A Cross-Sectional Survey of Pediatric Infectious Disease Physicians’ Approach to Congenital Cytomegalovirus Infection †
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Demographics
3.2. Knowledge of cCMV Symptoms and Diagnosis
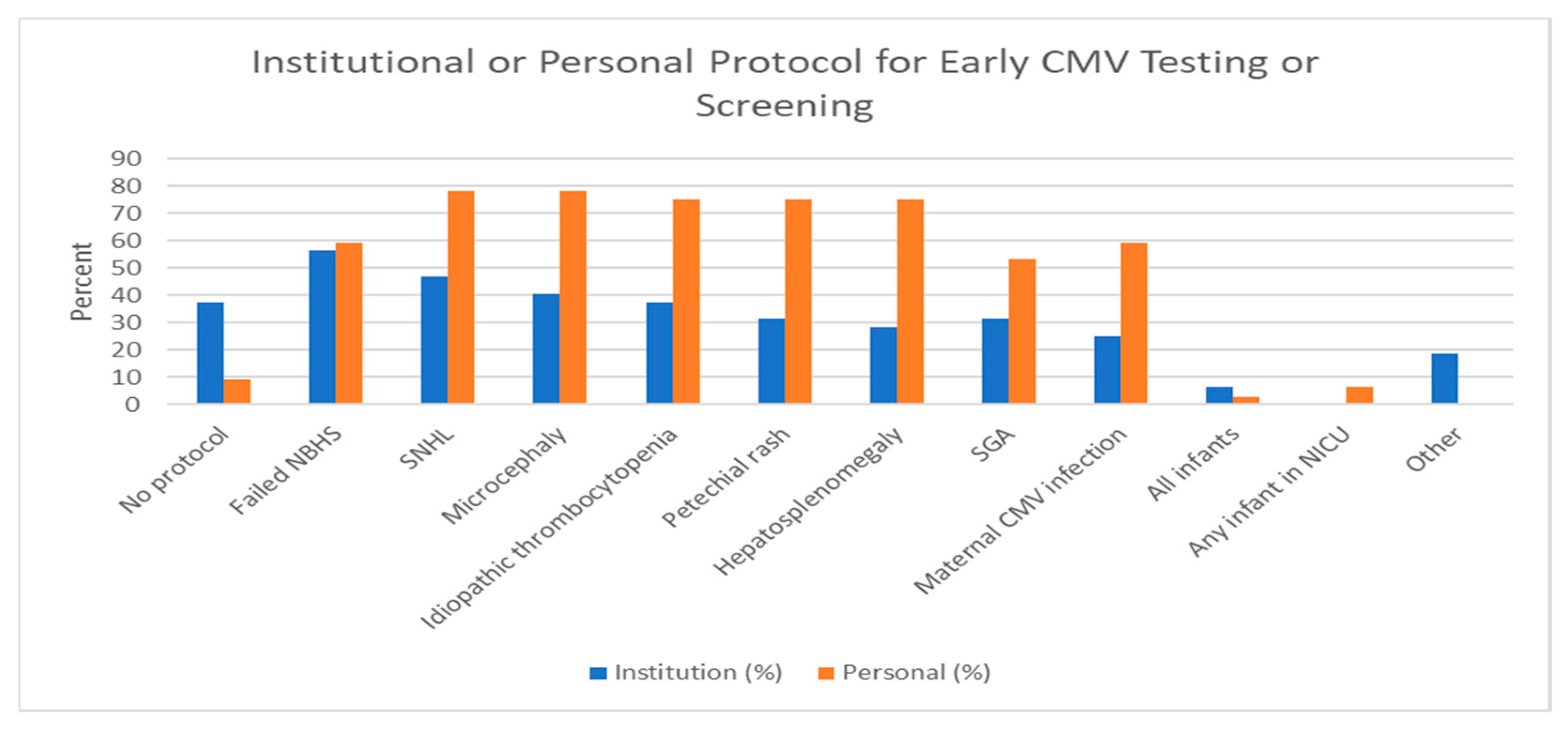
3.3. Screening and Testing at the Institutional and Physician Level
3.4. Evaluation and Treatment of Patients with Confirmed cCMV Infection
4. Discussion
4.1. Knowledge of cCMV Symptoms and Diagnosis
4.2. Screening and Testing at the Institutional and Physician Level
4.3. Evaluation and Treatment of Patients with Confirmed cCMV Infection
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kenneson, A.; Cannon, M.J. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 2007, 17, 253–276. [Google Scholar] [CrossRef]
- Ssentongo, P.; Hehnly, C.; Birungi, P.; Roach, M.A.; Spady, J.; Fronterre, C.; Wang, M.; Murray-Kolb, L.E.; Al-Shaar, L.; Chinchilli, V.M.; et al. Congenital Cytomegalovirus Infection Burden and Epidemiologic Risk Factors in Countries With Universal Screening: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e2120736. [Google Scholar] [CrossRef] [PubMed]
- Lanzieri, T.M.; Dollard, S.C.; Bialek, S.R.; Grosse, S.D. Systematic review of the birth prevalence of congenital cytomegalovirus infection in developing countries. Int. J. Infect. Dis. 2014, 22, 44–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreher, A.M.; Arora, N.; Fowler, K.B.; Novak, Z.; Britt, W.J.; Boppana, S.B.; Ross, S.A. Spectrum of disease and outcome in children with symptomatic congenital cytomegalovirus infection. J. Pediatr. 2014, 164, 855–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goderis, J.; De Leenheer, E.; Smets, K.; Van Hoecke, H.; Keymeulen, A.; Dhooge, I. Hearing loss and congenital CMV infection: A systematic review. Pediatrics 2014, 134, 972–982. [Google Scholar] [CrossRef] [Green Version]
- Rawlinson, W.D.; Boppana, S.B.; Fowler, K.B.; Kimberlin, D.W.; Lazzarotto, T.; Alain, S.; Daly, K.; Doutre, S.; Gibson, L.; Giles, M.L.; et al. Congenital cytomegalovirus infection in pregnancy and the neonate: Consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect. Dis. 2017, 17, e177–e188. [Google Scholar] [CrossRef]
- Luck, S.E.; Wieringa, J.W.; Blazquez-Gamero, D.; Henneke, P.; Schuster, K.; Butler, K.; Capretti, M.G.; Cilleruelo, M.J.; Curtis, N.; Garofoli, F.; et al. Congenital Cytomegalovirus: A European Expert Consensus Statement on Diagnosis and Management. Pediatr. Infect. Dis. J. 2017, 36, 1205–1213. [Google Scholar] [CrossRef]
- Kimberlin, D.W.; Jester, P.M.; Sanchez, P.J.; Ahmed, A.; Arav-Boger, R.; Michaels, M.G.; Ashouri, N.; Englund, J.A.; Estrada, B.; Jacobs, R.F.; et al. Valganciclovir for symptomatic congenital cytomegalovirus disease. N. Engl. J. Med. 2015, 372, 933–943. [Google Scholar] [CrossRef] [Green Version]
- Kimberlin, D.W.; Lin, C.Y.; Sanchez, P.J.; Demmler, G.J.; Dankner, W.; Shelton, M.; Jacobs, R.F.; Vaudry, W.; Pass, R.F.; Kiell, J.M.; et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: A randomized, controlled trial. J. Pediatr. 2003, 143, 16–25. [Google Scholar] [CrossRef]
- Muldoon, K.M.; Armstrong-Heimsoth, A.; Thomas, J. Knowledge of congenital cytomegalovirus (cCMV) among physical and occupational therapists in the United States. PLoS ONE 2017, 12, e0185635. [Google Scholar] [CrossRef] [Green Version]
- Dedhia, K.; Tomlinson, J.; Murray, N.; Park, A. Congenital Cytomegalovirus and Hearing Loss: A Pilot Cross-Sectional Survey of Otologists’ and Pediatric Otolaryngologists’ Knowledge. OTO Open 2019, 3, 2473974X19849874. [Google Scholar] [CrossRef] [Green Version]
- Pesch, M.H.; Muldoon, K.M. Congenital Cytomegalovirus Knowledge, Practices, and Beliefs Among Primary Care Physicians and Newborn Hospitalists. J. Prim. Care Community Health 2022, 13, 21501319221106880. [Google Scholar] [CrossRef]
- Dedhia, K.; Fifer, R.C.; Muldoon, K.M.; Park, A. A Cross-Sectional Survey Evaluating Awareness of Congenital Cytomegalovirus Among Audiologists and Speech-Language Pathologists. Am. J. Audiol. 2021, 30, 145–159. [Google Scholar] [CrossRef]
- Ross, S.A.; Michaels, M.G.; Ahmed, A.; Palmer, A.L.; Sanchez, P.J.; Bernstein, D.I.; Feja, K.; Stewart, A.; Boppana, S.B.; Fowler, K.B. Contribution of Breastfeeding to False-Positive Saliva Polymerase Chain Reaction for Newborn Congenital Cytomegalovirus Screening. J. Infect. Dis. 2018, 217, 1612–1615. [Google Scholar] [CrossRef]
- Puhakka, L.; Lappalainen, M.; Lonnqvist, T.; Niemensivu, R.; Lindahl, P.; Nieminen, T.; Seuri, R.; Nupponen, I.; Pati, S.; Boppana, S.; et al. The Burden of Congenital Cytomegalovirus Infection: A Prospective Cohort Study of 20 000 Infants in Finland. J. Pediatr. Infect. Dis. Soc. 2019, 8, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Gantt, S.; Dionne, F.; Kozak, F.K.; Goshen, O.; Goldfarb, D.M.; Park, A.H.; Boppana, S.B.; Fowler, K. Cost-effectiveness of Universal and Targeted Newborn Screening for Congenital Cytomegalovirus Infection. JAMA Pediatr. 2016, 170, 1173–1180. [Google Scholar] [CrossRef] [Green Version]
- Diener, M.L.; Zick, C.D.; McVicar, S.B.; Boettger, J.; Park, A.H. Outcomes From a Hearing-Targeted Cytomegalovirus Screening Program. Pediatrics 2017, 139, e20160789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haller, T.; Shoup, A.; Park, A.H. Should hearing targeted screening for congenital cytomegalovirus infection Be implemented? Int. J. Pediatr. Otorhinolaryngol. 2020, 134, 110055. [Google Scholar] [CrossRef] [PubMed]
- Suarez, D.; Nielson, C.; McVicar, S.; Sidesinger, M.; Ostrander, B.; O’Brien, E.; Ampofo, K.; Ling, C.; Miner, L.; Park, A. Analysis of an Expanded Targeted Early Cytomegalovirus Testing Program. Otolaryngol. Head Neck Surg. 2023, online ahead of print. [Google Scholar] [CrossRef]
- Keymeulen, A.; De Leenheer, E.; Casaer, A.; Cossey, V.; Herregods, N.; Laroche, S.; Mahieu, L.; Van Mol, C.; Vanhaesebrouck, S.; Walle, C.V.; et al. Cranial ultrasound and MRI: Complementary or not in the diagnostic assessment of children with congenital CMV infection? Eur. J. Pediatr. 2022, 181, 911–920. [Google Scholar] [CrossRef]
- Capretti, M.G.; Lanari, M.; Tani, G.; Ancora, G.; Sciutti, R.; Marsico, C.; Lazzarotto, T.; Gabrielli, L.; Guerra, B.; Corvaglia, L.; et al. Role of cerebral ultrasound and magnetic resonance imaging in newborns with congenital cytomegalovirus infection. Brain Dev. 2014, 36, 203–211. [Google Scholar] [CrossRef]
- Hranilovich, J.A.; Park, A.H.; Knackstedt, E.D.; Ostrander, B.E.; Hedlund, G.L.; Shi, K.; Bale, J.F., Jr. Brain Magnetic Resonance Imaging in Congenital Cytomegalovirus With Failed Newborn Hearing Screen. Pediatr. Neurol. 2020, 110, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Smiljkovic, M.; Renaud, C.; Tapiero, B.; Lamarre, V.; Kakkar, F. Head ultrasound, CT or MRI? The choice of neuroimaging in the assessment of infants with congenital cytomegalovirus infection. BMC Pediatr. 2019, 19, 180. [Google Scholar] [CrossRef] [Green Version]
- Kimberlin, D.W.; Aban, I.; Acosta, E.P. Valganciclovir for Congenital Cytomegalovirus. N. Engl. J. Med. 2015, 372, 2463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilavsky, E.; Shahar-Nissan, K.; Pardo, J.; Attias, J.; Amir, J. Hearing outcome of infants with congenital cytomegalovirus and hearing impairment. Arch. Dis. Child. 2016, 101, 433–438. [Google Scholar] [CrossRef]
- Pasternak, Y.; Ziv, L.; Attias, J.; Amir, J.; Bilavsky, E. Valganciclovir Is Beneficial in Children with Congenital Cytomegalovirus and Isolated Hearing Loss. J. Pediatr. 2018, 199, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.; Long, S.S.; Kimberlin, D.W. Closer to Universal Newborn Screening for Congenital Cytomegalovirus Infection but Far Away from Antiviral Therapy in All Infected Infants. J. Pediatr. 2018, 199, 7–9. [Google Scholar] [CrossRef]
- De Cuyper, E.; Acke, F.; Keymeulen, A.; Dhooge, I. The Effect of (Val)ganciclovir on Hearing in Congenital Cytomegalovirus: A Systematic Review. Laryngoscope 2022, 132, 2241–2250. [Google Scholar] [CrossRef]
- Lackner, A.; Acham, A.; Alborno, T.; Moser, M.; Engele, H.; Raggam, R.B.; Halwachs-Baumann, G.; Kapitan, M.; Walch, C. Effect on hearing of ganciclovir therapy for asymptomatic congenital cytomegalovirus infection: Four to 10 year follow up. J. Laryngol. Otol. 2009, 123, 391–396. [Google Scholar] [CrossRef]
- Lanzieri, T.M.; Chung, W.; Flores, M.; Blum, P.; Caviness, A.C.; Bialek, S.R.; Grosse, S.D.; Miller, J.A.; Demmler-Harrison, G. Hearing Loss in Children With Asymptomatic Congenital Cytomegalovirus Infection. Pediatrics 2017, 139, e20162610. [Google Scholar] [CrossRef] [Green Version]
- Torrecillas, V.; Allen, C.M.; Greene, T.; Park, A.; Chung, W.; Lanzieri, T.M.; Demmler-Harrison, G. Should You Follow the Better-Hearing Ear for Congenital Cytomegalovirus Infection and Isolated Sensorineural Hearing Loss? Otolaryngol. Head Neck Surg. 2020, 162, 114–120. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Pediatric Infectious Disease (n = 32) |
|---|---|
| Age | 49.0 years |
| SD = 10.2 | |
| range: 30–66 | |
| Gender | |
| Female | 15 (46.9%) |
| Male | 17 (53.1%) |
| Race | |
| Asian | 7 (21.9%) |
| White | 26 (81.3%) 1 |
| Years in Practice | |
| 0–5 | 4 (12.5%) |
| 6–10 | 3 (9.4%) |
| 11–15 | 6 (18.8%) |
| >15 | 16 (50%) |
| Still in training | 3 (9.4%) |
| Current Position | |
| PID private practice | 1 (3.1%) |
| PID academic | 28 (90.3%) |
| PID fellow in training | 3 (9.7%) |
| % Children covered by Medicaid insurance in practice 2 | |
| 0–25 | 2 (7.1%) |
| 26–50 | 9 (32.1%) |
| 51–75 | 6 (19.4%) |
| 76–100 | 2 (7.1%) |
| Unsure | 9 (32.1%) |
| Correct Responses | |||
|---|---|---|---|
| Symptom | Selected | Not Selected | Percentage Correct |
| Correct symptoms | |||
| Hearing Loss | 32 | 0 | 100.0% |
| Intellectual disability | 30 | 2 | 93.8% |
| Loss of Vision | 30 | 2 | 93.8% |
| Seizures | 28 | 4 | 87.5% |
| Petechiae | 32 | 0 | 100.0% |
| Incorrect symptoms | |||
| Oral cavity ulcers | 0 | 32 | 100.0% |
| Correct Responses | |||
|---|---|---|---|
| Incidence | Selected | Not Selected | Percentage Correct |
| Approximately 10% of children with asymptomatic cCMV will develop SNHL. | 27 | 5 | 84.4% |
| Approximately 33% of children with symptomatic cCMV will develop SNHL | 12 | 20 | 37.5% |
| Approximately 30% of children with asymptomatic cCMV will develop SNHL. | 4 | 28 | 87.5% |
| Approximately 95% children with symptomatic cCMV will develop SNHL. | 3 | 29 | 90.6% |
| Correct Responses | ||
|---|---|---|
| % Progressive Hearing Loss | Selected | Percentage Correct |
| 5 | 3 | 9.4 |
| 20 | 5 | 15.6 |
| 35 | 7 | 21.9 |
| 50 | 17 | 53.1 |
| Correct Responses | |||
|---|---|---|---|
| Selected | Not Selected | Percentage Correct | |
| Dried blood spot CMV PCR after 3 weeks of age. | 4 | 28 | 12.5% |
| Dried blood spot CMV PCR prior to 3 weeks of age. | 22 | 10 | 31.2% |
| Urine PCR/culture at any age | 0 | 32 | 100.0% |
| Urine PCR/culture prior to 3 weeks of age. | 31 | 1 | 96.9% |
| Saliva PCR/culture at any age | 1 | 31 | 96.9% |
| Saliva PCR/culture prior to 3 weeks of age | 25 | 7 | 21.9% |
| Saliva CMV culture with confirmatory urine PCR or culture at any age. | 0 | 32 | 100.0% |
| Saliva CMV culture with confirmatory urine PCR or culture prior to 3 weeks of age. | 15 | 17 | 46.9% |
| Serologic CMV IgG testing at any age. | 0 | 32 | 100.0% |
| Serologic CMV IgG testing prior to 3 weeks of age. | 0 | 32 | 100.0% |
| Correct Responses | |||
|---|---|---|---|
| Which Test(s) Can Definitively Establish a Diagnosis for cCMV in Children Greater than 3 Weeks of Age? | Selected | Not Selected | Percentage Correct |
| Dried blood spot CMV PCR testing | 20 | 12 | 68.8% |
| Serology for CMV IgG | 0 | 32 | 100.0% |
| Serology for CMV IgM | 2 | 30 | 93.8% |
| Imaging studies including CT and MRI | 7 | 25 | 78.1% |
| Urine PCR/culture for CMV | 0 | 32 | 100.0% |
| Saliva culture for CMV | 0 | 32 | 100.0% |
| Other (eye findings; history of maternal seroconversion and symptomatic infant) | 2 | 30 | 93.8% |
| Correct Responses | |||
|---|---|---|---|
| Transmission Route | Selected | Not Selected | Percentage Correct |
| Kissing | 31 | 1 | 96.9% |
| Changing diapers without hand washing afterwards | 31 | 1 | 96.9% |
| Drinking breast milk | 31 | 1 | 96.9% |
| Receiving a blood transfusion | 31 | 1 | 96.9% |
| Sexual intercourse | 25 | 7 | 78.1% |
| Sharing food | 20 | 12 | 62.5% |
| Responses | |||
|---|---|---|---|
| Neuroimaging | Selected | Not Selected | Percentage |
| Head ultrasound (HUS) for all cases | 11 | 21 | 34.4% |
| MRI brain for all cases | 7 | 25 | 21.9% |
| HUS for all cases followed by brain MRI if an abnormality is found | 22 | 10 | 68.8% |
| HUS except a brain MRI if infant has microcephaly | 7 | 25 | 21.9% |
| Other (positive SNHL and/or abnormal neurologic finding | 1 | 31 | 3.1% |
| Tests Ordered during Valganciclovir Treatment | Selected | Percentage |
|---|---|---|
| CBC with differential | 28 | 87.5% |
| Viral titers | 4 | 12.5% |
| Drug concentration or pharmacokinetic studies | 0 | 0.0% |
| CMP | 18 | 56.3% |
| Drug resistance | 1 | 3.1% |
| Other | 7 | 21.9% |
| Duration of Valganciclovir Administration | Selected | Percentage |
| 6 weeks | 0 | 0.0% |
| 6 months | 29 | 90.6% |
| 9 months | 0 | 0.0% |
| 12 months | 1 | 3.1% |
| Until urine viral titers are undetectable | 0 | 0.0% |
| Other | 2 | 6.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoki, C.; White, M.; Pesch, M.H.; Melvin, A.J.; Park, A.H. A Cross-Sectional Survey of Pediatric Infectious Disease Physicians’ Approach to Congenital Cytomegalovirus Infection. Int. J. Neonatal Screen. 2023, 9, 17. https://doi.org/10.3390/ijns9020017
Hoki C, White M, Pesch MH, Melvin AJ, Park AH. A Cross-Sectional Survey of Pediatric Infectious Disease Physicians’ Approach to Congenital Cytomegalovirus Infection. International Journal of Neonatal Screening. 2023; 9(2):17. https://doi.org/10.3390/ijns9020017
Chicago/Turabian StyleHoki, Chieko, Michelle White, Megan H. Pesch, Ann J. Melvin, and Albert H. Park. 2023. "A Cross-Sectional Survey of Pediatric Infectious Disease Physicians’ Approach to Congenital Cytomegalovirus Infection" International Journal of Neonatal Screening 9, no. 2: 17. https://doi.org/10.3390/ijns9020017
APA StyleHoki, C., White, M., Pesch, M. H., Melvin, A. J., & Park, A. H. (2023). A Cross-Sectional Survey of Pediatric Infectious Disease Physicians’ Approach to Congenital Cytomegalovirus Infection. International Journal of Neonatal Screening, 9(2), 17. https://doi.org/10.3390/ijns9020017





