Multiple 17-OHP Cutoff Co-Variates Fail to Improve 21-Hydroxylase Deficiency Screening Accuracy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimens
2.2. Screening Assay
2.3. Statistical Analysis
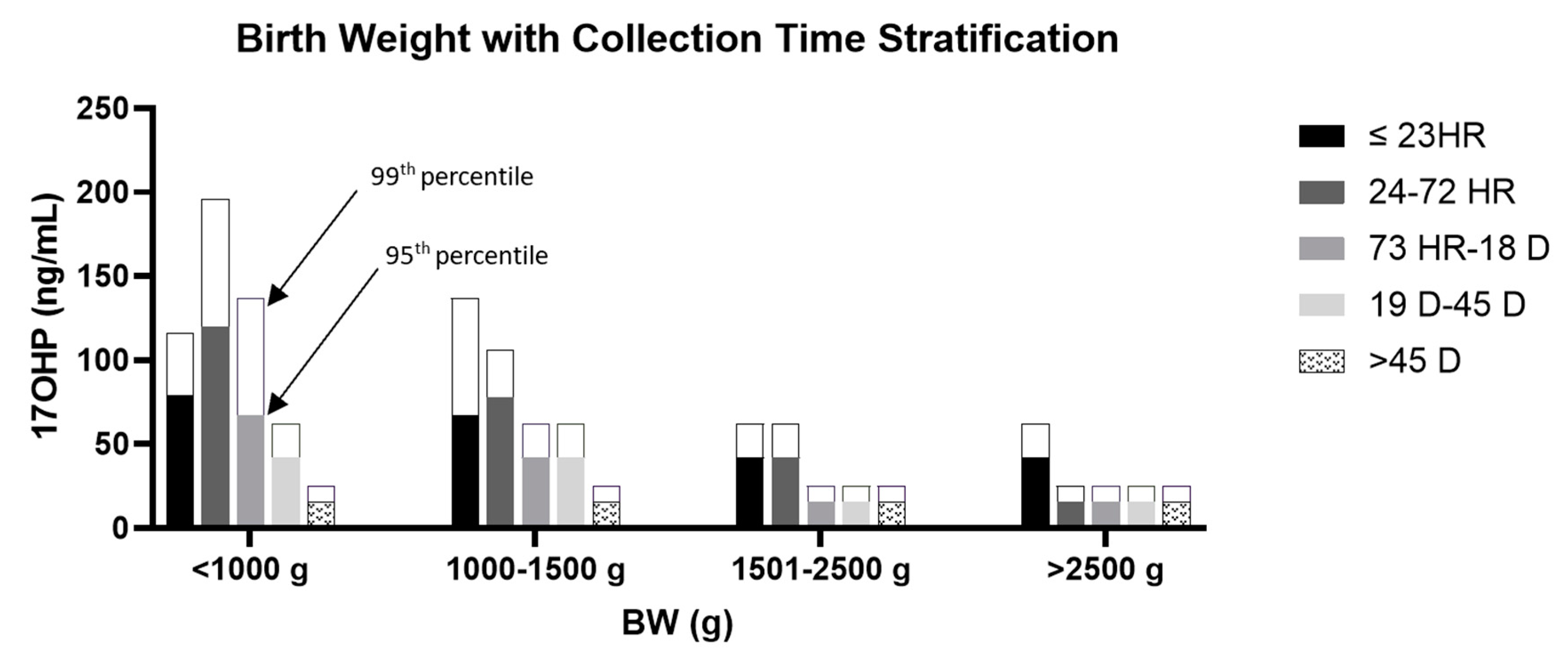
3. Results
3.1. Distribution of 17OHP in the Unaffected Population
3.2. Development and Application of 17OHP Concentration Cutoffs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antal, Z.; Zhou, P. Congenital adrenal hyperplasia: Diagnosis, evaluation, and management. Pediatr. Rev. 2009, 30, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Bialk, E.; Lasarev, M.; Held, P. Wisconsin’s screening algorithm for the identification of newborns with congenital adrenal hyperplasia. Int. J. Neonatal Screen. 2019, 5, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, S.; Spence, D.A.; New, M.I. Newborn screening for congenital adrenal hyperplasia with special reference to screening in Alaska. Ann. N. Y. Acad. Sci. 1985, 458, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Held, P.K.; Bird, I.M.; Heather, N.L. Newborn screening for congenital adrenal hyperplasia: Review of factors affecting screening accuracy. Int. J. Neonatal Screen. 2020, 6, 67. [Google Scholar] [CrossRef]
- Sarafoglou, K.; Banks, K.; Gaviglio, A.; Hietala, A.; McCann, M.; Thomas, W. Comparison of one-tier and two-tier newborn screening metrics for congenital adrenal hyperplasia. Pediatrics 2012, 130, 1261–1268. [Google Scholar] [CrossRef] [Green Version]
- Allen, D.B.; Hoffman, G.; Fitzpatrick, P.; Laessig, R.; Maby, S.; Slyper, A. Improved precision of newborn screening for congenital adrenal hyperplasia using weight-adjusted criteria for 17-hydroxyprogesterone levels. J. Pediatr. 1997, 130, 128–134. [Google Scholar] [CrossRef]
- Olgemoller, B.; Roscher, A.; Lieb, B.; Fingerhut, R. Screening for congenital adrenal hyperplasia: Adjustment of 17-hydroxyprogesterone cut-off values to both age and birth weight markedly improves the predictive value. J. Clin. Endocrinol. Metab. 2003, 88, 5790–5794. [Google Scholar] [CrossRef] [Green Version]
- Sarafoglou, K.; Banks, K.; Kyllo, J.; Pittock, S.; Thomas, W. Cases of congenital adrenal hyperplasia missed by newborn screening in Minnesota. JAMA 2012, 307, 2371–2374. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, G.; Carvalho, D.; de Miranda, M.; Faure, C.; Vallejos, C.; Brito, V.N.; Rodrigues, A.D.S.; Madureira, G.; Mendonca, B.B.; Bachega, T.A. Neonatal 17-hydroxyprogesterone levels adjusted according to age at sample collection and birth weight improve the efficacy of congenital adrenal hyperplasia newborn screening. Clin. Endocrinol. 2017, 86, 480–487. [Google Scholar] [CrossRef] [Green Version]
- van der Kamp, H.J.; Oudshoorn, C.G.M.; Elvers, B.H.; van Baarle, M.; Otten, B.J.; Wit, J.M.; Verkerk, P.H. Cutoff levels of 17-alpha-hydroxyprogesterone in neonatal screening for congenital adrenal hyperplasia should be based on gestational age rather than on birth weight. J. Clin. Endocr. Metab. 2005, 90, 3904–3907. [Google Scholar] [CrossRef]
- Pearce, M.; DeMartino, L.; McMahon, R.; Hamel, R.; Maloney, B.; Stansfield, D.M.; McGrath, E.C.; Occhionero, A.; Gearhart, A.; Caggana, M.; et al. Newborn screening for congenital adrenal hyperplasia in New York State. Mol. Genet. Metab. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Chan, C.; McFann, K.; Taylor, L.; Wright, D.; Zeitler, P.S.; Barker, J.M. Congenital adrenal hyperplasia and the second newborn screen. J. Pediatr. 2013, 163, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Speiser, P.W.; Chawla, R.; Chen, M.; Diaz-Thomas, A.; Finlayson, C.; Rutter, M.M.; Sandberg, D.E.; Shimy, K.; Talib, R.; Cerise, J.; et al. Newborn screening protocols and positive predictive value for congenital adrenal hyperplasia vary across the United States. Int. J. Neonatal Screen. 2020, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Pode-Shakked, N.; Blau, A.; Pode-Shakked, B.; Tiosano, D.; Weintrob, N.; Eyal, O.; Zung, A.; Levy-Khademi, F.; Tenenbaum-Rakover, Y.; Zangen, D.; et al. Combined gestational age and birth weight- adjusted cutoffs for newborn screening of congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 2019, 104, 3172–3180. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.Y.; Park, H.D.; Kim, J.W.; Oh, H.J.; Yang, J.S.; Chang, Y.S.; Park, W.S.; Lee, S.-Y. Steroid profiling for congenital adrenal hyperplasia by tandem mass spectrometry as a second-tier test reduces follow-up burdens in a tertiary care hospital: A retrospective and prospective evaluation. J. Perinat. Med. 2014, 42, 121–127. [Google Scholar] [CrossRef]
- Matern, D.; Tortorelli, S.; Oglesbee, D.; Gavrilov, D.; Rinaldo, P. Reduction of the false-positive rate in newborn screening by implementation of MS/MS-based second-tier tests: The Mayo Clinic experience (2004–2007). J. Inherit. Metab. Dis. 2007, 30, 585–592. [Google Scholar] [CrossRef]
- de Hora, M.R.; Heather, N.L.; Patel, T.; Bresnahan, L.G.; Webster, D.; Hofman, P.L. Measurement of 17-hydroxyprogeterone by LCMSMS improves newborn screening for CAH due to 21-hydroxylase deficiency in New Zealand. Int. J. Neonatal Screen. 2020, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, E.; Liu, A.; Randall, H.; Haslip, C.; Keune, F.; Murray, M.; Longo, N.; Pasquali, M. Use of steroid profiling by UPLC-MS/MS as a second-tier test in newborn screening for congenital adrenal hyperplasia: The Utah experience. Pediatr. Res. 2009, 66, 230–235. [Google Scholar] [CrossRef] [Green Version]
- White, P.C. Update on diagnosis and management of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Curr. Opin. Endocrinol. 2018, 25, 178–184. [Google Scholar]
- Held, P.K.; Bialk, E.R.; Lasarev, M.R.; Allen, D.B. 21-deoxycortisol is a key screening marker for 21-hydroxylase deficiency. J. Pediatr. 2022, 242, 213–219. [Google Scholar] [CrossRef]
- Turcu, A.; Mallappa, A.; Elman, M.; Avila, N.; Marko, J.; Rao, H.; Tsodikov, A.; Auchus, R.J.; Merke, D.P. 11-oxygenated androgens are biomarkers of adrenal volume and testicular adrenal rest tumors in 21- hydroxylase deficiency. J. Clin. Endocrinol. Metab. 2017, 102, 2701–2710. [Google Scholar] [CrossRef] [PubMed]
- Sarafoglou, K.; Lorentz, C.; Otten, N.; Oetting, W.; Grebe, S. Molecular testing in congenital adrenal hyperplasia due to 21 alpha-hydroxylase deficiency in the era of newborn screening. Clin. Genet. 2012, 82, 64–70. [Google Scholar] [CrossRef] [PubMed]
| 17OHP Cutoff at 99th Percentile | |||
| Presence of disease | |||
| 21OHD * | Unaffected† | ||
| Test results | Screened positive | 53 | 355 |
| Screened negative | 2 | 33,927 | |
| Sensitivity | 0.963 | ||
| Specificity | 0.989 | ||
| PPV | 0.130 | ||
| 17OHP cutoff at 95th percentile | |||
| Presence of disease | |||
| 21OHD * | Unaffected† | ||
| Test results | Screened positive | 54 | 1921 |
| Screened negative | 1 | 32,361 | |
| Sensitivity | 0.981 | ||
| Specificity | 0.943 | ||
| PPV | 0.027 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matharu, P.K.; Held, P.K.; Allen, D.B. Multiple 17-OHP Cutoff Co-Variates Fail to Improve 21-Hydroxylase Deficiency Screening Accuracy. Int. J. Neonatal Screen. 2022, 8, 57. https://doi.org/10.3390/ijns8040057
Matharu PK, Held PK, Allen DB. Multiple 17-OHP Cutoff Co-Variates Fail to Improve 21-Hydroxylase Deficiency Screening Accuracy. International Journal of Neonatal Screening. 2022; 8(4):57. https://doi.org/10.3390/ijns8040057
Chicago/Turabian StyleMatharu, Preet K., Patrice K. Held, and David B. Allen. 2022. "Multiple 17-OHP Cutoff Co-Variates Fail to Improve 21-Hydroxylase Deficiency Screening Accuracy" International Journal of Neonatal Screening 8, no. 4: 57. https://doi.org/10.3390/ijns8040057
APA StyleMatharu, P. K., Held, P. K., & Allen, D. B. (2022). Multiple 17-OHP Cutoff Co-Variates Fail to Improve 21-Hydroxylase Deficiency Screening Accuracy. International Journal of Neonatal Screening, 8(4), 57. https://doi.org/10.3390/ijns8040057






