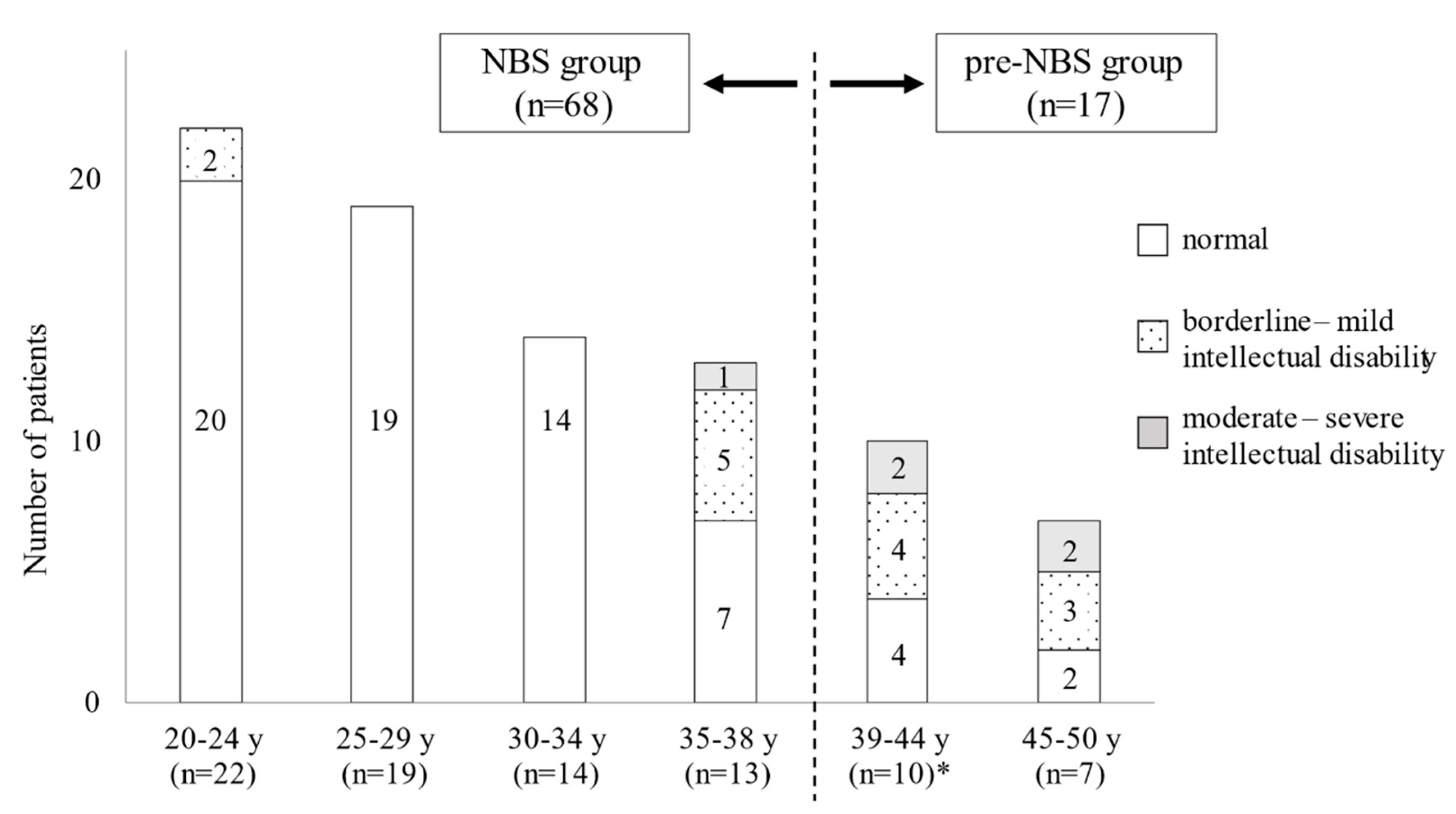
| NBS Group (n = 68) | Pre-NBS Group (n = 17) | ||
|---|---|---|---|
| Sex | |||
| male | 34 | 7 | |
| female | 33 | 10 | |
| unknown | 1 | 0 | |
| Median age (range) [years] | 28.5 (20.5–38.2) | 43.9 (37.7–50.8) | |
| 20–24 | 22 | 0 | |
| 25–29 | 19 | 0 | |
| 30–34 | 14 | 0 | |
| 35–39 | 13 | 3 | |
| 40–44 | 0 | 7 | |
| 45–49 | 0 | 6 | |
| 50- | 0 | 1 | |
| Clinical form | |||
| classic | 54 | 16 | |
| mild HPA | 6 | 0 | |
| BH4-responsive | 2 | 0 | |
| BH4 defect | 0 | 0 | |
| unknown | 6 | 1 | |
| Follow-up department | |||
| pediatrics | 66 | 16 | |
| internal medicine | 1 | 1 | |
| gynecology | 1 | 0 | |
| treatment interruption | 21 | 5 | |
| Reasons for Discontinuation (Number of Patients with the Same Comment) | Reasons for Restarting (Number of Patients with the Same Comment) |
|---|---|
| Economic problems (7) Self-judgment/personal circumstances (7) Unpleasant taste of the special formula (6) Recommendation of the attending physicians (5) Changes in one’s environment (3) | Pregnancy (8) Improvement of the medical subsidy system (4) Appearance of psychiatric abnormalities (3) Spontaneously restarted (2) |
| NBS Group (n = 68) | Pre-NBS Group (n = 17) | |||||||
|---|---|---|---|---|---|---|---|---|
| 20–24 y (n = 22) | 25–29 y (n = 19) | 30–34 y (n = 14) | 35–38 y (n = 13) | Total (%) | 39–44 (n = 10) * | 45–50 y (n = 7) | Total (%) | |
| Psychiatric status | ||||||||
| normal | 20 | 19 | 13 | 12 | 64 (94%) | 7 | 4 | 11 (65%) |
| transient impairment during treatment interruption | 2 | 0 | 1 | 0 | 3 (4%) | 0 | 0 | 0 (0%) |
| psychiatric disability | 0 | 0 | 0 | 1 | 1 (1%) | 3 | 3 | 6 (35%) |
| Physical characteristics | ||||||||
| normal | 19 | 13 | 14 | 11 | 57 (84%) | 5 | 7 | 12 (71%) |
| short stature | 1 | 0 | 0 | 0 | 1 (1%) | 1 | 0 | 1 (6%) |
| obesity | 2 | 3 | 0 | 1 | 6 (9%) | 2 | 0 | 2 (12%) |
| obesity and short stature | 0 | 2 | 0 | 0 | 2 (3%) | 0 | 0 | 0 (0%) |
| leanness | 0 | 1 | 0 | 1 | 2 (3%) | 1 | 0 | 1 (6%) |
| unknown | 0 | 0 | 0 | 0 | 0 (0%) | 1 | 0 | 1 (6%) |
| Education status | ||||||||
| university | 9 | 10 | 3 | 4 | 26 (38%) | 1 | 1 | 2 (12%) |
| technical school | 3 | 5 | 3 | 0 | 11 (16%) | 1 | 0 | 1 (6%) |
| high school # | 10 | 2 | 4 | 2 | 18 (26%) | 2 | 2 | 4 (24%) |
| junior high school | 0 | 0 | 0 | 1 | 1 (1%) | 0 | 0 | 0 (0%) |
| school for individuals with a disability | 0 | 0 | 0 | 1 | 1 (1%) | 3 | 2 | 5 (30%) |
| unknown | 0 | 2 | 4 | 5 | 11 (16%) | 3 | 2 | 5 (30%) |
| Employment status | ||||||||
| attending school | 4 | 1 | 0 | 0 | 5 (7%) | 0 | 0 | 0 (0%) |
| employed | 18 | 17 | 14 | 12 | 61 (90%) | 3 | 4 | 7 (41%) |
| unemployed | 0 | 1 | 0 | 1 | 2 (3%) | 4 | 1 | 5 (30%) |
| living in a house for individuals with a disability | 0 | 0 | 0 | 0 | 0 (0%) | 3 | 2 | 5 (30%) |
| unknown | 0 | 0 | 0 | 0 | 0 (0%) | 0 | 0 | 0 (0%) |
| Marital status | ||||||||
| married | 0 | 4 | 9 | 6 | 19 (28%) | 2 | 0 | 2 (12%) |
| unmarried or divorce | 14 | 8 | 4 | 5 | 31 (46%) | 8 | 5 | 13 (76%) |
| unknown | 8 | 7 | 1 | 2 | 18 (26%) | 0 | 2 | 2 (12%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, K.; Yamaguchi, S.; Yokoyama, K.; Aoki, K.; Taketani, T. Long-Term Neurological Outcomes of Adult Patients with Phenylketonuria before and after Newborn Screening in Japan. Int. J. Neonatal Screen. 2021, 7, 21. https://doi.org/10.3390/ijns7020021
Yamada K, Yamaguchi S, Yokoyama K, Aoki K, Taketani T. Long-Term Neurological Outcomes of Adult Patients with Phenylketonuria before and after Newborn Screening in Japan. International Journal of Neonatal Screening. 2021; 7(2):21. https://doi.org/10.3390/ijns7020021
Chicago/Turabian StyleYamada, Kenji, Seiji Yamaguchi, Kazunori Yokoyama, Kikumaro Aoki, and Takeshi Taketani. 2021. "Long-Term Neurological Outcomes of Adult Patients with Phenylketonuria before and after Newborn Screening in Japan" International Journal of Neonatal Screening 7, no. 2: 21. https://doi.org/10.3390/ijns7020021
APA StyleYamada, K., Yamaguchi, S., Yokoyama, K., Aoki, K., & Taketani, T. (2021). Long-Term Neurological Outcomes of Adult Patients with Phenylketonuria before and after Newborn Screening in Japan. International Journal of Neonatal Screening, 7(2), 21. https://doi.org/10.3390/ijns7020021






