Abstract
Autism spectrum disorder (ASD) is a neurodevelopmental condition with a prevalence rate of 2.78%, and it is characterized by deficits in sociability and communication and restricted patterns of interests and activities. Remarkably, this psychiatric disorder exhibits a pronounced gender bias, with 80% of children diagnosed with ASD being boys. In this review, we will present advancements in mouse models of ASD and their potential contributions to our understanding of the disorder. We will highlight how initial pre-clinical investigations focused solely on male mice due to the gender bias in ASD and explain why we believe that this approach might have had detrimental consequences regarding our understanding of ASD etiology and pathophysiology. We will highlight the evidence of two sensitive periods during brain development when differential exposure to gonadal hormones may result in sex differences in brain function and behavior: the perinatal period and the pre-pubertal period. Finally, we will suggest neuroinflammation as a feasible biological mechanism that may converge different ASD etiological factors and cellular mechanisms into a brain sexual differentiation context, thus accounting for the gender disparities observed in the disorder.
1. Introduction
Autism spectrum disorder (ASD) is typically diagnosed in toddlers and young children who exhibit social impairment, deficits in communication, and restricted patterns of interests and activities [1]. The spectral nature of the disorder is remarkable, with some individuals being highly functional and presenting only mild, although challenging, symptoms, while others require substantial support and develop no language skills. In recent decades, the incidence of ASD has increased [2], lately showing a prevalence rate of 2.78% [3]. Epidemiological data suggest that ASD results from the interaction of different genes during development, while symptoms and severity largely depend on environmental factors that act upon these genes and alter the trajectory of brain development [4,5,6].
Remarkably, ASD exhibits a strong gender bias, being diagnosed 3.8 times more frequently in boys than in girls [7,8]. This intriguing bias persists even in the context of the increased incidence of ASD observed in later years [3]. Females may be underdiagnosed due to their tendency to camouflage autistic traits [9] and the possibility that ASD manifests differently in females than in males, as proposed by the female autism phenotype theory [10]. These diagnostic challenges may partially explain the male bias, but various lines of evidence suggest that biological factors contribute to female resilience and male vulnerability to ASD.
On one hand, the female protective effect model suggests that females require a greater etiologic load, such as a higher genetic mutation burden, to exhibit ASD symptoms than males [11]. This model of female resilience is supported by the higher risk of autistic impairments observed in family members of ASD females than in those of ASD males [12,13]. Furthermore, women with ASD have a higher burden of copy number variants and autosomal single-nucleotide variants than males with ASD [14].
On the other hand, the extreme male brain theory suggests that males may be more vulnerable to developing ASD because the characteristics of the disorder represent an extreme form of the male pattern, while females are less likely to exhibit autistic impairments due to their inherently higher social skills and empathetic behaviors [15]. This theory is linked to the hypothesis that fetal testosterone and estrogen can influence tendency to express altered social behavior [16,17], although this hypothesis has been challenged [18].
This review will present advances in mouse models of ASD and outline how they can contribute to our understanding of the disorder. Initially, pre-clinical studies focused on male mice, and researchers justified this decision on the sex bias in ASD. However, this bias had profound consequences on translational research, especially in psychiatric disorders [19]. We believe that this approach had detrimental consequences on our understanding of ASD etiology and pathophysiology, as it ignored a biological factor that largely influenced the occurrence of the disorder. When both sexes were included in pre-clinical research into ASD, relevant differences emerged in terms of how male and female subjects responded to the pharmacological or genetic manipulations used to generate the models. Therefore, we will present the animal models of ASD, stressing where sex differences were studied and whether male and female subjects showed differences in the expression of ASD-relevant behaviors.
Next, we will present advancements in our understanding of brain sexual differentiation, focusing on the mechanisms involved and how this process can affect ASD-relevant behaviors. In this context, we will summarize evidence that shows two sensitive periods in brain masculinization: the perinatal and the pre-pubertal periods. Finally, we will propose possible biological mechanisms that may converge different etiological factors and pathophysiological pathways of ASD in a brain sexual differentiation context, thus accounting for sex differences in the disorder. We propose that immune cells and molecules play a central role in this process.
It is worth mentioning that this article is concerned with the effect of biological sex, which is determined genetically in mammals, rather than the effect of gender, which refers to the sexual identity or social role perceived by an individual. Although the role of gender in the development and expression of psychiatric disorders, particularly ASD, remains unknown, this article focuses on discussing animal models of ASD and does not address gender influence.
2. Mouse Models of ASD
2.1. Mouse Models of Psychiatric Disorders
Currently, no animal models have been proven to suffer from known psychiatric diseases, share their causes and symptoms, and respond similarly to treatments validated in humans. Thus, homologous models of psychiatric diseases are rare. Previously, the main utility of animal models in pre-clinical psychiatry was to validate drugs that target psychiatric symptoms. For example, rodents exposed to an open-field environment experience a conflict between their fear-induced thigmotaxis and their desire to explore the new surroundings [20]. A fearful animal would mainly explore the periphery of the field and avoid the center, particularly if it is brightly lit. For many years, this test, which is known as the open-field test, has been utilized to evaluate candidate anxiolytic drugs, demonstrating a high predictive value. Indeed, drugs that increased the exploration of the center of an open field in rodents demonstrated anxiolytic effects when administered to humans, although some exceptions were also observed [21].
Despite the lack of homologous models of psychiatric diseases, recent efforts have allowed the development of valuable animal models that serve as investigative tools [22,23]. Based on the original proposal of Willner [24], animal models of human mental disorders are required to fulfill construct, face, and predictive validity. Although no model completely accounts for all three requirements and other authors have proposed different levels of validity [25], researchers attempt to make their models as valuable as possible to ensure that they can translate their discoveries into our understanding of human disorders.
Construct validity ensures that the same etiological cause of the disease is responsible for the phenotype observed in the animal. To assess construct validity, it is necessary to understand the implicit or explicit hypothesis regarding the disease’s cause, such as the association between specific genes and their products and particular disorders. As a result, researchers have generated numerous genetically modified animals with genetic mutations similar to those found in humans or exposed animals to environmental factors associated with increased prevalence of certain pathologies. Behavioral and pharmacological analyses of these models are crucial in identifying associated phenotypic changes [26]. These analyses allow the comparison of different etiological hypotheses and the exploration of pathophysiological pathways [27].
Animal models are also expected to exhibit face validity, which means that the changes observed in the animal should be comparable to the symptoms seen in human patients. This requirement is based on the assumption that the phenotype observed in the animal arises from the same physiological processes as the human disease. However, this belief is not always accurate and can be difficult to confirm, especially since most psychiatric disorders have complex symptoms that may be caused by various genetic and environmental factors. To address this complexity, researchers have proposed the concept of endophenotype, which is a valuable tool that can advance our understanding of psychiatric disorders [28].
The predictive validity of an animal model is demonstrated when a treatment tested on the animal has comparable effects on humans, as exemplified using benzodiazepines [21]. Occasionally, these effects can be observed without thorough comprehension of the biological processes that underlie them. As a result, some researchers regard predictive models as only partially beneficial and subject to undesirable side effects, particularly when the mechanisms of the disease and treatment are not fully understood.
In summary, for an animal model of a psychiatric disorder to be valuable, it should have construct, face, and predictive validity, or at least some of these factors. The more the model imitates the etiological factors of a disease, manifests the phenotype/symptoms, and precisely forecasts the outcome of suggested treatments, the more advantageous it is to researchers who study the psychiatric disease.
2.2. Tools to Evaluate Face Validity of Mouse Models of ASD and Limitations Related to Sex
Mouse models of ASD are primarily evaluated based on their face validity. Initially, reports referred to three core symptoms of autism for diagnosis [29], while the latest version of the Diagnostic and Statistical Manual of Mental Illnesses (DSM) focuses on two symptoms: persistent deficits in social communication and social interactions across multiple contexts, as well as the expression of restricted and repetitive behaviors, interests, and activities (including hyper- or hypo-reactivity to sensory stimuli) [1]. Importantly, the severity of these symptoms varies highly between individuals with ASD, which is not usually considered in pre-clinical studies. Behavioral tests have been designed based on these diagnostic criteria and divided into two categories to measure these behaviors in rodents: (1) sociability and communication deficits and (2) repetitive and stereotyped behaviors [30,31].
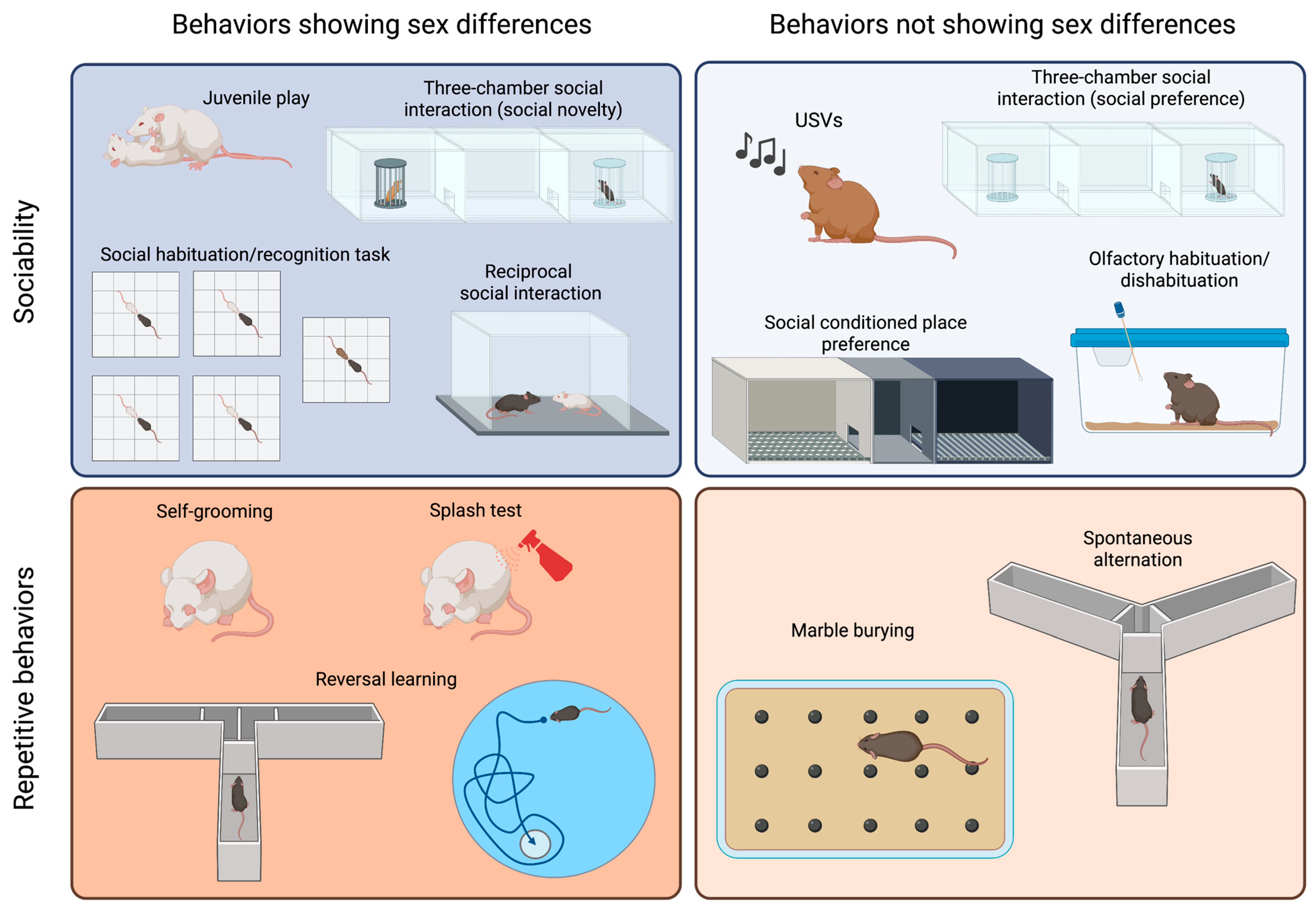
Initially, research into the influence of sex differences on behavior led to unintended consequences and the limiting of behavioral neuroscience research to male subjects because they were believed to be less influenced by the cyclic production of gonadal steroids that result from ovary function. Female behavior was only considered relevant in terms of understanding reproductive behavior, resulting in a poor understanding of sex differences in behavior and creating pre-clinical research biased in favor of male subjects [19]. However, our current understanding suggests that female behavior may not be much different or difficult to study than male behavior [32], and the extended mandate of funding agencies to include subjects of both sexes has opened a new wave of data that shows sex’s influence on a wide variety of behaviors, besides reproductive behaviors. In this section, we will discuss the tests most commonly used to evaluate ASD-relevant behaviors and specify whether there are differences in their applicability or results related to the sex of the animal (Figure 1).
Figure 1.
Sex differences in tests commonly used to assess sociability and repetitive behaviors in ASD models. The tests are categorized based on the core behavior that they measure (sociability or repetitive behaviors) and whether they yield different results in male and female animals. Created with BioRender.com.
2.2.1. Tests to Evaluate Sociability and Communication Deficits in Mice
The species Mus musculus is known to be highly social, exhibiting reciprocal social interactions, communal nesting, sexual and parenting behaviors, territorial scent marking, and aggressive behaviors [33,34,35,36]. Many social assays have been reported in the field of behavioral neuroscience. However, in this article, we will specifically discuss the tests commonly used to evaluate social impairments in mouse models of ASD.
It is probable that the most popular test to assess sociability is the three-chamber social interaction test, which has been chosen by numerous scientists because it allows for automatization of measurements and can be easily standardized [37,38]. In this test, animals are initially allowed to explore a cage divided into three compartments. A stimulus animal is then placed in one of the lateral compartments, known as the “social side”, and either the time that the test mouse spends exploring the social stimulus or the time spent in the social chamber is measured. Different modifications to this test have been proposed, including changing the size of the cage and placing an object into the other lateral compartment [39,40]. Usually, a young mouse of the same sex as the test mouse is employed as a stimulus. Although strain differences have been reported, most mice will prefer the social side and, therefore, spend more time in that compartment than in that containing an inanimate object [37,39,41]. This test has the advantage of placing the stimulus mouse in a cage, which is unreachable by the test mouse, and it can be performed with aggressive animals, with many male adult mice being aggressive. In addition, this test is routinely used to measure social memory, adding a third phase in which a novel stimulus replaces the object, and the test mouse then shows a preference for such novelty. This test has been used to assess sociability in both males and females, and both sexes show similar levels of exploration of the young and same-sex social stimulus [42,43,44] (Figure 1, right panel). However, social novelty recognition is influenced by the sex and strain of the animal [44] (Figure 1, left panel). Additionally, this test can be employed to assess sex preference by presenting a female and a male adult mouse. Sex preference is also influenced by the sex and the strain of the animal [44].
The reciprocal social interaction test is certainly more ethologically relevant, as it allows the animals to freely interact with each other [31]. However, it is also more time consuming, as videos should be scored manually (although automated measurement has been achieved using machine learning approaches [45]). Besides the advantage of allowing the characterization of the mode of social engagement between the mice, a main concern is how animals should be paired, as the partner may influence the outcome of the test [46,47]. Moreover, in strains such as CF1, CBA or CD1, which have been reported to exhibit high levels of aggressive behavior [48], this test cannot be applied to adult males, as their aggression may overshadow any potential differences in sociability.
A similar paradigm can be used to explore juvenile play, which is a social behavior typical of young animals. Although previous work on rats showed that females played less than males (reviewed in [49]), reports on mice are heterogenous. C57BL/6J (B6) and CD1 females solicit play activities more often than males [36,50], while outbred CF1 males performed more of these behaviors than females [43] (Figure 1, left panel). An evaluation of juvenile play in the four-core genotype (FCG) model in B6 background showed that XY males solicited play activities more often than any other group, suggesting that both gonadal hormones and sex chromosomes are relevant to the expression of this behavior [36].
In addition to performing a social novelty trial in the three-chamber social interaction test, social memory can be evaluated via a social habituation/recognition task [51]. In this test, animals are sequentially exposed to a stimulus mouse for a short period, showing habituation, and then exposed to a novel animal to evaluate their response to novelty. As for free social interaction, when aggressive animals are being assessed, stimuli can be presented in a small cage to avoid attacks.
Social conditioned place preference is a useful tool for the evaluation of the rewarding nature of social interactions in young mice [52,53]. However, some strains (e.g., BALB/c) do not display social conditioning. This test shows no sex differences when performed with young animals [52]. However, female and male adult mice exhibit a differential response to social conditioning that depends on previous social conditions, such as isolation versus group housing [53].
Olfactory habituation/dishabituation to social odors is a valuable tool for the assessment of the response to a social stimulus, as it lacks the confounding effect of a second mouse with its own sociability levels. Mice tend to sniff a novel odor and then reduce their exploration as they acclimatize to the stimulus [54]. A dishabituation effect is observed when a different odor is introduced, and animals reinstate a high level of sniffing. Using social odors, it is possible to evaluate whether animals can discriminate between the same and different social odors and assess whether these odors are more salient to some animals than others [55]. No sex differences were observed in response to non-social odors [56]. Females, however, are more sensitive than male to social odors [57], possibly due to sex differences in the development of the olfactory system [58].
Although it is not yet well understood how mice communicate, ultrasonic vocalizations (USVs) appear to contribute to the communication of information and social bonding [59,60,61,62,63,64]. This observation is especially relevant to pups, and the analysis of ultrasonic vocalizations in pups separated from their dam and nest is the gold standard method for the measurement of alterations in sociability in newborns [61,64]. Pups of different strains perform different numbers of USVs, and each strain seems to have a unique repertoire of syllables [59]. In young, adolescent, and adult mice, both males and females vocalize, but the frequency and quality of the vocalizations depend on the eliciting stimulus and strain [60,65].
2.2.2. Tests to Evaluate Repetitive and Stereotyped Behaviors in Mice
Mice show spontaneous motor stereotypies, including self-grooming and burying, which can develop into repetitive behaviors if they persist for prolonged periods [66]. Self-grooming is an innate behavior involved in hygiene maintenance and thermoregulation, which can be easily assessed in a home cage or novel environment [40,43,55,67,68]. Adult male mice have been reported to spend more time self-grooming than females [43]. The splash test is an alternative method used to elicit self-grooming behavior, where a sucrose solution is splashed on the back of the animal, and the sweetness of the solution sustains the grooming behavior [69]. The time spent grooming after the splash can be affected by the sex of the animal, although such an effect depends on the mouse strain being studied [70].
In the marble burying test, animals are exposed to an environment in which marble balls have been placed on top of a thick floor of bedding, which elicits the burying behavior in most mouse strains [71,72]. The test involves quantifying the marbles buried at different times, typically lasting 20 min [71]. Although most reports on marble burying behavior only involve males [72], it has been shown that the estrous cycle alters the burying response in rats [73].
Spontaneous alternation in the Y maze or T maze can also be evaluated in an attempt to measure repetitive behaviors because mice typically alternate at levels significantly above chance, indicating their willingness to explore novel environments [74,75]. These tests do not require training, though animals need to be active and explorative. However, an important confounder of these tests is that they depend on spatial working memory [76]. Male and female mice similarly alternate in the Y maze [43].
Perseverative behaviors are also relatively common in mice and can be evaluated by measuring the flexibility of a mouse in terms of switching from a learned habit to a new habit. These reversal learning tasks are usually evaluated in mazes, such as the T-maze, Morris water maze, or Clock maze [37,77]. These tests require a substantial amount of training, and they are time consuming and cannot be used in a short age period (e.g., adolescence), although some attempts have been made to develop shorter protocols [78]. Using the FCG model, it was shown that the sex–chromosome complement affects learning of a reversed task, with XY animals showing more perseverative errors [79].
The insistence on exploring a known object, subject, or area over a novel example can be interpreted as analogous to the restriction in interests or insistence on sameness observed in human subjects with ASD. To explore this observation, mice have been evaluated via the novel object recognition task [55], the social habituation/recognition task [51], or the nose poking in a hole board task [80]. Although males have mostly been evaluated in these tests, sex differences have been observed, with females exhibiting better performance when objects were similar in a novel object recognition test [81].
Hypo- and hyper-reactivity to sensory stimuli can be readily evaluated in mice. Acoustic startle, air puff startle, tail flick, and hot plate can be used to evaluate adult animals. In addition, the development of sensory capacity and its response can be evaluated during the post-natal period [43,82]. Males show a stronger startle reactivity than females [83], and some sex differences in the nociceptive response have been reported [84].
2.2.3. Tests for the Evaluation of Associated Symptoms
In a subset of individuals with ASD, there are associated symptoms related to other psychiatric disorders that exhibit high comorbidity with ASD, such as anxiety and depression. Various tests can be conducted to evaluate these behaviors, many of which show sex differences (reviewed in this issue, [85]). Additionally, ASD individuals may experience seizures, intellectual disability, sleep disruption, and gastrointestinal distress. Analogous phenotypes can be assessed in mice [86]. Seizures can be observed directly or recorded via electroencephalography (EEG). Different memory tasks that measure spatial learning (e.g., Morris water maze), contextual and cued fear conditioning, shock avoidance, object recognition, and operant tasks can be used to evaluate cognitive abilities. Running wheels and home cage monitoring systems can be used to evaluate sleep and circadian activity. Many of these parameters exhibit sex differences that should be considered when evaluating mouse models of ASD.
Evaluating associated behaviors can help to strengthen the phenotypes that correspond to the core symptom, though they can also identify potential confounders or artifacts. For instance, strong anxiety- or depression-related behaviors may lead to low exploration of social stimuli, rendering social interaction data meaningless. The same is true for memory deficits regarding social habituation or odor hyposensitivity and the observation of affected olfactory habituation or pup USVs. Deciding which phenotypes are relevant to associated symptoms and which phenotypes are artifacts that can confound the interpretation of tests related to diagnostic symptoms presents an internal contradiction that needs to be addressed on a case-by-case basis. Furthermore, these confounders may impact one sex more than the other, thus contributing to the sex bias observed in the model.
Attention-deficit/hyperactivity disorder (ADHD) is frequently observed as a comorbid condition with ASD [87]. However, there have been prior few studies that examined the occurrence of ADHD phenotypes in rodent models of ASD. ADHD primarily affects attention, learning, hyperactivity–impulsivity, and aggressiveness [1]. Various behavioral tests can be employed to assess behaviors associated with these symptoms [88,89]. Deficits in learning and memory can be evaluated using the Barnes maze or the novel object recognition task. Hyperactivity can be measured through locomotion assessments in the open-field test. Impulsivity and attention deficits can be evaluated in tests such as the spontaneous Y maze alternation test or the continuous performance test. Aggressiveness is typically assessed using the resident–intruder test. Interestingly, animals that lack the integrin CD103 exhibit both ASD- and ADHD-related behaviors, and their phenotypes also exhibit sex specificity [90]. Investigating ADHD-related behaviors in animal models of ASD can contribute to our understanding of the biological connections between these neurodevelopmental disorders.
2.2.4. Non-Behavioral Associated Symptoms
Mouse models of ASD recapitulate other symptoms observed in individuals with ASD, which may result from etiological factors and pathophysiological pathways and are worth investigating.
Human studies have shown the presence of activated glia, neuroinflammation, and expression of pro-inflammatory cytokines in the brains of individuals diagnosed with ASD [91,92], as well as elevated levels of pro-inflammatory cytokines, both basally and in response to an inflammatory stimulus, in the plasma of ASD patients [93]. Alterations in glial function, neuroinflammation, and an altered response to inflammatory stimuli have also been reported in mouse models of ASD [67,82]. In addition, immunological dysfunction, such as T cell dysfunction, autoantibody production, and augmentation of pro-inflammatory cytokines, has been proposed in the pathogenesis of ASD ([93,94,95,96], reviewed in [97]). Investigating this in topic animal models has shown that cytokines can participate in the post-natal programming of adult sociability, and they can also modulate this behavior in the adult brain [40,42,67,82]. Also, mouse models of ASD have been generated after pre- or neo-natal exposure to inflammatory stimuli (e.g., PolyI:C, LPS or virus) [77,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]. Interestingly, sex differences in inflammatory responses and glial development and function have been reported in both humans and animals [113].
The autistic brain is characterized by hyperconnectivity in local circuits and hypoconnectivity between brain regions [114]. This observation is consistent with ASD being diagnosed before the age of 5, when synaptogenesis is most active in humans, a process that is next followed by active synaptic pruning and elimination [115]. Indeed, autistic brains show increased spine density in the apical dendrites of cortical pyramidal neurons [116], and many genes identified as providing susceptibility to ASD code for synaptic proteins or affect the morphogenesis of dendritic spines (reviewed in [114,117]). Alterations in synaptic function and brain activity have been studied in some mouse models of ASD to understand the relevance of these findings [117,118,119]. Additionally, many genetic models of ASD are built on the alteration of proteins involved in synaptic function, including neuroligins [120,121,122,123,124,125,126], neurexins [127], and shank proteins [128,129,130,131,132,133,134,135,136]. As discussed below, differences in dendritic growth and synaptic formation represent a known mechanism of brain sexual differentiation.
2.3. Sex Differences in Mouse Models of ASD
Animal studies on ASD have traditionally favored male animals due to its higher incidence in boys, which has led to a lack of evidence on the impact of sex on these models, and we believe that this problem may have delayed discovery in ASD. However, recent research has identified sex differences in ASD models, shedding light on possible mechanisms involved in the etiology and pathophysiology of the disorder. In Table 1, we provide a summary of the sex differences in sociability and repetitive behaviors observed in the most studied mouse models of ASD. We have included specific notations to indicate the behavioral phenotypes observed in males and females when both sexes were analyzed, and the effect of sex was considered (violet was the male symbol and was the orange female symbol). For studies in which results were obtained from both male and female subjects, but the sex effect was not reported, we used black male and female symbols. It is important to note that when only male behavioral phenotypes are reported in a model, it indicates that we did not come across any reports that specifically addressed females in those studies.
Among the most extensively studied pharmacological and environmental models of ASD are those generated via pre-natal exposure to valproic acid (VPA) or maternal immune activation (MIA). Various doses of VPA can be administered to animals at different gestational ages, resulting in ASD-related behaviors (as reviewed in [137]). While most studies have used only male animals, evidence shows that VPA affects ASD-related phenotypes in males, but does not do so in females [42,138]. For example, male mice exposed to 600 mg/kg VPA at gestational day (GD) 12.5 displayed reduced sociability in the three-chamber test, while female social interaction was not affected [42]. However, VPA affects female mice, as they show signs of neuroinflammation during the post-natal period [82] and in adulthood [42]. Remarkably, repetitive behaviors were not assessed in females, and, hence, evidence of the sex-specific effect of VPA on behavior is lacking and warrants further investigation.
MIA models are generated by challenging the maternal immune system using an inflammatory stimulus. Commonly used inflammatory stimuli are the polyinosinic–polycytidylic acid (PolyI:C) that mimics viral infections and bacterial lipolysaccharides (LPS) that elicit an inflammatory response similar to the one triggered by a bacterial infection. When PolyI:C is administered at GD12, there is a consistent effect on sociability (reviewed in [139]). Although pre-natal PolyI:C exposure can affect social behavior in both male and female mice [107], some sex-specific differences can be observed that may depend on the gestational age at which the stimulus was administered, the strain of the mouse, and the dose and type of PolyI:C [139,140]. Similarly, while male mice pre-natally exposed to LPS show reduced sociability, female social behavior is unaffected [109,110,141]. Similar to the VPA model, LPS exposure leads to increased self-grooming in males, though this behavior has not been evaluated in females. When the human influenza virus is injected into pregnant dams at GD9.5 to elicit an inflammatory response, their adult offspring of both sexes exhibit reduced social interaction [98].
A neonatal inflammatory challenge also results in long-lasting effects on sociability, though the extent of the effect and occurrence of sex differences depends on factors such as mouse strain, drug dose, and age at administration [140,142,143,144,145].
Propionic acid (PPA) is a gut metabolite that can elicit neuroinflammatory responses [146,147]. It has been shown that intracerebroventricular (icv), subcutaneous (sc), or intraperitoneal (ip) administration of PPA can elicit behavioral alterations related to ASD, such as reduced social interactions and repetitive patterns of behavior [146,147,148,149]. Unfortunately, all studies on PPA have only used male subjects. However, maternal administration of PPA does not result in ASD-related behaviors in female and male offspring [150].
Male mice pre-natally exposed to a monoclonal antibody against contactin-associated protein-like 2 (Caspr2) show reduced sociability, increased repetitive behavior (marble burying), and inflexibility in learning [77]. However, the ASD-related behavioral phenotype of this model was not replicated when it was combined with the FCG model [104].
Several genetic models of ASD have been proposed. Many of them are constructed based on the notion that ASD results from synaptic alterations, and, thus, key synaptic proteins, such as neuroligins 1, 3, and 4; neurexin 1α; and shank proteins, have been targeted [120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136]. Other targeted molecules are peptides involved in social responses, such as oxytocin (OT) and vasopressin (AVP), and their receptors [151,152,153,154,155]. Others models are generated by replicating genetic alterations observed in human subjects with ASD or related disorders [156,157,158,159,160,161,162,163,164,165,166,167,168]. Finally, some mouse inbred strains, such as BTBR, Balb/c, and C58, exhibit reduced sociability and increased repetitive behaviors and are, thus, proposed to be studied as ASD models [39,59,60,166,169,170,171,172,173,174,175,176,177,178,179,180,181]. Unfortunately, many of these models have only been studied using male subjects, while the role of sex has often not been specifically analyzed. Although most ASD genetic models studied to date have shown no sex differences, some interesting exceptions can be observed in Table 1, such as the more evident expression of Pten haplosufficiency sociability and repetitive behaviors in males than in females [161,162]. As shown in Table 1, a more systematic characterization of these models, including the inclusion of female subjects, is necessary to identify potential sex differences in genetic rodent models of ASD.

Table 1.
Sex differences in sociability-associated and repetitive behaviors in mouse models of ASD: A review of pharmacological models, genetic models, and inbred strains. This table reviews sociability-associated alterations and repetitive behaviors observed in the most commonly used pharmacological and genetic models of ASD, as well as in specific inbred strains. When data on the sex effect on behavior are available, differences are specified by describing different outcomes in each sex (♂ or ♀).
Table 1.
Sex differences in sociability-associated and repetitive behaviors in mouse models of ASD: A review of pharmacological models, genetic models, and inbred strains. This table reviews sociability-associated alterations and repetitive behaviors observed in the most commonly used pharmacological and genetic models of ASD, as well as in specific inbred strains. When data on the sex effect on behavior are available, differences are specified by describing different outcomes in each sex (♂ or ♀).
| Pharmacological Animal Models | Sociability-Associated Behaviors | Repetitive Behaviors | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Tests | Results | Test | Results | ||||
| VPA Valproic acid maternal injection | rSI 3ch-SI 3ch-SN JSP SM | ↓ Social interaction ↓ Juvenile social play ↓ Social novelty preference | ♂ | SG MB | ↑ Self-grooming ↑ Marble burying | ♂ | [42,55,138,182,183,184,185,186] |
| = Social preference | ♀ | ND | ♀ | ||||
| MIA Maternal immune activation: polyinosinic-polycytidylic acid (PolyI:C) via maternal injection at GD12.5 or 3 injections (3×) at GD10.5–12.5–14.5 | 3ch-SI 3ch-SN rSI SM USVs | ↓ Social interaction ↓/= Social novelty preference (GD12.5/3 × GD10.5–12.5–14.5) ↓ USVs (young and adult) | ♂ | MB SG sT-maze | ↑ Self-grooming ↑ Marble burying ↓ Spontaneous alternation | ♂ | [98,99,100,102,105,106,107,108,112,140] |
| ↓/= Social interaction (GD12.5/3 × GD10.5–12.5–14.5) = Social novelty preference (3 × GD10.5–12.5–14.5) ↑ USVs (3 × GD10.5–12.5–14.5) | ♀ | ↑/= Self-grooming (GD12.5/3 × GD10.5–12.5–14.5) ↑ Marble burying ↓ Spontaneous alteration | ♀ | ||||
| Bacterial lipopolysaccharides (LPS) via maternal injection at GD17 | rSI JSP | ↓ Social interaction ↓ Juvenile social play | ♂ | HBT | = Hole-poke frequency ↑ Self-grooming | ♂ | [109,110,141] |
| ↓ Social interaction = Juvenile social play | ♀ | ND | ♀ | ||||
| Human influenza virus via maternal injection at GD9.5 | rSI | ↓ Social interaction | ♂♀ | ND | [98] | ||
| Neonatal LPS LPS injection at different post-natal ages | rSI SM USVs | ↓/= Social interaction ↓ USVs (pups and young) | ♂ | SG | ↑ Self-grooming (in open field) | ♂ | [140,142,143,144,145] |
| ↓/= Social interaction = USVs (pups) ↓ Social novelty preference | ♀ | = Self-grooming (in open field) | ♀ | ||||
| PPA Propionic acid (PPA) via icv, sc, or ip injection or pre-natal maternal injection | rSI JSP 3ch-SI | ↓/= Social interaction = Juvenile social play | ♂ | rT-maze | ↓ Reversal learning | ♂ | [146,147,148,149,150] |
| = Social interaction = Juvenile social play | ♀ | ND | ♀ | ||||
| C6 mice In utero exposure to a maternal antibody reactive to contactin-associated protein-like 2 (Caspr2). | 3ch-SI | =/↓ Social interaction | ♂ | SG MB Clock | = Self-grooming =/↑ Marble burying ↓ Reversal learning | ♂ | [77,104] |
| = Social interaction | ♀ | = Self-grooming = Marble burying | ♀ | ||||
| GENETIC ANIMAL MODELS | Test | Results | Test | Results | Ref. | ||
| Nlgn4 Null mutation of the murine ortholog of the human Nlgn4 (neuroligin-4) gene | 3ch-SI 3ch-SN rSI SM USVs Nesting | ↓ Social interaction ↓/= Social novelty preference ↓ USVs ↓ Nesting behavior | ♂ | SG MB | = Self-grooming = Marble burying ↑ Circling episodes (spontaneous) | ♂ | [120,121] |
| ↓ Social interaction ↓ Social novelty preference ↓ USVs = Nesting behavior (tendency) | ♀ | ↑ Self-grooming = Marble burying ↑ Circling episodes (spontaneous) | ♀ | ||||
| Nlgn3 Homozygous mutation of humanized R451C mutation of the Nlgn3 (neuroligin-3) gene | JSP 3ch-SI 3ch-SN rSI SM | ↓ Juvenile social play ↓/= Social interaction = Social novelty preference | ♂ | SG | = Self-grooming | ♂ | [122,123] |
| Null mutation in the murine ortholog of the human Nlgn3 gene | 3ch-SI 3ch-SN rSI SM | = Social interaction ↓ Social novelty preference ↓ USVs | ♂ | ND | [124,125] | ||
| Neurexin 1α Null mutation in the murine neurexin 1α gene | 3ch-SI 3ch-SN SM Nesting | = Social interaction = Social novelty preference ↓ Nesting behavior | ♂♀ | SG | ↑ Self-grooming | ♂♀ | [127] |
| Nlgn1 Null mutation in the murine ortholog of the human Nlgn1 (neuroligin-1) gene | 3ch-SI 3ch-SN rSI SM Nesting Olfactory | ↓/= Social interaction = Social novelty preference = Social olfaction ↓ Nesting behavior | ♂♀ | SG MB | ↑ Self-grooming = Marble burying | ♂♀ | [126] |
| Pten Conditional null mutation of the mouse ortholog of the human pten gene, which is inactivated in neurons of the cortex and hippocampus | 3ch-SI 3ch-SN rSI SM Nesting USVs | ↓ Social interaction ↓ Social novelty preference ↓ Social memory ↓ Nesting behavior = USVs | ♂♀ | MB HBT | ↓ Marble burying ↓ Hole-poke frequency | ♂♀ | [156,157] |
| Haploinsufficent mutant line in which exon 5, which encodes the core catalytic phosphatase domain, is deleted | 3ch-SI 3ch-SN SM | = Social interaction ↓ Social novelty preference | ♂ | MB | ↑ Marble burying | ♂ | [161,162] |
| ↓/= Social interaction = Social novelty preference | ♀ | = Marble burying | ♀ | ||||
| En2 Null mutation in the murine ortholog of the human En2 (engrailed 2) gene | JSP rSI | ↓ Juvenile social play =/↓ Social interaction | ♂ | SG | ↑ Self-grooming | ♂ | [166,187] |
| ↓ Juvenile social play =/↓ Social interaction | ♀ | ↑ Self-grooming | ♀ | ||||
| 15q11–13 Paternal duplication of the genomic region on mouse chromosome 7, which corresponds to the human genomic region 15q11-13, which is observed to be maternally duplicated in some cases of ASD | 3ch-SI 3ch-SN USVs SM | ↓ Social interaction ↓ Social novelty preference ↓ USVs | ♂ | MB | ↓ Marble burying | ♂ | [163,164] |
| 17p11.2 Duplication in the genomic region of murine chromosome 11, which is homologous to the human genomic region 17p11.2 | 3ch-SI 3ch-SN SM Nesting | ↓ Social interaction = Social novelty preference ↓ Nesting behavior | ♂ | ND | [165] | ||
| Slc6a4 Null mutation in the murine ortholog of the human serotonin transporter (Slc6a4) gene | 3ch-SI SM | ↓ Social interaction = Social novelty preference | ♂ | ND | [166] | ||
| ↓ Social interaction = Social novelty preference | ♀ | ||||||
| OT Null mutation in the murine oxytocin gene | 3ch-SI SM Olfactory | ↓/= Social interaction = Social novelty preference ↓ Social memory | ♂ | SG | ND | [151,152] | |
| = Social olfaction | ♂♀ | = Self-grooming | ♂♀ | ||||
| V1aR and V1bR Null mutations in the murine vasopressin receptors (Avpr1a or Avpr1b genes) | rSI SM USVs | ↓ Social interaction ↓ Social memory ↓ USVs | ♂ | ND | [153,154,155] | ||
| ↓ USVs | ♀ | ||||||
| Mecp2 Conditional mutation in methyl-CpG-binding protein 2 gene | rSI SM Nesting Olfactory | ↓ Social interaction ↓/= Social memory ↓ Nesting behavior = Social olfaction | ♂ | SG | = Self-grooming ↑ Forepaw stereotypical movements | ♂ | [167,168] |
| Fmr1 Null mutant mouse with a targeted mutation in the Fmr1 gene (phenotype is dependent on the genetic background) | 3ch-SI SM | ↓ Social interaction (FVB/129) = Social novelty preference | ♂ | SG MB | ↑ Self-grooming ↑/↓ Marble burying | ♂ | [158,159,160] |
| Shank1 Null mutation in the murine shank1 gene | 3ch-SI rSI Olfactory | ↓/= Social interaction = Social olfaction | ♂♀ | SG | = Self-grooming | ♂♀ | [128] |
| Shank2 Null mutation in the murine shank2 gene | 3ch-SI 3ch-SN SM USVs Olfactory Nesting | =/↓ Social interaction ↓ Social novelty preference ↓ USVs (adult) = Social olfaction ↓ Nesting behavior | ♂ | SG | = Self-grooming ↑ Repetitive jumping | ♂ | [129,130] |
| = Social interaction ↓ Social novelty preference = Social olfaction | ♀ | ↑/= Self-grooming ↑ Repetitive jumping | ♀ | ||||
| Shank3 Mutations in the ankyrin domain | 3ch-SI rSI SM USVs Olfactory | =/↓ Social interaction = Social novelty preference ↑/↓ USVs = Social olfaction | ♂ | SG HBT | ↑ Self-grooming ↑ Hole-poke frequency | ♂ | [131,132,133,134] |
| ↓ Social interaction = Social novelty preference ↓ USVs | ♀ | ↑ Self-grooming ↑ Hole-poke frequency | ♀ | ||||
| Mutations in the PDZ domain | 3ch-SI rSI SM | ↓ Social interaction (juvenile) ↓ Social novelty preference | ♂ | SG | ↑ Self-grooming (juvenile and adult) | ♂ | [132,136] |
| =/↓ Social interaction (juvenile) | ♀ | ↑ Self-grooming (juvenile) | ♀ | ||||
| Mutations in the Homer binding domain | 3ch-SI rSI SM USV Olfactory Nesting | ↓/= Social interaction ↓/= Social novelty preference = USVs (Adult) = Social olfaction ↓ Nesting | ♂ | SG MB | ↑ Self-grooming ↓ Marble burying | ♂ | [135] |
| = Social interaction ↓ Social novelty preference ↓ Nesting | ♀ | ↑ Self-grooming ↓ Marble burying | ♀ | ||||
| Chd8 Chromodomain helicase DNA-binding protein 8 haploinsuficiency | rSI 3ch-SN 3ch-SI | ↓/=/↑ Social interaction (rSI/3ch-SI/3ch-SI) ↓ Social novelty preference | ♂ | ND | [188,189,190] | ||
| = Social interaction ↑ Social novelty preference | ♀ | ||||||
| Arid1b AT-rich interaction domain 1B | SBHC 3ch-SI rSI USV | ↓ Social interaction ↓ Social interaction (juvenile) Altered USVs (pups) | ♂ | SG MB | ↑ Self-grooming ↓ Marble burying | ♂ | [191,192,193] |
| ND | ♀ | ↑ Self-grooming | ♀ | ||||
| Myt1l Myelin transcription factor 1-like gene | 3ch-SN USVs | ↓/= Social novelty preference ↓ Social interaction ↓ USVs (pups) | ♂♀ | SG MB | ↓ Self-grooming ↓ Marble burying | ♂♀ | [194,195,196] |
| Scn2a Nav1.2 gen, which is a member of the voltage-gated sodium channels family | 3ch-SI 3ch-SN USV | ↑Social interaction ↓ Social novelty preference ↓ USVs (pups and adults) | ♂ | SG MB | ↑ Self-grooming ↓ Marble burying | ♂ | [197,198,199,200,201] |
| = Social interaction ↓ Social novelty preference | ♀ | ↑ Self-grooming ↓ Marble burying | ♀ | ||||
| Adnp Activity-dependent neuroprotective protein | USVs 3ch-SI | ↓ USVs (pups) = Social interaction | ♂ | ND | [202,203,204] | ||
| ↓ USVs (pups) ↓ Social interaction | ♀ | ||||||
| INBRED STRAINS | Tests | Results | Test | Results | Ref. | ||
| BTBR + tf/J | 3ch-SI 3ch-SN rSI SM JSP Olfactory USVs | ↓ Social interaction = Social novelty preference ↓ Juvenile social play = Social olfaction ↓ USVs (adults) | ♂ | SG MB | ↑ Self-grooming ↑ Marble burying | ♂ | [59,169,170,176,177,178,179,180,181] |
| ↓ Social interaction | ♂♀ | ↑ Self-grooming | ♂♀ | ||||
| ↓ Juvenile social play ↓ Social interaction | ♀ | ↑ Self-grooming ↑ Marble burying | ♀ | ||||
| BALB/c | 3ch-SI SM JSP USVs | ↓ Social interaction = Social novelty preference ↓ USVs (juvenile) | ♂ | ND | [39,60,166,171,172] | ||
| ↓ Social interaction ↓ Juvenile social play | ♀ | ||||||
| C58/J | 3ch-SI SM Olfactory | ↓/= Social interaction = Social novelty preference = Social olfaction | ♂ | SG Revearsal-HBT | ↑ Self-grooming behavior ↑ Repetitive motor stereotypes ↑ Repetitive jumping = Reversal learning | ♂ | [172,173,174,175] |
| = Social interaction ↓ Social novelty preference = Social olfaction | ♀ | ↑ Self-grooming behavior ↑ Repetitive jumping = Reversal learning | ♀ | ||||
3ch-SI, three-chamber social interaction; 3ch-SN, three-chamber social novelty; Clock, clock maze test; HBT, hole board test; JSP, juvenile social play; MB, marble burying; rSI, reciprocal social interaction; rT-maze, reversal learning in T-maze; SG, self-grooming; SM, social memory; sT-maze, spontaneous T maze; USVs, ultrasonic vocalizations. Symbols: ↑, the model shows increased behavior compared to control mice of the same sex; ↓, the model shows decreased behavior compared to control mice of the same sex; =, the model shows no difference in behavior compared to control mice of the same sex;/, separate reports that obtained different results; ♀, effects observed in females; ♂, effects observed in males; ♂♀, both sexes were studied, but no sex differences were reported.
3. Brain Sexual Differentiation and Sex Differences: Relevance to Mouse Models of ASD
As we mentioned above, many behaviors relevant to ASD show different patterns in male mice than in female mice. In addition, the recent inclusion of female mice in analyses of animal models of ASD has proven that there are sex differences in these models. Although the biological basis for these differences remains largely unknown, we and other researchers believe that studying them could yield insights into ASD and other neurodevelopmental disorders. On one hand, by exploring the cellular and molecular mechanisms behind these differences, we could learn more about why males are more vulnerable to ASD, while females are typically resilient. On the other hand, understanding sex-related processes could help to illuminate the biological mechanisms that alter the brain’s developmental trajectory and lead to ASD symptoms.
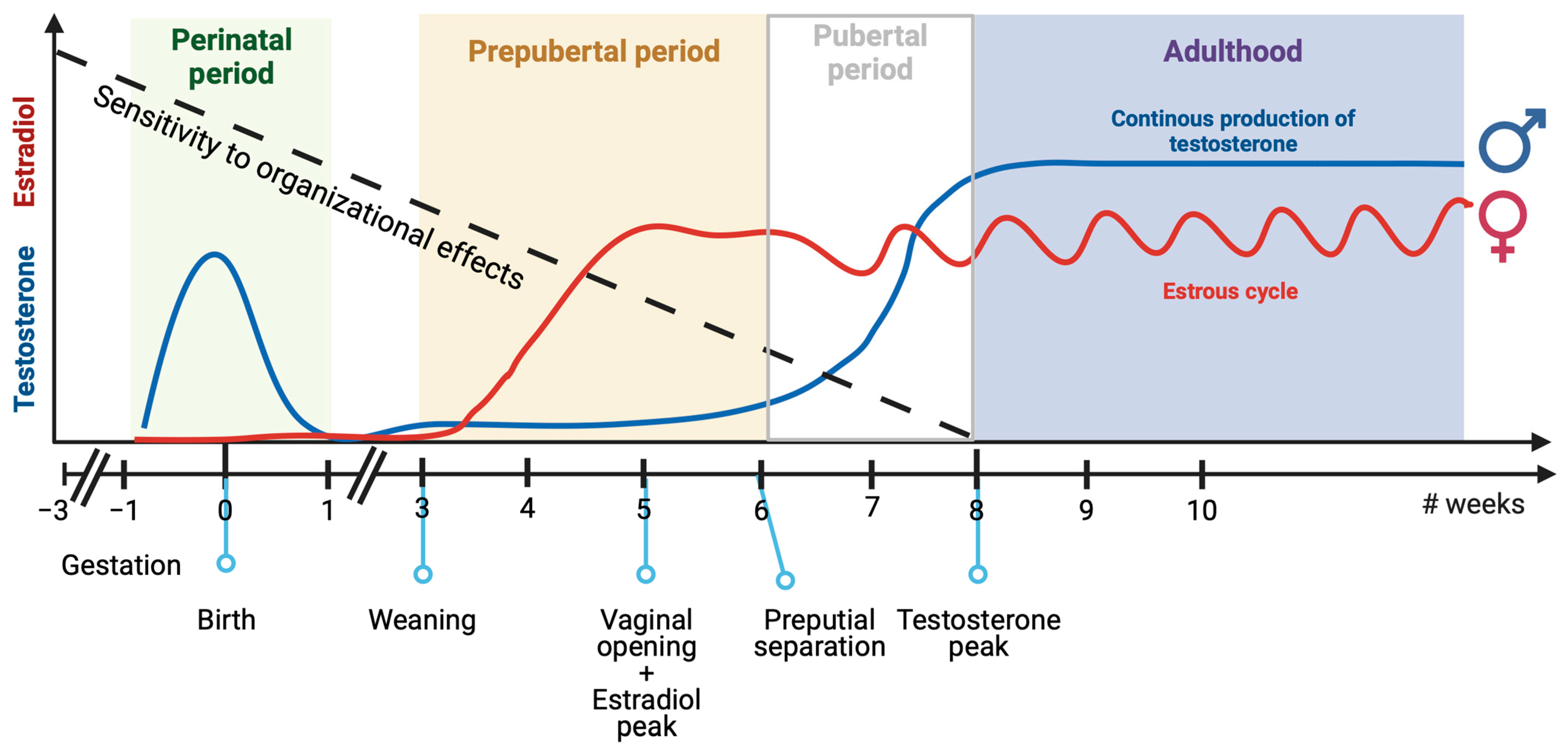
In this section, we will discuss the current understanding of the mechanisms governing brain sexual differentiation, including genetic and hormonal pathways, and their relevance to ASD. We will examine two periods in which sex affects brain development: the perinatal period and the juvenile/pre-pubertal period. In rodents, gonadal hormonal levels are different in these two periods. Males are exposed to testosterone during the perinatal period, while females produce no gonadal hormones. Conversely, as animals reach sexual maturity, ovaries start producing steroids weeks earlier than testicles (see Figure 2). Although earlier studies suggested that gonadal hormones only had a relevant organizational role on brain development during the perinatal period [205], it is now apparent that gonadal steroids can affect brain development throughout the post-natal period until sexual maturity is reached. This finding has changed the previous model to include a long sensitive period during which different genetic and hormonal factors act and result in long-lasting effects on brain function (Figure 2, based on what has been proposed by other researchers [206]). Therefore, we propose that both periods are relevant to the possible sex-specific modulation of brain differentiation based on genes and environment. We will discuss these topics in the context of the determination of social and repetitive behaviors, which are relevant to ASD.
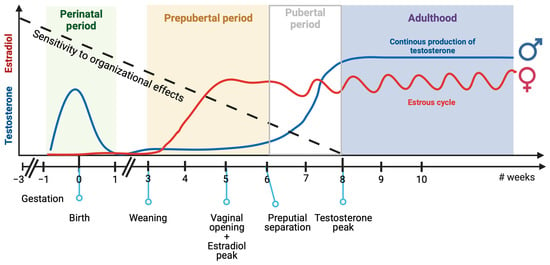
Figure 2.
Sensitivity to organizational effects of sex chromosome genes and gonadal hormones along the mouse life. Mice brains remain sensitive to organizational effects of sex chromosome genes and gonadal hormones from gestation to adulthood. There are two key periods in which male and female mice are naturally exposed to different levels of gonadal hormones. During the perinatal period, testosterone levels are high in males, while ovaries produce negligible levels of estrogen. Conversely, during the pre-pubertal period, ovaries start producing steroids weeks earlier than testicles, and females are exposed to higher levels of gonadal hormones than males. Created with BioRender.com.
3.1. Perinatal Sexual Differentiation of the Brain
The process of sexual differentiation of mammals is influenced by several biological factors. The first of these is the sex–chromosome complement, which determines whether the bipotential gonads will differentiate into testes or ovaries. The expression of the Sry gene (the sex-determining region of the Y chromosome) in males triggers the development of testes [207], which then release testosterone and anti-Müllerian hormone. This process promotes the development of Wolff ducts and leads to the regression of the Müllerian ducts. Dihydrotestosterone, which is produced by the action of the 5α-reductase on testosterone, is responsible for the development of other male sexual structures, such as the penis and scrotum. In females, sexual structures differentiate largely without hormonal influence, as a result of the absence of the Y chromosome (and Sry gene) and the action of transcription factors present in the X chromosome. Due to this tight relationship between sex chromosome-bearing genes and gonadal hormones in early development, the contributions of each factor are difficult to disentangle. Some experimental strategies have been developed and will be discussed below.
Genes present in sex chromosomes and gonadal hormones not only regulate the development of reproductive systems and other peripheric organs, but also influence brain development. To do so, they act on different levels of neuronal organization, ranging from altering the expression of specific molecules to affecting the function of neuronal circuits. This early organization of brain structures determines how the adult brain will respond to gonadal hormones and what kind of sexual behavior the animal will exhibit, a hypothesis known as the organizational–activational hypothesis of sexual differentiation of the brain [205]. Under this hypothesis, male perinatal exposure to testosterone masculinizes and/or defeminizes the developing brain permanently, while female brains are permanently feminized in the absence of perinatal gonadal hormones. After puberty, testicles produce continuous levels of testosterone that activate the adult male brain, triggering typical male sexual behaviors, such as mounting, when the animal is exposed to a receptive female. Conversely, ovaries produce estrogens and progestogens in a cyclic manner in adulthood, and they activate the female brain, triggering typical female sex behaviors, such as lordosis. Research in rats has shown that early exposure to gonadal hormones and genes present in sex chromosomes can affect not only reproductive systems and behaviors, but also a range of brain structures and capacities, including cognition, pain, feeding, social behavior, and emotion [208,209].
To distinguish the contribution of sex–chromosome complement from gonadal hormones on brain masculinization, the Four Core Genotype (FCG) model has been developed in mice [210,211,212]. This model combines animals with a Y chromosome in which the Sry gene has been deleted (Y-) with animals carrying a Sry transgene in an autosomal chromosome. Thus, this model can generate XX and XY gonadal males and XX and XY gonadal females, allowing the study of the four combinations of chromosomal complement and gonadal type. Using this model, it was demonstrated that many sex differences in behavior and brain structure were independent of sex–chromosome complement [211,213,214]. However, some interesting exceptions were found that are relevant to the study of ASD. For example, using a resident–intruder paradigm, it was shown that animals bearing two X chromosomes (XX females and XXSry males) interacted less with the intruder than mice bearing a Y chromosome [215]. Conversely, 21-day-old XX females played more with a sibling than all other groups, suggesting that this behavior is determined by both sex–chromosome complement and gonadal hormones [36]. Aggressive behavior and reduced parental care were observed in XY females, showing that these sexually dimorphic social behaviors are under the developmental control of not only gonadal hormones, but also sex chromosome genes [216]. These behavioral alterations correlate with increased arginine–vasopressin (AVP) fiber density in the lateral septum in mice bearing the Y chromosome, suggesting that this cellular dimorphism is regulated by genes carried on that chromosome [211,216]. Therefore, the evaluation of the FCG mouse model shows that chromosome–sex complement plays a role in determining sex biases in social behaviors, and this contribution may be relevant in the context of animal models of ASD. To our knowledge, only the C6 model of ASD has been combined with the FCG model, but, unfortunately, in that work, the effects of pre-natal exposure to anti-Caspr2 on behaviors relevant to ASD were not replicated [104].
Various studies have indicated that testosterone produced by the testicles during the perinatal period is the primary driver for brain masculinization. Testosterone can act on the developing nervous system directly through androgen receptors (AR) [217] or be aromatized locally in the brain and act through estrogen receptors (ER) [218]. To study the organizational effects of gonadal steroids, researchers either evaluate the masculinization or defeminization effects of injecting neonatal females with testosterone or estradiol or perinatally analyze the demasculinization or feminization effects in males deprived of their normal testicular secretions [219]. In mice, males display higher levels of offensive aggression in various laboratory tests, and both organizational and activational effects of testosterone contribute to these large sex differences [220]. To evaluate the effect of early exposure to estradiol on ASD-relevant behaviors, female animals were exposed to 5 μg of E2 at PD2, PD5, and PD8, following a protocol that masculinizes sexual behaviors in female mice [221]. Although there was a sex bias in juvenile play in the CF1 outbred strain used, E2 females exhibited normal levels of playful behavior [43]. Furthermore, no sex differences or neonatal E2 effects on social interaction were found. In the same report, self-grooming was studied as a measurement of repetitive behaviors, and it was observed that males spent more time in self-grooming than females. E2 females exhibited intermediate behavior, indicating that post-natal exposure to E2 can influence the expression of this behavior in adulthood. To the best of our knowledge, no environmental or genetic ASD model has been combined with methods that alter exposure to gonadal hormones around birth. Therefore, the contribution of these steroids to the phenotypes of ASD models needs to be studied.
As a result of sexual differentiation, structural sexual dimorphisms are observed later in life. These include sex differences in the number of neurons and glial cells, cell size, dendritic arborization, spine density, and myeline volume, both in specific brain regions and structures. Social behavior is a result of the action of a complex neuronal network in both rodents [222] and humans [223], making numerous brain structures relevant to study in this context. Gonadal hormones can regulate the function of some limbic and hypothalamic areas that project to cortical areas that are relevant for the processing of environmental information [224]. Different areas either show a sexually dimorphic pattern of expression of AR and ER or exhibit structural dimorphism [225,226]. Some cellular and molecular mechanisms that mediate the organizational effects of gonadal hormones on brain cells have been described, albeit mostly in rats [227,228].
During the perinatal period, testosterone is converted into estradiol in the brain, which then impacts the development of various hypothalamic structures. One example of this process is seen in the rat’s arcuate nucleus, where estradiol stimulates the expression of the glutamate decarboxylase enzyme, leading to an increase in the synthesis of GABA. This process ultimately results in structural and functional changes in the surrounding astrocytes, which suppress the formation of dendritic spines in the neurons [229]. Conversely, in the ventromedial nucleus, activation of ERs leads to an increase in dendrite ramification and the number of dendrite spines, though the exact mechanism behind this shift remains unknown, and it may be mediated by NMDA receptors [230].
Male rats have a larger sexually dimorphic nucleus (SDN) of the preoptic area and a smaller anteroventral periventricular nucleus (AVPV) of the hypothalamus than females. This dimorphism is determined perinatally by testosterone, which reduces apoptosis in the male SDN and increases cell loss in the AVPV, thereby masculinizing and defeminizing the brain [231]. Recent evidence suggests that microglial cells may play a role in the sexual differentiation of the SDN, either by phagocytosing apoptotic neurons or causing their apoptosis through a process called phagoptosis [232]. The AVPV is essential for the female preovulatory surge of gonadotropin-releasing hormone (GnRH) and luteinizing hormone (LH) [233], which are activated in females only after puberty. The SDN, on the other hand, is involved in male sexual behavior and the preference for female partners [234].
In the preoptic area (POA) of the rat, two types of immune cells are involved in the masculinization process: mast cells and microglial cells. Mast cells, which originate in the bone marrow and secrete histamine and serotonin, among other signaling molecules, are more abundant in the male brain during the perinatal period of sexual differentiation. In the POA, they release histamine in response to estradiol [235], which acts on surrounding microglial cells. Microglial cells, i.e., the resident macrophages of the brain, are also more abundant in males during the sensitive period [236]. They respond to histamine inducing the expression and activity of cyclo-oxygenase-2 (Cox-2) and, thus, locally increase the synthesis of prostaglandin E2 (PGE2) [237]. PGE2, in turn, leads to the phosphorylation of AMPA receptors, their insertion at the membrane [238], and an increase in the density of dendritic spines in the neurons [237].
The rodent posterodorsal subnucleus of the medial amygdala (MePD) plays a vital role in integrating environmental and internal stimuli, such as hormones and metabolism, to regulate social behaviors. Lesioning the MePD at PD21 in young female rats (PD29-33) results in increased social interaction and reduced play fighting [239]. Both rats and mice exhibit sexual dimorphism in the MePD, with adult males having a larger MePD than females [240,241], and this difference is already evident in pre-pubertal animals [241]. This increased volume in males occurs due to the presence of more astrocytes in the MePD [96,97], which is already observed in young animals (PD25) [242]. This sexual dimorphism is also mediated by microglial cells, which are more abundant and phagocytic in the developing male brain and selectively eliminate astrocytes in the amygdala [243]. Interestingly, blocking microglial phagocytosis results in male rats playing less and exhibiting behavior more similar to that of females.
The regulation of social behavior is complex and involves the interaction of various molecules affected by hormones such as estrogen and androgen. Among these molecules, oxytocin (OT) and arginine vasopressin (AVP) play a key role [244]. While both neuropeptides are pro-social in both sexes [245], AVP is more involved in the expression of male social behaviors, such as scent marking, aggression, parental behavior, pair bonding, and social recognition (reviewed in [246]), while OT is essential for the manifestation of many female social behaviors, such as maternal care, pair bond formation, social recognition, and social motivation (reviewed in [247]). It is important to note that although different neuropeptides may be required for social behavior in different sexes, activity of both systems can influence social behavior. For example, OT is necessary for pair bond formation in female prairie voles [248], whereas AVP is necessary for this behavior to occur in males of the same species [249]. While the link between animal sex and OT/AVP regulation of social behaviors has not been fully elucidated, some relevant facts can be noted. For instance, OT- and AVP-neurons within limbic–hypothalamic areas express ERs [250]. Studies in mice have shown that social recognition is disrupted if the genes ERα, ERβ, or OT are deleted [251], and this trend appears to depend on estradiol augmenting the expression of OT receptors, both in the amygdala and the hypothalamus [252]. The importance of OT and AVP for social behavior in lower animals has led to an interest in their functions in humans. Studies in people have found links between neuropeptide activity in the amygdala and the expression of more complex emotions, like love, fear, and trust [253]. As mentioned above, mice deficient in OT or AVP receptors have been proposed as models of ASD, as they show altered sociability [151,152,153,154,155]. In addition, polymorphisms in OT and AVP, as well as their receptors, have also been associated with ASD (reviewed in [254]). However, the direct contribution of sex differences in OT/AVP function to ASD-relevant behaviors has not been studied.
Numerous studies have linked the cerebellum to ASD (reviewed in [255]), and this structure is affected in different rodent models of ASD [67,256,257]. When studying sex differences in the cerebellum, researchers found that the synaptic and intrinsic properties of the neurons in the cerebellar nuclei differ between sexes at PD17–24 [258]. Intracerebellar administration of nimesulide, which is a Cox-2 inhibitor, during the second post-natal week resulted in a reduction in play in male rats at PD25–38, reaching levels similar to females [259]. Interestingly, an inflammatory stimulus (LPS) injected peripherally at PD10 and PD12 provokes a reduction in social play and affects cerebellar parameters in young male rats, including increasing aromatase activity, increasing estradiol content, and reducing dendritic length in Purkinje neurons [260]. Female animals were not affected by this treatment. Also relevant to this topic is the fact that different genetic mutations that affect cerebellar function show different incidences in males and females. For example, mutations that lead to Purkinje cell degeneration, such as those present in the reeler and staggerer mice, have earlier and more profound effects in heterozygous males than in females, and these effects are observed in young animals [261,262]. In addition, the FCG model was used to demonstrate that the sex difference in calbindin expression in the cerebellum and pre-frontal cortex (PFC), where females have more of this calcium-binding protein than males at PD21–25, is a consequence of the sex–chromosome complement, as XY females have calbindin levels similar to males [263]. Therefore, sex affects cerebellar development both through sex chromosome genes and gonadal hormones. The specific contributions of these differences in mouse models of ASD need to be studied further.
In summary, different brain regions and neuronal circuits associated with ASD are affected by sex–chromosome complement and gonadal hormones during the perinatal period. By determining the survival and connectivity of neurons and the survival and activational state of glia, biological sex can also affect different behaviors relevant to ASD. As demonstrated in this section, employing animal models can help us to understand the link between biological sex and behavior and identify early events that can have long-lasting effects.
3.2. Pre-Pubertal Sexual Differentiation of the Brain
There is a second developmental period in mice when gonadal hormone levels significantly differ between males and females (Figure 2). Female ovaries start to produce estrogen and progesterone at an earlier point in the juvenile period than male testicles start to produce testosterone [264]. In rats, female production of luteinizing hormone (LH) and estradiol begins at PD21 and peaks at around PD35 [265], while testosterone in males only slightly increases from PD21 to PD45 and peaks in adulthood (PD60) [266,267]. The age of vaginal opening and preputial separation, as well as the age at which gonadal hormones increase before achieving sexual maturation, varies greatly between different mouse strains [268,269]. Similar to rats, female C57BL/6J mice also experience an increase in circulating estradiol at around PD26-29 [270], while testosterone gradually increases from PD35 and becomes significant at PD70 [271]. Although the consequences of this hormone-level difference have been studied less extensively than perinatal masculinization, some reports support the notion that this process is critical for brain feminization and, thus, the later expression of certain sex-related behavioral differences (as reviewed in [206,272,273,274]).
The organizational effects of estrogen during adolescence have been demonstrated using mice deficient of aromatase (ArKO mice) that were treated with estrogen during different pre-pubertal periods [275]. Female ArKO mice show reduced lordosis when tested as adults. This deficit can be reversed by treating mice with estradiol between PD15 and PD25, whereas earlier treatment (between PD5 and PD15) had no such effect (and, actually, resulted in reduced lordosis in WT females, showing a masculinizing effect).
During adolescence, testosterone is produced in lower quantities, though it still appears to have some organizational effects. Experiments with Syrian hamsters demonstrated that castrating males before puberty but after the post-natal period of sexual brain differentiation results in animals with reduced sexual and aggressive behavior compared to intact males, even when gonadal hormones were replaced in adulthood [276,277]. Additionally, mice that are castrated at PD30 show decreased aggressiveness, although they exhibit normal levels of sexual behavior [278]. Furthermore, female rats treated between PD15 and PD30 with testosterone show reduced lordosis and proceptive behaviors, along with increased mounting and intromission, when compared with control females [279]. In male rats that were gonadectomized neonatally, injection with estradiol and progesterone in adulthood induced female sexual behavior, though this feminization effect was reduced if animals were treated between PD15 and PD30 with testosterone, and typical male sexual behavior was observed in this case [280]. In summary, during the pre-pubertal period, both estradiol and testosterone appear to have organizational effects that are different from each other.
Gonadal hormones that act during the juvenile period not only affect sexual behavior, but also affect other behaviors. Female and male mice gonadectomized at PD25 display reduced parental behavior, which can be restored if animals are treated with estradiol, though not testosterone, during adolescence [281]. In a familiar environment, adult male rats spend more time in social interaction than female rats, while the opposite is observed in an unfamiliar environment [282]. Male rats gonadectomized at PD19 spent a similar amount of time in social interaction in both environments, a behavioral effect that can be reversed via treatment with testosterone propionate. Male rats gonadectomized at PD22 made less playful attacks and initiated fewer play fights than intact males between PD31 and PD35, ages at which the rate of rough-and-tumble play is maximal [283]. To the extent of our knowledge, no study has evaluated the role of gonadal hormones during this pre-pubertal period on mouse models of ASD. As most models show behavioral and brain alterations early in life, it would be interesting to evaluate whether estrogen levels during the juvenile period could have a protective effect on females or their absence could explain the bigger impact in males, specifically in models that show sex differences.
During adolescence, hormones play a role in organizing the brain through the same developmental processes that occur during the perinatal organizational period. These hormones regulate brain maturation by controlling neuronal proliferation [284], neuronal death [285], and myelinization [285]. For instance, although neonatal testosterone promotes cell death in the AVPV of the rat’s hypothalamus, the sex difference only becomes apparent during puberty [209], when neurons in the female AVPV proliferate [284]. As mentioned before, rat males have a larger SDN of the hypothalamus than females, and this dimorphism is determined perinatally due to testosterone reducing apoptosis in males. However, proliferation in that region also occurs during adolescence in males [284]. Similarly, although the dimorphism in MePD is observed early in life, testosterone is necessary during puberty to maintain and augment the sex differences by further increasing the number of astrocytes in males [242,286]. In addition, gonadectomization of rats at PD22 results in reduced frequency of mEPSCs and dendritic spine density in the adult MePD [283], indicating that gonadal hormones are necessary during adolescence to achieve proper excitatory/inhibitory balance in this structure. These effects of testicular hormones are mediated by the AR, as they are not observed when animals carry the testicular feminization mutation of the AR [242,286].
Structural sex differences in the cerebral cortex are smaller and less evident than those in the hypothalamus. Many of these differences emerge in female animals during puberty as a result of exposure to ovarian hormones. Early studies found that most cortical regions are larger in males than in females [287] due to increased neuronal number [288]. However, this sex difference is not observed if female rats are gonadectomized at PD20 [289], suggesting that ovarian hormones around puberty promote cell death. Additionally, ovarian hormones modulate synaptic pruning in the cortex, as evidenced by the observation that female rats ovariectomized at PD30 have more dendritic spines than intact females [290]. Studies specifically examining the ventral mPFC have found that adult male rats have more neurons in this region than females, although this difference is not observed at PD35 [285]. This sex difference results from the increased death of neurons in females between PD35 and PD90 and the bigger increase in white matter in males [285,291]. Additionally, the number of glial cells in the ventral mPFC was similar in both sexes at PD35, but increased in males at PD90. Interestingly, gonadectomy at PD20–22 resulted in an increased number of neurons and glial cells in females, along with a bigger white matter volume, suggesting that ovarian hormones that act during adolescence trigger cell loss [292]. Male ventral mPFC parameters were not affected via gonadectomy. The mPFC is particular relevant to studies of ASD models because it is involved in the regulation of social play and dominance [293,294].
It is surprising that puberty and adolescence were long disregarded as developmental periods in animals, while in humans, puberty is considered the primary period in which normal sex differences in physiology and behavior emerge. Additionally, different psychopathologies manifest sex differences in susceptibility during that period, including mood disorders, eating disorders, and schizophrenia (reviewed in [295]). However, it is essential to notice that the extension of the developmental window during which gonadal hormones can affect brain function would depend on the temporal maturation of the gonads and the dynamics of brain circuit refinement and consolidation, which are different in humans than in rodents [269,296]. Rodents are altricial species, and much of their post-natal development corresponds to processes that occur in the human fetus. However, some brain structures mature later in rodents than in humans (e.g., the cerebellum [297] and the mPFC [298]), making them particularly sensitive to environmental or pharmacological factors that act during the juvenile period. Thus, identifying mechanisms and factors that affect the trajectories of brain maturation during the pre-pubertal period may be relevant not only to psychiatric disorders that emerge at adolescence, but also to neurodevelopmental disorders [295].
4. Brain Inflammation as a Mechanism of Convergence of Diverse ASD Etiological Factors and Brain Sexual Differentiation
In previous sections, we discussed how biological sex, through genes present in sex chromosomes and gonadal hormones, affects the brain and behavior. This regulation occurs during the perinatal period, as well as throughout the juvenile period. Although the exact mechanisms are not fully understood, glial cells and inflammatory molecules play a fundamental role in regulating cell death and survival, dendritic growth, and synapse formation. These processes are involved in the development of ASD-relevant behaviors, and both periods that are sensitive to gonadal hormones are critical for this development.
Most pharmacological models of ASD involve administering the stimulus pre-natally, typically at around GD12. Interestingly, many of these models directly elicit a maternal immune response and result in neuroinflammation in the fetal brain [97]. Additionally, models like the VPA model alter the neuroinflammatory state in the brain, despite not directly targeting the immune cells [42,67,82]. Moreover, neonatal and early inflammatory stimuli can have long-lasting deleterious consequences on ASD-relevant behaviors [40,140,142,143,144,260], with some reports indicating sex differences in their effects [144,145].
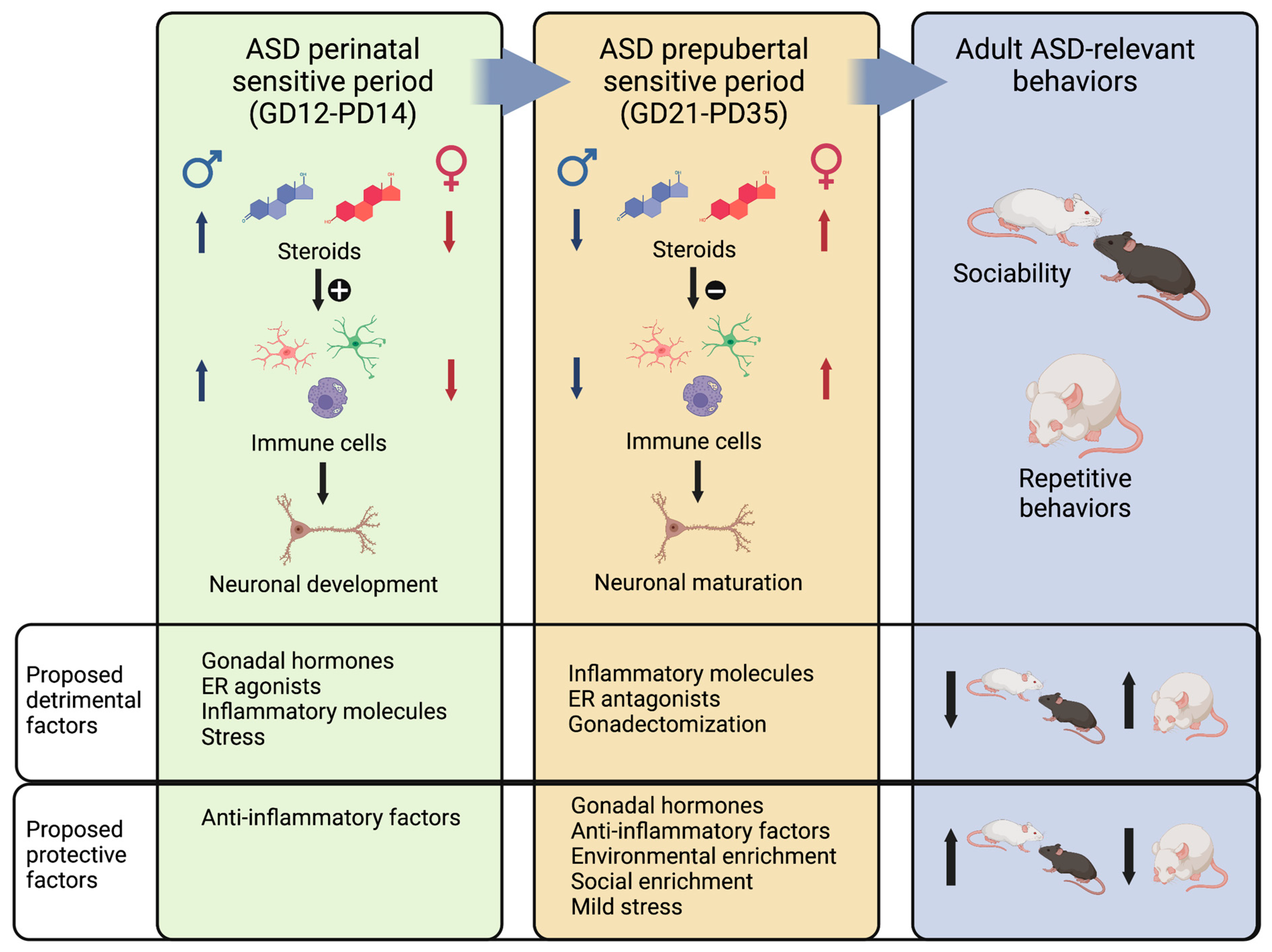
During development, males exhibit a higher presence of astrocytes in the arcuate nucleus [243] and the preoptic area [299]. In addition, males have a greater number of microglial cells in the cortex, hippocampus, and amygdala [300], which also display increased phagocytic activity [243]. These disparities in glial cells result from the stimulatory effect of testosterone, as evidenced by the partial reversal of these differences in castrated males or females treated with testosterone [299,301,302]. Such differences may account for the observation that males are more affected by early-life immune activation compared to females. For instance, a low dose of Escherichia coli on PD4 profoundly impacts learning and memory in adult males, but it has no effect on females [303]. Based on these findings, we propose that gonadal hormones and factors that trigger an inflammatory response during this period increase the likelihood of developing ASD-related impairments later in life (Figure 3, left panel). Given males’ heightened basal inflammatory state, they are more vulnerable to the influence of these factors, while females display greater resilience. Furthermore, the administration of anti-inflammatory agents during early-life stages could prevent the emergence of ASD-related behaviors later in life.
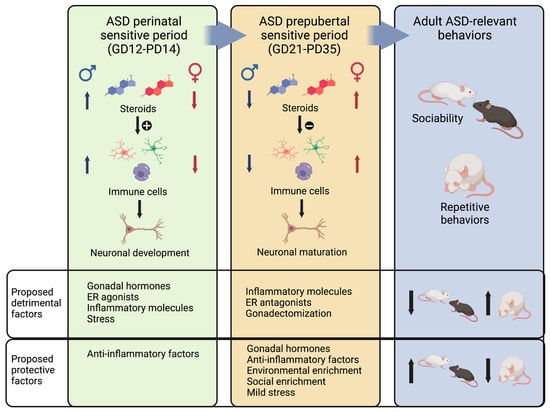
Figure 3.
Proposed mechanism that illustrates how gonadal hormones modulate immune cells to affect ASD-relevant neuronal development and maturation during two sensitive periods. During the ASD perinatal sensitive period, males are exposed to higher levels of steroids than females, and hormones stimulate immune cells, which are essential to neuronal development. In this period, excessive gonadal hormones, ER agonists, inflammatory molecules, and stress would be detrimental, resulting in adult ASD-related behaviors. In contrast, anti-inflammatory factors and low levels of gonadal hormones would be protective. During the ASD pre-puberal period, females are exposed to higher levels of gonadal hormones, and at this age, steroids are anti-inflammatory. Immune cells participate in neuronal maturation during this period, though a more anti-inflammatory phenotype may be needed. Thus, protective factors related to the expression of ASD-related behaviors later in life include gonadal hormones, anti-inflammatory factors, environmental enrichment, social enrichment, and mild stress. Detrimental factors include inflammatory molecules, ER antagonists, and gonadectomy. Created with BioRender.com.
The pre-pubertal period, on the other hand, has been much less studied, and few studies have explored the differential roles and effects of gonadal hormones and inflammatory processes on ASD-relevant behaviors. However, some evidence suggests that during this period, social and repetitive behaviors are consolidated, and this process is sensitive to external and internal factors. For example, social or environmental enrichment after weaning (PD21) can rescue social deficits in the VPA model [55,183,186] and the BTBR strain [177], as well as behavioral deficits in genetic mouse models of ASD [304]. In addition, short handling of animals between PD22 and PD34 also rescues pre-natal VPA effects on adult male sociability [184]. Finally, interventions during this period, such as environmental enrichment, can rescue repetitive behaviors [175,183,305].
Sex differences in glial cells and neuroimmune responses have been much less studied during the pre-pubertal period. Nevertheless, the examination of adult brain cells suggests that profound changes occur in these cell populations over the course of juvenile development. On one hand, the adult female brain harbors a larger number of microglial cells and astrocytes than the adult male brain [306]. However, male microglial cells show larger soma sizes [307], which have been associated with increased activation [308] and may represent a primed phenotype that is predisposed to a heightened response [309,310]. Conversely, microglia in the female brain exert an anti-inflammatory effect [311], which is potentially influenced by the impact of estrogens on these cells [312]. Based on these findings, we propose that the higher levels of gonadal hormones, particularly estradiol, observed in females during the pre-pubertal period contribute to an anti-inflammatory state in the brain. This state may account for the resilience observed in female animals to factors that trigger ASD-related behaviors (Figure 3, middle panel). Other interventions known to reverse the ASD phenotypes may also operate via modulating the neuroinflammatory state. For instance, environmental enrichment has been shown to result in animals exhibiting a blunted inflammatory response in the brain [313].
In conclusion, we propose that sex influences neuroimmune function throughout development, altering the effect of immune cells on the development of neuronal circuits that determine sociability levels and the expression of repetitive behaviors (Figure 3). During the perinatal period, gonadal hormones are permissive, and they even stimulate immune and glial cells to masculinize the brain. Abnormally increased levels of gonadal steroids or inflammatory stimuli during this period can affect the development of brain circuits, resulting in maladaptive function and behavior. Under this hypothesis, females might be resilient to these deleterious effects because they are normally exposed to low levels of gonadal hormones and have less active glial cells than males. During the juvenile period, we propose that gonadal hormones and mild inflammatory processes are beneficial to brain consolidation and refinement of circuits, as well as the establishment of normal levels of sociability and repetitive behaviors. Higher levels of gonadal hormones in females during this period make them resilient to the development of ASD-relevant behaviors, while males lack this beneficial influence. Figure 3 illustrates this proposed mechanism of convergence of diverse ASD etiological factors and brain sexual differentiation.
While further research is needed to confirm or refute these hypotheses, they provide a framework for the comprehensive study of gonadal hormones and neuroinflammatory processes in the context of ASD models. We hope that such studies could help identify possible causes of the sex bias in ASD and, most importantly, lead to the development of diagnosis tools and treatments to help individuals with ASD.
5. Limitations and Future Prospects
ASD shows sex differences in phenotype and incidence [7,8,10]. In this review, we have explored the study of sex differences in mouse models of ASD and collected evidence from the literature that support this idea. To conduct this analysis, an extensive literature search was performed using multiple databases and keywords to identify pertinent studies and their key findings. Although the inclusion of both sexes in pre-clinical studies is still limited, we have identified intriguing cases that have allowed the investigation of the underlying biological causes of sex differences and their impact on ASD-relevant behaviors. We believe that studying the biological mechanisms behind these differential effects in male and female mice can enhance our understanding of similar processes in humans.
One significant mechanism through which sex can influence ASD is through the role of gonadal hormones. We have reviewed the current understanding of how sex steroids can impact behavior, focusing on the brain sexual differentiation model and considering the perinatal and pre-pubertal organizational periods. It is important to note that the developmental trajectories of gonads and brains differ between humans and mice [269,296], and, therefore, processes that occur during the juvenile period in rodents may have critical implications for neurodevelopment in humans [295]. Additionally, we discuss the involvement of immune cells and inflammatory processes in the hormonal regulation of behavior. We believe that such studies can generate novel hypotheses and contribute to our understanding of factors relevant to ASD.
It is crucial to acknowledge the limitations of pre-clinical research. The validity of rodent models for psychiatric diseases continues to be debated [314], and the translation of pre-clinical findings to human health management remains limited to few successful examples [315]. However, it is worth noting that our understanding of the underlying processes that contribute to psychiatric disorders has significantly improved in recent decades, and this knowledge is essential for scientific and clinical advancements. Therefore, animal models of psychiatric disorders have the potential to impact psychiatry and enhance our understanding of the role of biological sex in influencing these disorders.
Author Contributions
Conceptualization, A.M.D.; investigation, A.S., V.M. and A.M.D.; writing—original draft preparation, V.M. and A.M.D.; writing—review and editing, A.M.D.; funding acquisition, A.M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación, grant number PICT2019-1279, and the Universidad de Buenos Aires, grant number 20020190100102BA. A.M.D. and V.M. are full-time members and A.S. is a fellow of the Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created.
Acknowledgments
ChatGPT (GPT-3, OpenAI’s large-scale language-generation model) was used to improve the writing style of this article. Authors reviewed, edited, and revised the ChatGPT generated texts to their own liking, improving accuracy, and take ultimate responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Arlington, TX, USA, 2013; ISBN 0-89042-555-8. [Google Scholar]
- Chiarotti, F.; Venerosi, A. Epidemiology of Autism Spectrum Disorders: A Review of Worldwide Prevalence Estimates since 2014. Brain Sci. 2020, 10, 274. [Google Scholar] [CrossRef]
- Maenner, M.J.; Warren, Z.; Williams, A.R.; Amoakohene, E.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Fitzgerald, R.T.; Furnier, S.M.; Hughes, M.M.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. MMWR. Surveill. Summ. 2023, 72, 1–14. [Google Scholar] [CrossRef]
- Muhle, R.; Trentacoste, S.V.; Rapin, I. The Genetics of Autism. Pediatrics 2004, 113, e472–e486. [Google Scholar] [CrossRef]
- Hallmayer, J.; Cleveland, S.; Torres, A.; Phillips, J.; Cohen, B.; Torigoe, T.; Miller, J.; Fedele, A.; Collins, J.; Smith, K.; et al. Genetic Heritability and Shared Environmental Factors among Twin Pairs with Autism. Arch. Gen. Psychiatry 2011, 68, 1095–1102. [Google Scholar] [CrossRef]
- Sandin, S.; Lichtenstein, P.; Kuja-Halkola, R.; Larsson, H.; Hultman, C.M.; Reichenberg, A. The Familial Risk of Autism. JAMA J. Am. Med. Assoc. 2014, 311, 1770–1777. [Google Scholar] [CrossRef]
- Elsabbagh, M. Linking Risk Factors and Outcomes in Autism Spectrum Disorder: Is There Evidence for Resilience? BMJ 2020, 368, l6880. [Google Scholar] [CrossRef]
- Loomes, R.; Hull, L.; Mandy, W.P.L. What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 466–474. [Google Scholar] [CrossRef]
- Hull, L.; Petrides, K.V.; Allison, C.; Smith, P.; Baron-Cohen, S.; Lai, M.C.; Mandy, W. “Putting on My Best Normal”: Social Camouflaging in Adults with Autism Spectrum Conditions. J. Autism Dev. Disord. 2017, 47, 2519–2534. [Google Scholar] [CrossRef] [PubMed]
- Hull, L.; Petrides, K.V.; Mandy, W. The Female Autism Phenotype and Camouflaging: A Narrative Review. Rev. J. Autism Dev. Disord. 2020, 7, 306–317. [Google Scholar] [CrossRef]
- Skuse, D.H. Imprinting, the X-Chromosome, and the Male Brain: Explaining Sex Differences in the Liability to Autism. Pediatr. Res. 2000, 47, 9. [Google Scholar] [CrossRef]
- Robinson, E.B.; Lichtenstein, P.; Anckarsäter, H.; Happé, F.; Ronald, A. Examining and Interpreting the Female Protective Effect against Autistic Behavior. Proc. Natl. Acad. Sci. USA 2013, 110, 5258–5262. [Google Scholar] [CrossRef] [PubMed]
- Wigdor, E.M.; Weiner, D.J.; Grove, J.; Fu, J.M.; Thompson, W.K.; Carey, C.E.; Baya, N.; van der Merwe, C.; Walters, R.K.; Satterstrom, F.K.; et al. The Female Protective Effect against Autism Spectrum Disorder. Cell Genomics 2022, 2, 100134. [Google Scholar] [CrossRef] [PubMed]
- Jacquemont, S.; Coe, B.P.; Hersch, M.; Duyzend, M.H.; Krumm, N.; Bergmann, S.; Beckmann, J.S.; Rosenfeld, J.A.; Eichler, E.E. A Higher Mutational Burden in Females Supports a “Female Protective Model” in Neurodevelopmental Disorders. Am. J. Hum. Genet. 2014, 94, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, D.M.; Warrier, V.; Allison, C.; Baron-Cohen, S. Testing the Empathizing–Systemizing Theory of Sex Differences and the Extreme Male Brain Theory of Autism in Half a Million People. Proc. Natl. Acad. Sci. USA 2018, 115, 12152–12157. [Google Scholar] [CrossRef]
- Auyeung, B.; Baron-Cohen, S.; Chapman, E.; Knickmeyer, R.; Taylor, K.; Hackett, G. Foetal Testosterone and the Child Systemizing Quotient. Eur. J. Endocrinol. Suppl. 2006, 155, 123–130. [Google Scholar] [CrossRef]
- Auyeung, B.; Baron-Cohen, S.; Ashwin, E.; Knickmeyer, R.; Taylor, K.; Hackett, G.; Baron-Cohen, S.; Ashwin, E.; Knickmeyer, R.; Taylor, K.; et al. Fetal Testosterone and Autistic Traits. Br. J. Psychol. 2009, 100, 1–22. [Google Scholar] [CrossRef]
- Kung, K.T.F.; Spencer, D.; Pasterski, V.; Neufeld, S.; Glover, V.; O’Connor, T.G.; Hindmarsh, P.C.; Hughes, I.A.; Acerini, C.L.; Hines, M. No Relationship between Prenatal Androgen Exposure and Autistic Traits: Convergent Evidence from Studies of Children with Congenital Adrenal Hyperplasia and of Amniotic Testosterone Concentrations in Typically Developing Children. J. Child Psychol. Psychiatry 2016, 57, 1455–1462. [Google Scholar] [CrossRef]
- Beery, A.K.; Zucker, I. Sex Bias in Neuroscience and Biomedical Research. Neurosci. Biobehav. Rev. 2011, 35, 565–572. [Google Scholar] [CrossRef]
- Belzung, C.; Griebel, G. Measuring Normal and Pathological Anxiety-like Behaviour in Mice: A Review. Behav. Brain Res. 2001, 125, 141–149. [Google Scholar] [CrossRef]
- Prut, L.; Belzung, C. The Open Field as a Paradigm to Measure the Effects of Drugs on Anxiety-like Behaviors: A Review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Nestler, E.J.; Hyman, S.E. Animal Models of Neuropsychiatric Disorders. Nat. Neurosci. 2010, 13, 1161–1169. [Google Scholar] [CrossRef]
- Kaiser, T.; Feng, G. Modeling Psychiatric Disorders for Developing Effective Treatments. Nat. Med. 2015, 21, 979–988. [Google Scholar] [CrossRef]
- Willner, P. The Validity of Animal Models of Depression. Psychopharmacology 1984, 83, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Belzung, C.; Lemoine, M. Criteria of Validity for Animal Models of Psychiatric Disorders: Focus on Anxiety Disorders and Depression. Biol. Mood Anxiety Disord. 2011, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Lipska, B.K.; Weinberger, D.R. To Model a Psychiatric Disorder in Animals: Schizophrenia as a Reality Test. Neuropsychopharmacology 2000, 23, 223–239. [Google Scholar] [CrossRef]
- Uliana, D.L.; Zhu, X.; Gomes, F.V.; Grace, A.A. Using Animal Models for the Studies of Schizophrenia and Depression: The Value of Translational Models for Treatment and Prevention. Front. Behav. Neurosci. 2022, 16, 935320. [Google Scholar] [CrossRef]
- Gottesman, I.I.; Gould, T.D. The Endophenotype Concept in Psychiatry: Etymology and Strategic Intentions. Am. J. Psychiatry 2003, 160, 636–645. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. DSM-IV-TR; American Psychiatric Association: Arlington, TX, USA, 2000; ISBN 0-89042-024-6. [Google Scholar]
- Crawley, J.N. Translational Animal Models of Autism and Neurodevelopmental Disorders. Dialogues Clin. Neurosci. 2012, 14, 293–305. [Google Scholar] [CrossRef]
- Silverman, J.L.; Yang, M.; Lord, C.; Crawley, J.N. Behavioural Phenotyping Assays for Mouse Models of Autism. Nat. Rev. Neurosci. 2010, 11, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Beery, A.K. Inclusion of Females Does Not Increase Variability in Rodent Research Studies. Curr. Opin. Behav. Sci. 2018, 23, 143–149. [Google Scholar] [CrossRef]
- Moles, A.; Kieffer, B.L.; D’Amato, F.R. Deficit in Attachment Behavior in Mice Lacking the μ-Opioid Receptor Gene. Science 2004, 304, 1983–1986. [Google Scholar] [CrossRef] [PubMed]
- Miczek, K.A.; Maxson, S.C.; Fish, E.W.; Faccidomo, S. Aggressive Behavioral Phenotypes in Mice. Behav. Brain Res. 2001, 125, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Burns-Cusato, M.; Scordalakes, E.M.; Rissman, E.F. Of Mice and Missing Data: What We Know (and Need to Learn) about Male Sexual Behavior. Physiol. Behav. 2004, 83, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.H.; Rissman, E.F. Sex Differences in Juvenile Mouse Social Behavior Are Influenced by Sex Chromosomes and Social Context. Genes Brain Behav. 2011, 10, 465–472. [Google Scholar] [CrossRef]
- Moy, S.S.; Nadler, J.J.; Young, N.B.; Perez, A.; Holloway, L.P.; Barbaro, R.P.; Barbaro, J.R.; Wilson, L.M.; Threadgill, D.W.; Lauder, J.M.; et al. Mouse Behavioral Tasks Relevant to Autism: Phenotypes of 10 Inbred Strains. Behav. Brain Res. 2007, 176, 4–20. [Google Scholar] [CrossRef]
- Nadler, J.J.; Moy, S.S.; Dold, G.; Trang, D.; Simmons, N.; Perez, A.; Young, N.B.; Barbaro, R.P.; Piven, J.; Magnuson, T.R.; et al. Automated Apparatus for Quantitation of Social Approach Behaviors in Mice. Genes Brain Behav. 2004, 3, 303–314. [Google Scholar] [CrossRef]
- Brodkin, E.S.; Hagemann, A.; Nemetski, S.M.; Silver, L.M. Social Approach-Avoidance Behavior of Inbred Mouse Strains towards DBA/2 Mice. Brain Res. 2004, 1002, 151–157. [Google Scholar] [CrossRef]
- Depino, A.M.; Lucchina, L.; Pitossi, F. Early and Adult Hippocampal TGF-Β1 Overexpression Have Opposite Effects on Behavior. Brain. Behav. Immun. 2011, 25. [Google Scholar] [CrossRef]
- Pietropaolo, S.; Guilleminot, A.; Martin, B.; D’Amato, F.R.; Crusio, W.E. Genetic-Background Modulation of Core and Variable Autistic-like Symptoms in Fmr1 Knock-out Mice. PLoS ONE 2011, 6, e17073. [Google Scholar] [CrossRef]
- Kazlauskas, N.; Seiffe, A.; Campolongo, M.; Zappala, C.; Depino, A.M. Sex-Specific Effects of Prenatal Valproic Acid Exposure on Sociability and Neuroinflammation: Relevance for Susceptibility and Resilience in Autism. Psychoneuroendocrinology 2019, 110, 104441. [Google Scholar] [CrossRef]
- Seiffe, A.; Federico Ramirez, M.; Darío Barrios, C.; Milagros Albarrán, M.; Mara Depino, A.; Ramirez, M.F.; Barrios, C.D.; Albarrán, M.M.; Depino, A.M. Early Estradiol Exposure Masculinizes Disease-relevant Behaviors in Female Mice. Eur. J. Neurosci. 2021, 53, 2483–2499. [Google Scholar] [CrossRef] [PubMed]
- Kopachev, N.; Netser, S.; Wagner, S. Sex-Dependent Features of Social Behavior Differ between Distinct Laboratory Mouse Strains and Their Mixed Offspring. iScience 2022, 25, 103735. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Kennedy, A.; Burgos-Artizzu, X.P.; Zelikowsky, M.; Navonne, S.G.; Perona, P.; Anderson, D.J. Automated Measurement of Mouse Social Behaviors Using Depth Sensing, Video Tracking, and Machine Learning. Proc. Natl. Acad. Sci. USA 2015, 112, E5351–E5360. [Google Scholar] [CrossRef] [PubMed]
- Argue, K.J.; McCarthy, M.M. Utilization of Same- vs. Mixed-Sex Dyads Impacts the Observation of Sex Differences in Juvenile Social Play Behavior. Curr. Neurobiol. 2000, 6, 17–23. [Google Scholar] [CrossRef]
- Argue, K.J.; McCarthy, M.M. Characterization of Juvenile Play in Rats: Importance of Sex of Self and Sex of Partner. Biol. Sex Differ. 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Lidster, K.; Owen, K.; Browne, W.J.; Prescott, M.J. Cage Aggression in Group-Housed Laboratory Male Mice: An International Data Crowdsourcing Project. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Auger, A.P.; Olesen, K.M. Brain Sex Differences and the Organisation of Juvenile Social Play Behaviour. J. Neuroendocrinol. 2009, 21, 519–525. [Google Scholar] [CrossRef]
- Terranova, M.L.; Laviola, G.; Alleva, E. Ontogeny of Amicable Social Behavior in the Mouse: Gender Differences and Ongoing Isolation Outcomes. Dev. Psychobiol. 1993, 26, 467–481. [Google Scholar] [CrossRef]
- Hörnberg, H.; Pérez-Garci, E.; Schreiner, D.; Hatstatt-Burklé, L.; Magara, F.; Baudouin, S.; Matter, A.; Nacro, K.; Pecho-Vrieseling, E.; Scheiffele, P. Rescue of Oxytocin Response and Social Behaviour in a Mouse Model of Autism. Nature 2020, 584, 252–256. [Google Scholar] [CrossRef]
- Panksepp, J.B.; Lahvis, G.P. Social Reward among Juvenile Mice. Genes Brain Behav. 2007, 6, 661–671. [Google Scholar] [CrossRef]
- Cann, C.; Venniro, M.; Hope, B.T.; Ramsey, L.A. Parametric Investigation of Social Place Preference in Adolescent Mice. Behav. Neurosci. 2020, 134, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Crawley, J.N. Simple Behavioral Assessment of Mouse Olfaction. Curr. Protoc. Neurosci. 2009, 48. [Google Scholar] [CrossRef] [PubMed]
- Campolongo, M.; Kazlauskas, N.; Falasco, G.; Urrutia, L.; Salgueiro, N.; Höcht, C.; Depino, A.M. Sociability Deficits after Prenatal Exposure to Valproic Acid Are Rescued by Early Social Enrichment. Mol. Autism 2018, 9, 36. [Google Scholar] [CrossRef]
- Pankevich, D.E.; Bale, T.L. Stress and Sex Influences on Food-Seeking Behaviors. Obesity 2008, 16, 1539–1544. [Google Scholar] [CrossRef]
- Baum, M.J.; Keverne, E.B. Sex Difference in Attraction Thresholds for Volatile Odors from Male and Estrous Female Mouse Urine. Horm. Behav. 2002, 41, 213–219. [Google Scholar] [CrossRef]
- Kass, M.D.; Czarnecki, L.A.; Moberly, A.H.; McGann, J.P. Differences in Peripheral Sensory Input to the Olfactory Bulb between Male and Female Mice. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Scattoni, M.L.; Gandhy, S.U.; Ricceri, L.; Crawley, J.N. Unusual Repertoire of Vocalizations in the BTBR T+tf/J Mouse Model of Autism. PLoS ONE 2008, 3, 48–52. [Google Scholar] [CrossRef]
- Panksepp, J.B.; Jochman, K.A.; Kim, J.U.; Koy, J.K.; Wilson, E.D.; Chen, Q.; Wilson, C.R.; Lahvis, G.P. Affiliative Behavior, Ultrasonic Communication and Social Reward Are Influenced by Genetic Variation in Adolescent Mice. PLoS ONE 2007, 2, e351. [Google Scholar] [CrossRef]
- Branchi, I.; Santucci, D.; Alleva, E. Ultrasonic Vocalisation Emitted by Infant Rodents: A Tool for Assessment of Neurobehavioural Development. Behav. Brain Res. 2001, 125, 49–56. [Google Scholar] [CrossRef]
- Egnor, S.E.R.; Seagraves, K.M. The Contribution of Ultrasonic Vocalizations to Mouse Courtship. Curr. Opin. Neurobiol. 2016, 38, 1–5. [Google Scholar] [CrossRef]
- Moles, A.; Costantini, F.; Garbugino, L.; Zanettini, C.; D’Amato, F.R. Ultrasonic Vocalizations Emitted during Dyadic Interactions in Female Mice: A Possible Index of Sociability? Behav. Brain Res. 2007, 182, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Scattoni, M.L.; Crawley, J.; Ricceri, L. Ultrasonic Vocalizations: A Tool for Behavioural Phenotyping of Mouse Models of Neurodevelopmental Disorders. Neurosci. Biobehav. Rev. 2009, 33, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Faure, A.; Pittaras, E.; Nosjean, A.; Chabout, J.; Cressant, A.; Granon, S. Social Behaviors and Acoustic Vocalizations in Different Strains of Mice. Behav. Brain Res. 2017, 320, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, T.; Lee, C.C. Neural Mechanisms Underlying Repetitive Behaviors in Rodent Models of Autism Spectrum Disorders. Front. Cell. Neurosci. 2021, 14, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Lucchina, L.; Depino, A.M. Altered Peripheral and Central Inflammatory Responses in a Mouse Model of Autism. Autism Res. 2014, 7, 273–289. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Stewart, A.M.; Song, C.; Berridge, K.C.; Graybiel, A.M.; Fentress, J.C. Neurobiology of Rodent Self-Grooming and Its Value for Translational Neuroscience. Nat. Rev. Neurosci. 2016, 17, 45–59. [Google Scholar] [CrossRef]
- Teissier, A.; Le Magueresse, C.; Olusakin, J.; Andrade da Costa, B.L.S.; De Stasi, A.M.; Bacci, A.; Imamura Kawasawa, Y.; Vaidya, V.A.; Gaspar, P. Early-Life Stress Impairs Postnatal Oligodendrogenesis and Adult Emotional Behaviour through Activity-Dependent Mechanisms. Mol. Psychiatry 2020, 25, 1159–1174. [Google Scholar] [CrossRef]
- Pitzer, C.; Kurpiers, B.; Eltokhi, A. Sex Differences in Depression-Like Behaviors in Adult Mice Depend on Endophenotype and Strain. Front. Behav. Neurosci. 2022, 16, 1–8. [Google Scholar] [CrossRef]
- Thomas, A.; Burant, A.; Bui, N.; Graham, D.; Yuva-Paylor, L.A.; Paylor, R. Marble Burying Reflects a Repetitive and Perseverative Behavior More than Novelty-Induced Anxiety. Psychopharmacology 2009, 204, 361–373. [Google Scholar] [CrossRef]
- de Brouwer, G.; Fick, A.; Harvey, B.H.; Wolmarans, D.W. A Critical Inquiry into Marble-Burying as a Preclinical Screening Paradigm of Relevance for Anxiety and Obsessive–Compulsive Disorder: Mapping the Way Forward. Cogn. Affect. Behav. Neurosci. 2019, 19, 1–39. [Google Scholar] [CrossRef]
- Schneider, T.; Popik, P. Attenuation of Estrous Cycle-Dependent Marble Burying in Female Rats by Acute Treatment with Progesterone and Antidepressants. Psychoneuroendocrinology 2007, 32, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Dember, W.N.; Fowler, H. Spontaneous Alternation Behavior. Psychol. Bull. 1958, 55, 412–428. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, R. The Neurobiological Basis of Spontaneous Alternation. Neurosci. Biobehav. Rev. 2002, 26, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Isseroff, A. Limited Recovery of Spontaneous Alternation after Extensive Hippocampal Damage: Evidence for a Memory Impairment. Exp. Neurol. 1979, 64, 284–294. [Google Scholar] [CrossRef]
- Brimberg, L.; Mader, S.; Jeganathan, V.; Berlin, R.; Coleman, T.R.; Gregersen, P.K.; Huerta, P.T.; Volpe, B.T.; Diamond, B. Caspr2-Reactive Antibody Cloned from a Mother of an ASD Child Mediates an ASD-like Phenotype in Mice. Mol. Psychiatry 2016, 21, 1663–1671. [Google Scholar] [CrossRef]
- Remmelink, E.; Smit, A.B.; Verhage, M.; Loos, M. Measuring Discrimination- and Reversal Learning in Mouse Models within 4 Days and without Prior Food Deprivation. Learn. Mem. 2016, 23, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Aarde, S.M.; Genner, R.M.; Hrncir, H.; Arnold, A.P.; Jentsch, J.D. Sex Chromosome Complement Affects Multiple Aspects of Reversal-Learning Task Performance in Mice. Genes Brain Behav. 2021, 20, 1–11. [Google Scholar] [CrossRef]
- Moy, S.S.; Nadler, J.J.; Poe, M.D.; Nonneman, R.J.; Young, N.B.; Koller, B.H.; Crawley, J.N.; Duncan, G.E.; Bodfish, J.W. Development of a Mouse Test for Repetitive, Restricted Behaviors: Relevance to Autism. Behav. Brain Res. 2008, 188, 178–194. [Google Scholar] [CrossRef]
- Bettis, T.; Jacobs, L.F. Sex Differences in Object Recognition Are Modulated by Object Similarity. Behav. Brain Res. 2012, 233, 288–292. [Google Scholar] [CrossRef]
- Kazlauskas, N.; Campolongo, M.; Lucchina, L.; Zappala, C.; Depino, A.M. Postnatal Behavioral and Inflammatory Alterations in Female Pups Prenatally Exposed to Valproic Acid. Psychoneuroendocrinology 2016, 72, 11–21. [Google Scholar] [CrossRef]
- Plappert, C.F.; Rodenbücher, A.M.; Pilz, P.K.D. Effects of Sex and Estrous Cycle on Modulation of the Acoustic Startle Response in Mice. Physiol. Behav. 2005, 84, 585–594. [Google Scholar] [CrossRef]
- Mitrovic, I.; Margeta-Mitrovic, M.; Bader, S.; Stoffel, M.; Jan, L.Y.; Basbaum, A.I. Contribution of GIRK2-Mediated Postsynaptic Signaling to Opiate and A2-Adrenergic Analgesia and Analgesic Sex Differences. Proc. Natl. Acad. Sci. USA 2003, 100, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Mir, F.R.; Rivarola, M.A. Sex Differences in Anxiety and Depression: What Can (and Cannot) Preclinical Studies Tell Us? Sexes 2022, 3, 141–163. [Google Scholar] [CrossRef]
- Crawley, J.N.; Paylor, R. A Proposed Test Battery and Constellations of Specific Behavioral Paradigms to Investigate the Behavioral Phenotypes of Transgenic and Knockout Mice. Horm. Behav. 1997, 31, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.C.; Kassee, C.; Besney, R.; Bonato, S.; Hull, L.; Mandy, W.; Szatmari, P.; Ameis, S.H. Prevalence of Co-Occurring Mental Health Diagnoses in the Autism Population: A Systematic Review and Meta-Analysis. Lancet Psychiatry 2019, 6, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, N.; Ganster, T.; O’Leary, A.; Popp, S.; Freudenberg, F.; Reif, A.; Soler Artigas, M.; Ribasés, M.; Ramos-Quiroga, J.A.; Lesch, K.P.; et al. Dissociation of Impulsivity and Aggression in Mice Deficient for the ADHD Risk Gene Adgrl3: Evidence for Dopamine Transporter Dysregulation. Neuropharmacology 2019, 156, 107557. [Google Scholar] [CrossRef]
- Dougnon, G.; Matsui, H. Modelling Autism Spectrum Disorder (ASD) and Attention-Deficit/Hyperactivity Disorder (ADHD) Using Mice and Zebrafish. Int. J. Mol. Sci. 2022, 23, 7550. [Google Scholar] [CrossRef]
- Jhun, M.; Panwar, A.; Cordner, R.; Irvin, D.K.; Veiga, L.; Yeager, N.; Pechnick, R.N.; Schubloom, H.; Black, K.L.; Wheeler, C.J. CD103 Deficiency Promotes Autism (ASD) and Attention-Deficit Hyperactivity Disorder (ADHD) Behavioral Spectra and Reduces Age-Related Cognitive Decline. Front. Neurol. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial Activation and Neuroinflammation in the Brain of Patients with Autism. Ann. Neurol. 2005, 57, 67–81. [Google Scholar] [CrossRef]
- Casanova, M.F. The Neuropathology of Autism. Brain Pathol. 2007, 17, 422–433. [Google Scholar] [CrossRef]
- Jyonouchi, H.; Sun, S.; Le, H. Proinflammatory and Regulatory Cytokine Production Associated with Innate and Adaptive Immune Responses in Children with Autism Spectrum Disorders and Developmental Regression. J. Neuroimmunol. 2001, 120, 170–179. [Google Scholar] [CrossRef]
- Gupta, S.; Aggarwal, S.; Rashanravan, B.; Lee, T. Th1- and Th2-like Cytokines in CD4+ and CD8+ T Cells in Autism. J. Neuroimmunol. 1998, 85, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Warren, R.; Averett, R.; Ghaziuddin, M. Circulating Autoantibodies to Neuronal and Glial Filament Proteins in Autism. Pediatr. Neurol. 1997, 17, 88–90. [Google Scholar] [CrossRef]
- Vojdani, A.; Campbell, A.; Anyanwu, E.; Kashanian, A.; Bock, K.; Vojdani, E. Erratum: Antibodies to Neuron-Specific Antigens in Children with Autism: Possible Cross-Reaction with Encephalitogenic Proteins from Milk, Chlamydia Pneumoniae and Streptococcus Group A (Journal of Neuroimmunology (2002) 129 (168) S0165572802001807). J. Neuroimmunol. 2002, 130, 248. [Google Scholar] [CrossRef]
- Depino, A.M. Peripheral and Central Inflammation in Autism Spectrum Disorders. Mol. Cell. Neurosci. 2013, 53. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Fatemi, S.H.; Sidwell, R.W.; Patterson, P.H. Maternal Influenza Infection Causes Marked Behavioral and Pharmacological Changes in the Offspring. J. Neurosci. 2003, 23, 297–302. [Google Scholar] [CrossRef]
- Weiser, M.J.; Mucha, B.; Denheyer, H.; Atkinson, D.; Schanz, N.; Vassiliou, E.; Benno, R.H. Dietary Docosahexaenoic Acid Alleviates Autistic-like Behaviors Resulting from Maternal Immune Activation in Mice. Prostaglandins Leukot. Essent. Fat. Acids 2016, 106, 27–37. [Google Scholar] [CrossRef]
- Ruskin, D.N.; Murphy, M.I.; Slade, S.L.; Masino, S.A. Ketogenic Diet Improves Behaviors in a Maternal Immune Activation Model of Autism Spectrum Disorder. PLoS ONE 2017, 12, e0171643. [Google Scholar] [CrossRef]
- Fortunato, J.J.; da Rosa, N.; Martins Laurentino, A.O.; Goulart, M.; Michalak, C.; Borges, L.P.; da Cruz Cittadin Soares, E.; Reis, P.A.; de Castro Faria Neto, H.C.; Petronilho, F. Effects of ω-3 Fatty Acids on Stereotypical Behavior and Social Interactions in Wistar Rats Prenatally Exposed to Lipopolysaccarides. Nutrition 2017, 35, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Pendyala, G.; Chou, S.; Jung, Y.; Coiro, P.; Spartz, E.; Padmashri, R.; Li, M.; Dunaevsky, A. Maternal Immune Activation Causes Behavioral Impairments and Altered Cerebellar Cytokine and Synaptic Protein Expression. Neuropsychopharmacology 2017, 42, 1435–1446. [Google Scholar] [CrossRef]
- Kirsten, T.B.; Chaves-Kirsten, G.P.; Chaible, L.M.; Silva, A.C.; Martins, D.O.; Britto, L.R.G.; Dagli, M.L.Z.; Torrão, A.S.; Palermo-Neto, J.; Bernardi, M.M. Hypoactivity of the Central Dopaminergic System and Autistic-like Behavior Induced by a Single Early Prenatal Exposure to Lipopolysaccharide. J. Neurosci. Res. 2012, 90, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Gata-Garcia, A.; Porat, A.; Brimberg, L.; Volpe, B.T.; Huerta, P.T.; Diamond, B. Contributions of Sex Chromosomes and Gonadal Hormones to the Male Bias in a Maternal Antibody-Induced Model of Autism Spectrum Disorder. Front. Neurol. 2021, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.P.; Li, J.; Garbett, K.; Mirnics, K.; Patterson, P.H. Maternal Immune Activation Alters Fetal Brain Development through Interleukin-6. J. Neurosci. 2007, 27, 10695–10702. [Google Scholar] [CrossRef]
- Malkova, N.V.; Yu, C.Z.; Hsiao, E.Y.; Moore, M.J.; Patterson, P.H. Maternal Immune Activation Yields Offspring Displaying Mouse Versions of the Three Core Symptoms of Autism. Brain. Behav. Immun. 2012, 26, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Tartaglione, A.M.; Villani, A.; Ajmone-Cat, M.A.; Minghetti, L.; Ricceri, L.; Pazienza, V.; De Simone, R.; Calamandrei, G. Maternal Immune Activation Induces Autism-like Changes in Behavior, Neuroinflammatory Profile and Gut Microbiota in Mouse Offspring of Both Sexes. Transl. Psychiatry 2022, 12, 384. [Google Scholar] [CrossRef]
- Wu, W.-L.; Hsiao, E.Y.; Yan, Z.; Mazmanian, S.K.; Patterson, P.H. The Placental Interleukin-6 Signaling Controls Fetal Brain Development and Behavior. Brain. Behav. Immun. 2017, 62, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Golan, H.M.; Lev, V.; Hallak, M.; Sorokin, Y.; Huleihel, M. Specific Neurodevelopmental Damage in Mice Offspring Following Maternal Inflammation during Pregnancy. Neuropharmacology 2005, 48, 903–917. [Google Scholar] [CrossRef]
- Hava, G.; Vered, L.; Yael, M.; Mordechai, H.; Mahoud, H. Alterations in Behavior in Adult Offspring Mice Following Maternal Inflammation during Pregnancy. Dev. Psychobiol. 2006, 48, 162–168. [Google Scholar] [CrossRef]
- Kirsten, T.B.; Taricano, M.; Maiorka, P.C.; Palermo-Neto, J.; Bernardi, M.M. Prenatal Lipopolysaccharide Reduces Social Behavior in Male Offspring. Neuroimmunomodulation 2010, 17, 240–251. [Google Scholar] [CrossRef]
- Wu, W.-L.; Adams, C.E.; Stevens, K.E.; Chow, K.-H.; Freedman, R.; Patterson, P.H. The Interaction between Maternal Immune Activation and Alpha 7 Nicotinic Acetylcholine Receptor in Regulating Behaviors in the Offspring. Brain. Behav. Immun. 2015, 46, 192–202. [Google Scholar] [CrossRef]
- Han, J.; Fan, Y.; Zhou, K.; Blomgren, K.; Harris, R.A. Uncovering Sex Differences of Rodent Microglia. J. Neuroinflammation 2021, 18, 1–11. [Google Scholar] [CrossRef]
- Geschwind, D.H.; Levitt, P. Autism Spectrum Disorders: Developmental Disconnection Syndromes. Curr. Opin. Neurobiol. 2007, 17, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Khazipov, R.; Luhmann, H.J. Early Patterns of Electrical Activity in the Developing Cerebral Cortex of Humans and Rodents. Trends Neurosci. 2006, 29, 414–418. [Google Scholar] [CrossRef]
- Hutsler, J.J.; Zhang, H. Increased Dendritic Spine Densities on Cortical Projection Neurons in Autism Spectrum Disorders. Brain Res. 2010, 1309, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Bonsi, P.; De Jaco, A.; Fasano, L.; Gubellini, P. Postsynaptic Autism Spectrum Disorder Genes and Synaptic Dysfunction. Neurobiol. Dis. 2022, 162, 105564. [Google Scholar] [CrossRef]
- Coiro, P.; Padmashri, R.; Suresh, A.; Spartz, E.; Pendyala, G.; Chou, S.; Jung, Y.; Meays, B.; Roy, S.; Gautam, N.; et al. Impaired Synaptic Development in a Maternal Immune Activation Mouse Model of Neurodevelopmental Disorders. Brain. Behav. Immun. 2015, 50, 249–258. [Google Scholar] [CrossRef]
- Sgritta, M.; Vignoli, B.; Pimpinella, D.; Griguoli, M.; Santi, S.; Bialowas, A.; Wiera, G.; Zacchi, P.; Malerba, F.; Marchetti, C.; et al. Impaired Synaptic Plasticity in an Animal Model of Autism Exhibiting Early Hippocampal GABAergic-BDNF/TrkB Signaling Alterations. iScience 2023, 26, 105728. [Google Scholar] [CrossRef] [PubMed]
- Jamain, S.; Radyushkin, K.; Hammerschmidt, K.; Granon, S.; Boretius, S.; Varoqueaux, F.; Ramanantsoa, N.; Gallego, J.; Ronnenberg, A.; Winter, D.; et al. Reduced Social Interaction and Ultrasonic Communication in a Mouse Model of Monogenic Heritable Autism. Proc. Natl. Acad. Sci. USA 2008, 105, 1710–1715. [Google Scholar] [CrossRef]
- El-Kordi, A.; Winkler, D.; Hammerschmidt, K.; Kästner, A.; Krueger, D.; Ronnenberg, A.; Ritter, C.; Jatho, J.; Radyushkin, K.; Bourgeron, T.; et al. Development of an Autism Severity Score for Mice Using Nlgn4 Null Mutants as a Construct-Valid Model of Heritable Monogenic Autism. Behav. Brain Res. 2013, 251, 41–49. [Google Scholar] [CrossRef]
- Chadman, K.K.; Gong, S.; Scattoni, M.L.; Boltuck, S.E.; Gandhy, S.U.; Heintz, N.; Crawley, J.N. Minimal Aberrant Behavioral Phenotypes of Neuroligin-3 R451C Knockin Mice. Autism Res. 2008, 1, 147–158. [Google Scholar] [CrossRef]
- Tabuchi, K.; Blundell, J.; Etherton, M.R.; Hammer, R.E.; Liu, X.; Powell, C.M.; Südhof, T.C. A Neuroligin-3 Mutation Implicated in Autism Increases Inhibitory Synaptic Transmission in Mice. Science 2007, 318, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Radyushkin, K.; Hammerschmidt, K.; Boretius, S.; Varoqueaux, F.; El-Kordi, A.; Ronnenberg, A.; Winter, D.; Frahm, J.; Fischer, J.; Brose, N.; et al. Neuroligin-3-Deficient Mice: Model of a Monogenic Heritable Form of Autism with an Olfactory Deficit. Genes Brain Behav. 2009, 8, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.E.; Fuccillo, M.V.; Maxeiner, S.; Hayton, S.J.; Gokce, O.; Lim, B.K.; Fowler, S.C.; Malenka, R.C.; Südhof, T.C. Autism-Associated Neuroligin-3 Mutations Commonly Impair Striatal Circuits to Boost Repetitive Behaviors. Cell 2014, 158, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.; Blaiss, C.A.; Etherton, M.R.; Espinosa, F.; Tabuchi, K.; Walz, C.; Bolliger, M.F.; Südhof, T.C.; Powell, C.M. Neuroligin-1 Deletion Results in Impaired Spatial Memory and Increased Repetitive Behavior. J. Neurosci. 2010, 30, 2115–2129. [Google Scholar] [CrossRef]
- Etherton, M.R.; Blaiss, C.A.; Powell, C.M.; Südhof, T.C. Mouse Neurexin-1α Deletion Causes Correlated Electrophysiological and Behavioral Changes Consistent with Cognitive Impairments. Proc. Natl. Acad. Sci. USA 2009, 106, 17998–18003. [Google Scholar] [CrossRef]
- Silverman, J.L.; Turner, S.M.; Barkan, C.L.; Tolu, S.S.; Saxena, R.; Hung, A.Y.; Sheng, M.; Crawley, J.N. Sociability and Motor Functions in Shank1 Mutant Mice. Brain Res. 2011, 1380, 120–137. [Google Scholar] [CrossRef]
- Schmeisser, M.J.; Ey, E.; Wegener, S.; Bockmann, J.; Stempel, A.V.; Kuebler, A.; Janssen, A.-L.; Udvardi, P.T.; Shiban, E.; Spilker, C.; et al. Autistic-like Behaviours and Hyperactivity in Mice Lacking ProSAP1/Shank2. Nature 2012, 486, 256–260. [Google Scholar] [CrossRef]
- Won, H.; Lee, H.-R.; Gee, H.Y.; Mah, W.; Kim, J.-I.; Lee, J.; Ha, S.; Chung, C.; Jung, E.S.; Cho, Y.S.; et al. Autistic-like Social Behaviour in Shank2-Mutant Mice Improved by Restoring NMDA Receptor Function. Nature 2012, 486, 261–265. [Google Scholar] [CrossRef]
- Bozdagi, O.; Sakurai, T.; Papapetrou, D.; Wang, X.; Dickstein, D.L.; Takahashi, N.; Kajiwara, Y.; Yang, M.; Katz, A.M.; Scattoni, M.L.; et al. Haploinsufficiency of the Autism-Associated Shank3 Gene Leads to Deficits in Synaptic Function, Social Interaction, and Social Communication. Mol. Autism 2010, 1, 15. [Google Scholar] [CrossRef]
- Peça, J.; Feliciano, C.; Ting, J.T.; Wang, W.; Wells, M.F.; Venkatraman, T.N.; Lascola, C.D.; Fu, Z.; Feng, G. Shank3 Mutant Mice Display Autistic-like Behaviours and Striatal Dysfunction. Nature 2011, 472, 437–442. [Google Scholar] [CrossRef]
- Wang, X.; McCoy, P.A.; Rodriguiz, R.M.; Pan, Y.; Je, H.S.; Roberts, A.C.; Kim, C.J.; Berrios, J.; Colvin, J.S.; Bousquet-Moore, D.; et al. Synaptic Dysfunction and Abnormal Behaviors in Mice Lacking Major Isoforms of Shank3. Hum. Mol. Genet. 2011, 20, 3093–3108. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, C.; Chen, Q.; van der Goes, M.-S.; Hawrot, J.; Yao, A.Y.; Gao, X.; Lu, C.; Zang, Y.; Zhang, Q.; et al. Striatopallidal Dysfunction Underlies Repetitive Behavior in Shank3-Deficient Model of Autism. J. Clin. Investig. 2017, 127, 1978–1990. [Google Scholar] [CrossRef] [PubMed]
- Kouser, M.; Speed, H.E.; Dewey, C.M.; Reimers, J.M.; Widman, A.J.; Gupta, N.; Liu, S.; Jaramillo, T.C.; Bangash, M.; Xiao, B.; et al. Loss of Predominant Shank3 Isoforms Results in Hippocampus-Dependent Impairments in Behavior and Synaptic Transmission. J. Neurosci. 2013, 33, 18448–18468. [Google Scholar] [CrossRef]
- Balaan, C.; Corley, M.J.; Eulalio, T.; Leite-ahyo, K.; Pang, A.P.S.; Fang, R.; Khadka, V.S.; Maunakea, A.K.; Ward, M.A. Juvenile Shank3b Deficient Mice Present with Behavioral Phenotype Relevant to Autism Spectrum Disorder. Behav. Brain Res. 2019, 356, 137–147. [Google Scholar] [CrossRef]
- Nicolini, C.; Fahnestock, M. The Valproic Acid-Induced Rodent Model of Autism. Exp. Neurol. 2018, 299, 217–227. [Google Scholar] [CrossRef]
- Schneider, T.; Roman, A.; Basta-Kaim, A.; Kubera, M.; Budziszewska, B.; Schneider, K.; Przewłocki, R. Gender-Specific Behavioral and Immunological Alterations in an Animal Model of Autism Induced by Prenatal Exposure to Valproic Acid. Psychoneuroendocrinology 2008, 33, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Kentner, A.C.; Bilbo, S.D.; Brown, A.S.; Hsiao, E.Y.; McAllister, A.K.; Meyer, U.; Pearce, B.D.; Pletnikov, M.V.; Yolken, R.H.; Bauman, M.D. Maternal Immune Activation: Reporting Guidelines to Improve the Rigor, Reproducibility, and Transparency of the Model. Neuropsychopharmacology 2019, 44, 245–258. [Google Scholar] [CrossRef]
- Carlezon, W.A.; Kim, W.; Missig, G.; Finger, B.C.; Landino, S.M.; Alexander, A.J.; Mokler, E.L.; Robbins, J.O.; Li, Y.; Bolshakov, V.Y.; et al. Maternal and Early Postnatal Immune Activation Produce Sex-Specific Effects on Autism-like Behaviors and Neuroimmune Function in Mice. Sci. Rep. 2019, 9, 1–18. [Google Scholar] [CrossRef]
- Hsueh, P.-T.; Lin, H.-H.; Wang, H.-H.; Liu, C.-L.; Ni, W.-F.; Liu, J.-K.; Chang, H.-H.; Sun, D.-S.; Chen, Y.-S.; Chen, Y.-L. Immune Imbalance of Global Gene Expression, and Cytokine, Chemokine and Selectin Levels in the Brains of Offspring with Social Deficits via Maternal Immune Activation. Genes, Brain Behav. 2018, 17, e12479. [Google Scholar] [CrossRef]
- MacRae, M.; Macrina, T.; Khoury, A.; Migliore, M.M.; Kentner, A.C. Tracing the Trajectory of Behavioral Impairments and Oxidative Stress in an Animal Model of Neonatal Inflammation. Neuroscience 2015, 298, 455–466. [Google Scholar] [CrossRef]
- Kentner, A.C.; Scalia, S.; Shin, J.; Migliore, M.M.; Rondón-Ortiz, A.N. Targeted Sensory Enrichment Interventions Protect against Behavioral and Neuroendocrine Consequences of Early Life Stress. Psychoneuroendocrinology 2018, 98, 74–85. [Google Scholar] [CrossRef]
- Smith, C.J.; Kingsbury, M.A.; Dziabis, J.E.; Hanamsagar, R.; Malacon, K.E.; Tran, J.N.; Norris, H.A.; Gulino, M.; Bordt, E.A.; Bilbo, S.D. Neonatal Immune Challenge Induces Female-Specific Changes in Social Behavior and Somatostatin Cell Number. Brain. Behav. Immun. 2020, 90, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Custódio, C.S.; Mello, B.S.F.; Filho, A.J.M.C.; de Carvalho Lima, C.N.; Cordeiro, R.C.; Miyajima, F.; Réus, G.Z.; Vasconcelos, S.M.M.; Barichello, T.; Quevedo, J.; et al. Neonatal Immune Challenge with Lipopolysaccharide Triggers Long-Lasting Sex- and Age-Related Behavioral and Immune/Neurotrophic Alterations in Mice: Relevance to Autism Spectrum Disorders. Mol. Neurobiol. 2018, 55, 3775–3788. [Google Scholar] [CrossRef] [PubMed]
- Macfabe, D.F.; Cain, N.E.; Boon, F.; Ossenkopp, K.; Cain, D.P. Effects of the Enteric Bacterial Metabolic Product Propionic Acid on Object-Directed Behavior, Social Behavior, Cognition, and Neuroinflammation in Adolescent Rats: Relevance to Autism Spectrum Disorder. Behav. Brain Res. 2011, 217, 47–54. [Google Scholar] [CrossRef]
- Shultz, S.R.; Aziz, N.A.B.; Yang, L.; Sun, M.; Macfabe, D.F.; Brien, T.J.O. Intracerebroventricular Injection of Propionic Acid, an Enteric Metabolite Implicated in Autism, Induces Social Abnormalities That Do Not Differ between Seizure-Prone (FAST) and Seizure-Resistant (SLOW) Rats. Behav. Brain Res. 2015, 278, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, S.; Won, J.; Jin, Y.; Hong, Y.; Hur, T.; Kim, J.; Lee, S.; Hong, Y. Pathophysiological and Neurobehavioral Characteristics of a Propionic Acid-Mediated Autism-like Rat Model. PLoS ONE 2018, 13, e0192925. [Google Scholar] [CrossRef]
- Shams, S.; Foley, K.A.; Kavaliers, M.; Macfabe, D.F.; Ossenkopp, K.P. Systemic Treatment with the Enteric Bacterial Metabolic Product Propionic Acid Results in Reduction of Social Behavior in Juvenile Rats: Contribution to a Rodent Model of Autism Spectrum Disorder. Dev. Psychobiol. 2019, 61, 688–699. [Google Scholar] [CrossRef]
- Foley, K.A.; MacFabe, D.F.; Vaz, A.; Ossenkopp, K.; Kavaliers, M. Sexually Dimorphic Effects of Prenatal Exposure to Propionic Acid and Lipopolysaccharide on Social Behavior in Neonatal, Adolescent, and Adult Rats: Implications for Autism Spectrum Disorders. Int. J. Dev. Neurosci. 2014, 39, 68–78. [Google Scholar] [CrossRef]
- Ferguson, J.N.; Young, L.J.; Hearn, E.F.; Matzuk, M.M.; Insel, T.R.; Winslow, J.T. Social Amnesia in Mice Lacking the Oxytocin Gene. Nat. Genet. 2000, 25, 284–288. [Google Scholar] [CrossRef]
- Crawley, J.N.; Chen, T.; Puri, A.; Washburn, R.; Sullivan, T.L.; Hill, J.M.; Young, N.B.; Nadler, J.J.; Moy, S.S.; Young, L.J.; et al. Social Approach Behaviors in Oxytocin Knockout Mice: Comparison of Two Independent Lines Tested in Different Laboratory Environments. Neuropeptides 2007, 41, 145–163. [Google Scholar] [CrossRef]
- Scattoni, M.; Mcfarlane, H.; Zhodzishsky, V.; Caldwell, H.; Young, W.; Ricceri, L.; Crawley, J. Reduced Ultrasonic Vocalizations in Vasopressin 1b Knockout Mice. Behav. Brain Res. 2008, 187, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Bielsky, I.F.; Hu, S.-B.; Szegda, K.L.; Westphal, H.; Young, L.J. Profound Impairment in Social Recognition and Reduction in Anxiety-Like Behavior in Vasopressin V1a Receptor Knockout Mice. Neuropsychopharmacology 2004, 29, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Bielsky, I.F.; Hu, S.-B.; Ren, X.; Terwilliger, E.F.; Young, L.J. The V1a Vasopressin Receptor Is Necessary and Sufficient for Normal Social Recognition: A Gene Replacement Study. Neuron 2005, 47, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.-H.; Luikart, B.W.; Powell, C.M.; Zhou, J.; Matheny, S.A.; Zhang, W.; Li, Y.; Baker, S.J.; Parada, L.F. Pten Regulates Neuronal Arborization and Social Interaction in Mice. Neuron 2006, 50, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Lugo, J.N.; Smith, G.D.; Arbuckle, E.P.; White, J.; Holley, A.J.; Floruta, C.M.; Ahmed, N.; Gomez, M.C.; Okonkwo, O. Deletion of PTEN Produces Autism-like Behavioral Deficits and Alterations in Synaptic Proteins. Front. Mol. Neurosci. 2014, 7, 1–13. [Google Scholar] [CrossRef]
- Spencer, C.M.; Graham, D.F.; Yuva-Paylor, L.A.; Nelson, D.L.; Paylor, R. Social Behavior in Fmr1 Knockout Mice Carrying a Human FMR1 Transgene. Behav. Neurosci. 2008, 122, 710–715. [Google Scholar] [CrossRef]
- Spencer, C.M.; Alekseyenko, O.; Serysheva, E.; Yuva-Paylor, L.A.; Paylor, R. Altered Anxiety-Related and Social Behaviors in the Fmr1 Knockout Mouse Model of Fragile X Syndrome. Genes, Brain Behav. 2005, 4, 420–430. [Google Scholar] [CrossRef]
- McNaughton, C.H.; Moon, J.; Strawderman, M.S.; Maclean, K.N.; Evans, J.; Strupp, B.J. Evidence for Social Anxiety and Impaired Social Cognition in a Mouse Model of Fragile X Syndrome. Behav. Neurosci. 2008, 122, 293–300. [Google Scholar] [CrossRef]
- Page, D.T.; Kuti, O.J.; Prestia, C.; Sur, M. Haploinsufficiency for Pten and Serotonin Transporter Cooperatively Influences Brain Size and Social Behavior. Proc. Natl. Acad. Sci. USA 2009, 106, 1989–1994. [Google Scholar] [CrossRef]
- Clipperton-Allen, A.E.; Page, D.T. Pten Haploinsufficient Mice Show Broad Brain Overgrowth but Selective Impairments in Autism-Relevant Behavioral Tests. Hum. Mol. Genet. 2014, 23, 3490–3505. [Google Scholar] [CrossRef]
- Nakatani, J.; Tamada, K.; Hatanaka, F.; Ise, S.; Ohta, H.; Inoue, K.; Tomonaga, S.; Watanabe, Y.; Chung, Y.J.; Banerjee, R.; et al. Abnormal Behavior in a Chromosome- Engineered Mouse Model for Human 15q11-13 Duplication Seen in Autism. Cell 2009, 137, 1235–1246. [Google Scholar] [CrossRef]
- Tamada, K.; Tomonaga, S.; Hatanaka, F.; Nakai, N.; Takao, K.; Miyakawa, T.; Nakatani, J.; Takumi, T. Decreased Exploratory Activity in a Mouse Model of 15q Duplication Syndrome; Implications for Disturbance of Serotonin Signaling. PLoS ONE 2010, 5, e15126. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.; Carmona-Mora, P.; Chrast, J.; Krall, P.M.; Canales, C.P.; Lupski, J.R.; Reymond, A.; Walz, K. Abnormal Social Behaviors and Altered Gene Expression Rates in a Mouse Model for Potocki-Lupski Syndrome. Hum. Mol. Genet. 2008, 17, 2486–2495. [Google Scholar] [CrossRef] [PubMed]
- Moy, S.S.; Nadler, J.J.; Young, N.B.; Nonneman, R.J.; Grossman, A.W.; Murphy, D.L.; D’Ercole, A.J.; Crawley, J.N.; Magnuson, T.R.; Lauder, J.M. Social Approach in Genetically Engineered Mouse Lines Relevant to Autism. Genes, Brain Behav. 2009, 8, 129–142. [Google Scholar] [CrossRef]
- Gemelli, T.; Berton, O.; Nelson, E.D.; Perrotti, L.I.; Jaenisch, R.; Monteggia, L.M. Postnatal Loss of Methyl-CpG Binding Protein 2 in the Forebrain Is Sufficient to Mediate Behavioral Aspects of Rett Syndrome in Mice. Biol. Psychiatry 2006, 59, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Moretti, P.; Bouwknecht, J.A.; Teague, R.; Paylor, R.; Zoghbi, H.Y. Abnormalities of Social Interactions and Home-Cage Behavior in a Mouse Model of Rett Syndrome. Hum. Mol. Genet. 2005, 14, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Wöhr, M.; Roullet, F.I.; Crawley, J.N. Reduced Scent Marking and Ultrasonic Vocalizations in the BTBR T+tf/J Mouse Model of Autism. Genes, Brain Behav. 2011, 10, 35–43. [Google Scholar] [CrossRef]
- Coretti, L.; Cristiano, C.; Florio, E.; Scala, G.; Lama, A.; Keller, S.; Cuomo, M.; Russo, R.; Pero, R.; Paciello, O.; et al. Sex-Related Alterations of Gut Microbiota Composition in the BTBR Mouse Model of Autism Spectrum Disorder. Sci. Rep. 2017, 7, 45356. [Google Scholar] [CrossRef]
- Chen, Q.; Panksepp, J.B.; Lahvis, G.P. Empathy Is Moderated by Genetic Background in Mice. PLoS ONE 2009, 4, e4387. [Google Scholar] [CrossRef]
- Moy, S.S.; Nadler, J.J.; Young, N.B.; Nonneman, R.J.; Segall, S.K.; Andrade, G.M.; Crawley, J.N.; Magnuson, T.R. Social Approach and Repetitive Behavior in Eleven Inbred Mouse Strains. Behav. Brain Res. 2008, 191, 118–129. [Google Scholar] [CrossRef]
- Ryan, B.C.; Young, N.B.; Crawley, J.N.; Bodfish, J.W.; Moy, S.S. Social Deficits, Stereotypy and Early Emergence of Repetitive Behavior in the C58/J Inbred Mouse Strain. Behav. Brain Res. 2010, 208, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Muehlmann, A.M.; Edington, G.; Mihalik, A.C.; Buchwald, Z.; Koppuzha, D.; Korah, M.; Lewis, M.H. Further Characterization of Repetitive Behavior in C58 Mice: Developmental Trajectory and Effects of Environmental Enrichment. Behav. Brain Res. 2012, 235, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, C.M.; Curry-Pochy, L.S.; Shafer, R.; Rudy, J.; Lewis, M.H. Reversal Learning in C58 Mice: Modeling Higher Order Repetitive Behavior. Behav. Brain Res. 2017, 332, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.L.; Tolu, S.S.; Barkan, C.L.; Crawley, J.N. Repetitive Self-Grooming Behavior in the BTBR Mouse Model of Autism Is Blocked by the MGluR5 Antagonist MPEP. Neuropsychopharmacology 2010, 35, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Perry, K.; Weber, M.D.; Katz, A.M.; Crawley, J.N. Social Peers Rescue Autism-Relevant Sociability Deficits in Adolescent Mice. Autism Res. 2011, 4, 17–27. [Google Scholar] [CrossRef]
- McFarlane, H.G.; Kusek, G.K.; Yang, M.; Phoenix, J.L.; Bolivar, V.J.; Crawley, J.N. Autism-like Behavioral Phenotypes in BTBR T+tf/J Mice. Genes, Brain Behav. 2008, 7, 152–163. [Google Scholar] [CrossRef]
- Silverman, J.L.; Smith, D.G.; Rizzo, S.J.S.; Karras, M.N.; Turner, S.M.; Tolu, S.S.; Bryce, D.K.; Smith, D.L.; Fonseca, K.; Ring, R.H.; et al. Negative Allosteric Modulation of the MGluR5 Receptor Reduces Repetitive Behaviors and Rescues Social Deficits in Mouse Models of Autism. Sci. Transl. Med. 2012, 4. [Google Scholar] [CrossRef]
- Bolivar, V.; Walters, S.; Phoenix, J. Assessing Autism-like Behavior in Mice: Variations in Social Interactions among Inbred Strains. Behav. Brain Res. 2007, 176, 21–26. [Google Scholar] [CrossRef]
- Yang, M.; Clarke, A.M.; Crawley, J.N. Postnatal Lesion Evidence against a Primary Role for the Corpus Callosum in Mouse Sociability. Eur. J. Neurosci. 2009, 29, 1663–1677. [Google Scholar] [CrossRef]
- Schneider, T.; Przewłocki, R. Behavioral Alterations in Rats Prenatally to Valproic Acid: Animal Model of Autism. Neuropsychopharmacology 2005, 30, 80–89. [Google Scholar] [CrossRef]
- Schneider, T.; Turczak, J.; Przewłocki, R. Environmental Enrichment Reverses Behavioral Alterations in Rats Prenatally Exposed to Valproic Acid: Issues for a Therapeutic Approach in Autism. Neuropsychopharmacology 2006, 31, 36–46. [Google Scholar] [CrossRef]
- Seiffe, A.; Ramírez, M.F.; Sempé, L.; Depino, A.M. Juvenile Handling Rescues Autism-Related Effects of Prenatal Exposure to Valproic Acid. Sci. Rep. 2022, 12, 7174. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.C.; Kim, P.; Go, H.S.; Choi, C.S.; Yang, S.-I.; Cheong, J.H.; Shin, C.Y.; Ko, K.H. The Critical Period of Valproate Exposure to Induce Autistic Symptoms in Sprague–Dawley Rats. Toxicol. Lett. 2011, 201, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Hara, Y.; Ago, Y.; Takano, E.; Hasebe, S.; Nakazawa, T.; Hashimoto, H.; Matsuda, T.; Takuma, K. Environmental Enrichment Attenuates Behavioral Abnormalities in Valproic Acid-Exposed Autism Model Mice. Behav. Brain Res. 2017, 333, 67–73. [Google Scholar] [CrossRef]
- Cheh, M.A.; Millonig, J.H.; Roselli, L.M.; Ming, X.; Jacobsen, E.; Kamdar, S.; Wagner, G.C. En2 Knockout Mice Display Neurobehavioral and Neurochemical Alterations Relevant to Autism Spectrum Disorder. Brain Res. 2006, 1116, 166–176. [Google Scholar] [CrossRef]
- Katayama, Y.; Nishiyama, M.; Shoji, H.; Ohkawa, Y.; Kawamura, A.; Sato, T.; Suyama, M.; Takumi, T.; Miyakawa, T.; Nakayama, K.I. CHD8 Haploinsufficiency Results in Autistic-like Phenotypes in Mice. Nature 2016, 537, 675–679. [Google Scholar] [CrossRef]
- Cherepanov, S.M.; Gerasimenko, M.; Yuhi, T.; Furuhara, K.; Tsuji, C.; Yokoyama, S.; Nakayama, K.I.; Nishiyama, M.; Higashida, H. Oxytocin Ameliorates Impaired Social Behavior in a Chd8 Haploinsufficiency Mouse Model of Autism. BMC Neurosci. 2021, 22, 32. [Google Scholar] [CrossRef]
- Jiménez, J.A.; Simon, J.M.; Hu, W.; Moy, S.S.; Harper, K.M.; Liu, C.-W.; Lu, K.; Zylka, M.J. Developmental Pyrethroid Exposure and Age Influence Phenotypes in a Chd8 Haploinsufficient Autism Mouse Model. Sci. Rep. 2022, 12, 5555. [Google Scholar] [CrossRef]
- Celen, C.; Chuang, J.-C.; Luo, X.; Nijem, N.; Walker, A.K.; Chen, F.; Zhang, S.; Chung, A.S.; Nguyen, L.H.; Nassour, I.; et al. Arid1b Haploinsufficient Mice Reveal Neuropsychiatric Phenotypes and Reversible Causes of Growth Impairment. eLife 2017, 6, e25730. [Google Scholar] [CrossRef]
- Jung, E.-M.; Moffat, J.J.; Liu, J.; Dravid, S.M.; Gurumurthy, C.B.; Kim, W.-Y. Arid1b Haploinsufficiency Disrupts Cortical Interneuron Development and Mouse Behavior. Nat. Neurosci. 2017, 20, 1694–1707. [Google Scholar] [CrossRef]
- Shibutani, M.; Horii, T.; Shoji, H.; Morita, S.; Kimura, M.; Terawaki, N.; Miyakawa, T.; Hatada, I. Arid1b Haploinsufficiency Causes Abnormal Brain Gene Expression and Autism-Related Behaviors in Mice. Int. J. Mol. Sci. 2017, 18, 1872. [Google Scholar] [CrossRef]
- Weigel, B.; Tegethoff, J.F.; Grieder, S.D.; Lim, B.; Nagarajan, B.; Liu, Y.-C.; Truberg, J.; Papageorgiou, D.; Adrian-Segarra, J.M.; Schmidt, L.K.; et al. MYT1L Haploinsufficiency in Human Neurons and Mice Causes Autism-Associated Phenotypes That Can Be Reversed by Genetic and Pharmacologic Intervention. Mol. Psychiatry 2023, 1–14. [Google Scholar] [CrossRef]
- Kim, S.; Oh, H.; Choi, S.H.; Yoo, Y.-E.; Noh, Y.W.; Cho, Y.; Im, G.H.; Lee, C.; Oh, Y.; Yang, E.; et al. Postnatal Age-Differential ASD-like Transcriptomic, Synaptic, and Behavioral Deficits in Myt1l-Mutant Mice. Cell Rep. 2022, 40, 111398. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lambo, M.E.; Ge, X.; Dearborn, J.T.; Liu, Y.; McCullough, K.B.; Swift, R.G.; Tabachnick, D.R.; Tian, L.; Noguchi, K.; et al. A MYT1L Syndrome Mouse Model Recapitulates Patient Phenotypes and Reveals Altered Brain Development Due to Disrupted Neuronal Maturation. Neuron 2021, 109, 3775–3792.e14. [Google Scholar] [CrossRef]
- Wang, H.-G.; Bavley, C.C.; Li, A.; Jones, R.M.; Hackett, J.E.; Bayleyen, Y.; Lee, F.S.; Rajadhyaksha, A.M.; Pitt, G.S. Scn2a Severe Hypomorphic Mutation Decreases Excitatory Synaptic Input and Causes Autism-Associated Behaviors. JCI Insight 2021, 6, e150698. [Google Scholar] [CrossRef]
- Indumathy, J.; Pruitt, A.; Gautier, N.M.; Crane, K.; Glasscock, E. Kv1.1 Deficiency Alters Repetitive and Social Behaviors in Mice and Rescues Autistic-like Behaviors Due to Scn2a Haploinsufficiency. Brain Behav. 2021, 11, e02041. [Google Scholar] [CrossRef]
- Léna, I.; Mantegazza, M. NaV1.2 Haploinsufficiency in Scn2a Knock-out Mice Causes an Autistic-like Phenotype Attenuated with Age. Sci. Rep. 2019, 9, 12886. [Google Scholar] [CrossRef] [PubMed]
- Spratt, P.W.E.; Ben-Shalom, R.; Keeshen, C.M.; Burke, K.J.; Clarkson, R.L.; Sanders, S.J.; Bender, K.J. The Autism-Associated Gene Scn2a Contributes to Dendritic Excitability and Synaptic Function in the Prefrontal Cortex. Neuron 2019, 103, 673–685.e5. [Google Scholar] [CrossRef] [PubMed]
- Tatsukawa, T.; Raveau, M.; Ogiwara, I.; Hattori, S.; Miyamoto, H.; Mazaki, E.; Itohara, S.; Miyakawa, T.; Montal, M.; Yamakawa, K. Scn2a Haploinsufficient Mice Display a Spectrum of Phenotypes Affecting Anxiety, Sociability, Memory Flexibility and Ampakine CX516 Rescues Their Hyperactivity. Mol. Autism 2019, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Hacohen-Kleiman, G.; Yizhar-Barnea, O.; Touloumi, O.; Lagoudaki, R.; Avraham, K.B.; Grigoriadis, N.; Gozes, I. Atypical Auditory Brainstem Response and Protein Expression Aberrations Related to ASD and Hearing Loss in the Adnp Haploinsufficient Mouse Brain. Neurochem. Res. 2019, 44, 1494–1507. [Google Scholar] [CrossRef] [PubMed]
- Hacohen-Kleiman, G.; Sragovich, S.; Karmon, G.; Gao, A.Y.L.; Grigg, I.; Pasmanik-Chor, M.; Le, A.; Korenková, V.; McKinney, R.A.; Gozes, I. Activity-Dependent Neuroprotective Protein Deficiency Models Synaptic and Developmental Phenotypes of Autism-like Syndrome. J. Clin. Investig. 2018, 128, 4956–4969. [Google Scholar] [CrossRef]
- Malishkevich, A.; Amram, N.; Hacohen-Kleiman, G.; Magen, I.; Giladi, E.; Gozes, I. Activity-Dependent Neuroprotective Protein (ADNP) Exhibits Striking Sexual Dichotomy Impacting on Autistic and Alzheimer’s Pathologies. Transl. Psychiatry 2015, 5, e501. [Google Scholar] [CrossRef] [PubMed]
- Phoenix, C.H.; Goy, R.W.; Gerall, A.A.; Young, W.C. Organizing Action of Prenatally Administered Testosterone Propionate on the Tissues Mediating Mating Behavior in the Female Guinea Pig. Endocrinology 1959, 65, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.M.; Molenda-Figueira, H.A.; Sisk, C.L. Back to the Future: The Organizational-Activational Hypothesis Adapted to Puberty and Adolescence. Horm. Behav. 2009, 55, 597–604. [Google Scholar] [CrossRef]
- Koopman, P.; Münsterberg, A.; Capel, B.; Vivian, N.; Lovell-Badge, R. Expression of a Candidate Sex-Determining Gene during Mouse Testis Differentiation. Nature 1990, 348, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Juraska, J.M. Sex Differences in “Cognitive” Regions of the Rat Brain. Psychoneuroendocrinology 1991, 16, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.C.; Shryne, J.E.; Gorski, R.A. Structural Sexual Dimorphisms in the Anteroventral Periventricular Nucleus of the Rat Hypothalamus Are Sensitive to Gonadal Steroids Perinatally, but Develop Peripubertally. Neuroendocrinology 1996, 63, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.P.; Chen, X. What Does the “Four Core Genotypes” Mouse Model Tell Us about Sex Differences in the Brain and Other Tissues? Front. Neuroendocrinol. 2009, 30, 1–9. [Google Scholar] [CrossRef]
- De Vries, G.J.; Simerly, R.B. Anatomy, Development, and Function of Sexually Dimorphic Neural Circuits in the Mammalian Brain. Horm. Brain Behav. 2002, 4, 137–139, XXVII–XXIX. [Google Scholar] [CrossRef]
- De Vries, G.J.; Rissman, E.F.; Simerly, R.B.; Yang, L.Y.; Scordalakes, E.M.; Auger, C.J.; Swain, A.; Lovell-Badge, R.; Burgoyne, P.S.; Arnold, A.P. A Model System for Study of Sex Chromosome Effects on Sexually Dimorphic Neural and Behavioral Traits. J. Neurosci. 2002, 22, 9005–9014. [Google Scholar] [CrossRef]
- Wagner, C.K.; Xu, J.; Pfau, J.L.; Quadros, P.S.; De Vries, G.J.; Arnold, A.P. Neonatal Mice Possessing an Sry Transgene Show a Masculinized Pattern of Progesterone Receptor Expression in the Brain Independent of Sex Chromosome Status. Endocrinology 2004, 145, 1046–1049. [Google Scholar] [CrossRef] [PubMed]
- Markham, J.A.; Jurgens, H.A.; Auger, C.J.; De Vries, G.J.; Arnold, A.P.; Juraska, J.M. Sex Differences in Mouse Cortical Thickness Are Independent of the Complement of Sex Chromosomes. Neuroscience 2003, 116, 71–75. [Google Scholar] [CrossRef] [PubMed]
- McPhie-Lalmansingh, A.A.; Tejada, L.D.; Weaver, J.L.; Rissman, E.F. Sex Chromosome Complement Affects Social Interactions in Mice. Horm. Behav. 2008, 54, 565–570. [Google Scholar] [CrossRef]
- Gatewood, J.D.; Wills, A.; Shetty, S.; Xu, J.; Arnold, A.P.; Burgoyne, P.S.; Rissman, E.F. Sex Chromosome Complement and Gonadal Sex Influence Aggressive and Parental Behaviors in Mice. J. Neurosci. 2006, 26, 2335–2342. [Google Scholar] [CrossRef]
- Sato, T.; Matsumoto, T.; Kawano, H.; Watanabe, T.; Uematsu, Y.; Sekine, K.; Fukuda, T.; Aihara, K.I.; Krust, A.; Yamada, T.; et al. Brain Masculinization Requires Androgen Receptor Function. Proc. Natl. Acad. Sci. USA 2004, 101, 1673–1678. [Google Scholar] [CrossRef] [PubMed]
- Raisman, G.; Field, P.M. Sexual Dimorphism in the Preoptic Area of the Rat. Science 1971, 173, 731–733. [Google Scholar] [CrossRef]
- Becker, J.B.; Arnold, A.P.; Berkley, K.J.; Blaustein, J.D.; Eckel, L.A.; Hampson, E.; Herman, J.P.; Marts, S.; Sadee, W.; Steiner, M.; et al. Strategies and Methods for Research on Sex Differences in Brain and Behavior. Endocrinology 2005, 146, 1650–1673. [Google Scholar] [CrossRef] [PubMed]
- Grgurevic, N.; Büdefeld, T.; Rissman, E.F.; Tobet, S.A.; Majdic, G. Aggressive Behaviors in Adult SF-1 Knockout Mice That Are Not Exposed to Gonadal Steroids during Development. Behav. Neurosci. 2008, 122, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.V.; Manoli, D.S.; Fraser, E.J.; Coats, J.K.; Tollkuhn, J.; Honda, S.I.; Harada, N.; Shah, N.M. Estrogen Masculinizes Neural Pathways and Sex-Specific Behaviors. Cell 2009, 139, 61–72. [Google Scholar] [CrossRef]
- Lee, N.S.; Beery, A.K. Neural Circuits Underlying Rodent Sociality: A Comparative Approach. In Brain Imaging in Behavioral Neuroscience; Springer: Berlin/Heidelberg, Germany, 2019; pp. 211–238. ISBN 9783642209246. [Google Scholar]
- Johnson, M.H.; Griffin, R.; Csibra, G.; Halit, H.; Farroni, T.; de Haan, M.; Tucker, L.A.; Baron-Cohen, S.; Richards, J. The Emergence of the Social Brain Network: Evidence from Typical and Atypical Development. Dev. Psychopathol. 2005, 17, 599–619. [Google Scholar] [CrossRef]
- Amodio, D.M.; Frith, C.D. Meeting of Minds: The Medial Frontal Cortex and Social Cognition. Nat. Rev. Neurosci. 2006, 7, 268–277. [Google Scholar] [CrossRef]
- Shepard, K.N.; Michopoulos, V.; Toufexis, D.J.; Wilson, M.E. Genetic, Epigenetic and Environmental Impact on Sex Differences in Social Behavior. Physiol. Behav. 2009, 97, 157–170. [Google Scholar] [CrossRef]
- Newman, S.W. The Medial Extended Amygdala in Male Reproductive Behavior. A Node in the Mammalian Social Behavior Network. Ann. N. Y. Acad. Sci. 1999, 877, 242–257. [Google Scholar] [CrossRef]
- Schwarz, J.M. Sex and the Developing Brain; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780128021149. [Google Scholar]
- McCarthy, M.M. Estradiol and the Developing Brain. Physiol. Rev. 2008, 88, 91–124. [Google Scholar] [CrossRef]
- Mong, J.A.; Nuñez, J.L.; McCarthy, M.M. GABA Mediates Steroid-Induced Astrocyte Differentiation in the Neonatal Rat Hypothalamus. J. Neuroendocrinol. 2002, 14, 45–55. [Google Scholar] [CrossRef]
- Todd, B.J.; Schwarz, J.M.; Mong, J.A.; McCarthy, M.M. Glutamate AMPA/Kainate Receptors, Not GABAA Receptors, Mediate Estradiol-Induced Sex Differences in the Hypothalamus. Dev. Neurobiol. 2007, 67, 304–315. [Google Scholar] [CrossRef]
- Davis, E.C.; Popper, P.; Gorski, R.A. The Role of Apoptosis in Sexual Differentiation of the Rat Sexually Dimorphic Nucleus of the Preoptic Area. Brain Res. 1996, 734, 10–18. [Google Scholar] [CrossRef]
- Pickett, L.A.; VanRyzin, J.W.; Marquardt, A.E.; McCarthy, M.M. Microglia Phagocytosis Mediates the Volume and Function of the Rat Sexually Dimorphic Nucleus of the Preoptic Area. Proc. Natl. Acad. Sci. USA 2023, 120, 2017. [Google Scholar] [CrossRef] [PubMed]
- Wintermantel, T.M.; Campbell, R.E.; Porteous, R.; Bock, D.; Gröne, H.J.; Todman, M.G.; Korach, K.S.; Greiner, E.; Pérez, C.A.; Schütz, G.; et al. Definition of Estrogen Receptor Pathway Critical for Estrogen Positive Feedback to Gonadotropin-Releasing Hormone Neurons and Fertility. Neuron 2006, 52, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Houtsmuller, E.J.; Brand, T.; de Jonge, F.H.; Joosten, R.N.J.M.A.; van de Poll, N.E.; Slob, A.K. SDN-POA Volume, Sexual Behavior, and Partner Preference of Male Rats Affected by Perinatal Treatment with ATD. Physiol. Behav. 1994, 56, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Lenz, K.M.; Pickett, L.A.; Wright, C.L.; Davis, K.T.; Joshi, A.; McCarthy, M.M. Mast Cells in the Developing Brain Determine Adult Sexual Behavior. J. Neurosci. 2018, 38, 8044–8059. [Google Scholar] [CrossRef] [PubMed]
- Lenz, K.M.; Nugent, B.M.; Haliyur, R.; McCarthy, M.M. Microglia Are Essential to Masculinization of Brain and Behavior. J. Neurosci. 2013, 33, 2761–2772. [Google Scholar] [CrossRef] [PubMed]
- Amateau, S.K.; McCarthy, M.M. Induction of PGE2 by Estradiol Mediates Developmental Masculinization of Sex Behavior. Nat. Neurosci. 2004, 7, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Lenz, K.M.; Wright, C.L.; Martin, R.C.; McCarthy, M.M. Prostaglandin E2 Regulates AMPA Receptor Phosphorylation and Promotes Membrane Insertion in Preoptic Area Neurons and Glia during Sexual Differentiation. PLoS ONE 2011, 6, e18500. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Hu, M.H.; Hanley, B.P.; Lin, Y.S.; Poston, L.; Lightman, S.L.; O’Byrne, K.T. The Posterodorsal Medial Amygdala Regulates the Timing of Puberty Onset in Female Rats. Endocrinology 2015, 156, 3725–3736. [Google Scholar] [CrossRef]
- Morris, J.A.; Jordan, C.L.; King, Z.A.; Northcutt, K.V.; Breedlove, S.M. Sexual Dimorphism and Steroid Responsiveness of the Posterodorsal Medial Amygdala in Adult Mice. Brain Res. 2008, 1190, 115–121. [Google Scholar] [CrossRef]
- Cooke, B.M.; Stokas, M.R.; Woolley, C.S. Morphological Sex Differences and Laterality in the Prepubertal Medial Amygdala. J. Comp. Neurol. 2007, 501, 904–915. [Google Scholar] [CrossRef]
- Johnson, R.T.; Breedlove, S.M.; Jordan, C.L. Androgen Receptors Mediate Masculinization of Astrocytes in the Rat Posterodorsal Medial Amygdala during Puberty. J. Comp. Neurol. 2013, 521, 2298–2309. [Google Scholar] [CrossRef]
- VanRyzin, J.W.; Marquardt, A.E.; Argue, K.J.; Vecchiarelli, H.A.; Ashton, S.E.; Arambula, S.E.; Hill, M.N.; McCarthy, M.M. Microglial Phagocytosis of Newborn Cells Is Induced by Endocannabinoids and Sculpts Sex Differences in Juvenile Rat Social Play. Neuron 2019, 102, 435–449.e6. [Google Scholar] [CrossRef]
- Donaldson, Z.R.; Young, L.J. Oxytocin, Vasopressin, and the Neurogenetics of Sociality. Science 2008, 322, 900–904. [Google Scholar] [CrossRef]
- Young, L.J.; Wang, Z. The Neurobiology of Pair Bonding. Nat. Neurosci. 2004, 7, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Goodson, J.L.; Bass, A.H. Social Behavior Functions and Related Anatomical Characteristics of Vasotocin/Vasopressin Systems in Vertebrates. Brain Res. Rev. 2001, 35, 246–265. [Google Scholar] [CrossRef]
- Lim, M.M.; Young, L.J. Neuropeptidergic Regulation of Affiliative Behavior and Social Bonding in Animals. Horm. Behav. 2006, 50, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.R.; Insel, T.R.; Harbaugh, C.R.; Carter, C.S. Oxytocin Administered Centrally Facilitates Formation of a Partner Preference in Female Prairie Voles (Microtus Ochrogaster). J. Neuroendocrinol. 1994, 6, 247–250. [Google Scholar] [CrossRef]
- Winslow, J.T.; Hastings, N.; Carter, C.S.; Harbaugh, C.R.; Insel, T.R. A Role for Central Vasopressin in Pair Bonding in Monogamous Prairie Voles. Nature 1993, 365, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Hrabovszky, E.; Kalló, I.; Steinhauser, A.; Merchenthaler, I.; Coen, C.W.; Petersen, S.L.; Liposits, Z. Estrogen Receptor-β in Oxytocin and Vasopressin Neurons of the Rat and Human Hypothalamus: Immunocytochemical and in Situ Hybridization Studies. J. Comp. Neurol. 2004, 473, 315–333. [Google Scholar] [CrossRef]
- Choleris, E.; Gustafsson, J.Å.; Korach, K.S.; Muglia, L.J.; Pfaff, D.W.; Ogawa, S. An Estrogen-Dependent Four-Gene Micronet Regulating Social Recognition: A Study with Oxytocin and Estrogen Receptor-α and -β Knockout Mice. Proc. Natl. Acad. Sci. USA 2003, 100, 6192–6197. [Google Scholar] [CrossRef]
- Young, L.J. The Neurobiology of Social Recognition, Approach, and Avoidance. Biol. Psychiatry 2002, 51, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, P.; Esslinger, C.; Chen, Q.; Mier, D.; Lis, S.; Siddhanti, S.; Gruppe, H.; Mattay, V.S.; Gallhofer, B.; Meyer-Lindenberg, A. Oxytocin Modulates Neural Circuitry for Social Cognition and Fear in Humans. J. Neurosci. 2005, 25, 11489–11493. [Google Scholar] [CrossRef]
- Cataldo, I.; Azhari, A.; Esposito, G. A Review of Oxytocin and Arginine-Vasopressin Receptors and Their Modulation of Autism Spectrum Disorder. Front. Mol. Neurosci. 2018, 11, 27. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Aldinger, K.A.; Ashwood, P.; Bauman, M.L.; Blaha, C.D.; Blatt, G.J.; Chauhan, A.; Chauhan, V.; Dager, S.R.; Dickson, P.E.; et al. Consensus Paper: Pathological Role of the Cerebellum in Autism. Cerebellum 2012, 11, 777–807. [Google Scholar] [CrossRef] [PubMed]
- Piochon, C.; Kloth, A.D.; Grasselli, G.; Titley, H.K.; Nakayama, H.; Hashimoto, K.; Wan, V.; Simmons, D.H.; Eissa, T.; Nakatani, J.; et al. Cerebellar Plasticity and Motor Learning Deficits in a Copy-Number Variation Mouse Model of Autism. Nat. Commun. 2014, 5, 5586. [Google Scholar] [CrossRef]
- Tsai, P.T.; Hull, C.; Chu, Y.; Greene-Colozzi, E.; Sadowski, A.R.; Leech, J.M.; Steinberg, J.; Crawley, J.N.; Regehr, W.G.; Sahin, M. Autistic-like Behaviour and Cerebellar Dysfunction in Purkinje Cell Tsc1 Mutant Mice. Nature 2012, 488, 647–651. [Google Scholar] [CrossRef]
- Mercer, A.A.; Palarz, K.J.; Tabatadze, N.; Woolley, C.S.; Raman, I.M. Sex Differences in Cerebellar Synaptic Transmission and Sex-Specific Responses to Autism-Linked Gabrb3 Mutations in Mice. eLife 2016, 5. [Google Scholar] [CrossRef]
- Dean, S.L.; Knutson, J.F.; Krebs-Kraft, D.L.; Mccarthy, M.M. Prostaglandin E2 Is an Endogenous Modulator of Cerebellar Development and Complex Behavior during a Sensitive Postnatal Period. Eur. J. Neurosci. 2012, 35, 1218–1229. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.F.; Wright, C.L.; McCarthy, M.M. A Critical Period in Purkinje Cell Development Is Mediated by Local Estradiol Synthesis, Disrupted by Inflammation, and Has Enduring Consequences Only for Males. J. Neurosci. 2016, 36, 10039–10049. [Google Scholar] [CrossRef]
- Lemaigre-dubreuil, Y.; Doulazmi, M.; Fre, F.; Hadj-sahraoui, N.; Delhaye-bouchaud, N.; Mariani, J. Span of the Heterozygous Staggerer Mouse (Rora/Rora Sg) Is Gender-Related. J. Comp. Neurol. 1999, 411, 267–273. [Google Scholar]
- Hadj-Sahraoui, N.; Frédéric, F.; Delhaye-Bouchaud, N.; Mariani, J. Gender Effect on Purkinje Cell Loss in the Cerebellum of the Heterozygous Reeler Mouse. J. Neurogenet. 1996, 11, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Abel, J.M.; Witt, D.M.; Rissman, E.F. Sex Differences in the Cerebellum and Frontal Cortex: Roles of Estrogen Receptor Alpha and Sex Chromosome Genes. Neuroendocrinology 2011, 93, 230–240. [Google Scholar] [CrossRef] [PubMed]
- McCormick, C.M.; Mathews, I.Z. Adolescent Development, Hypothalamic-Pituitary-Adrenal Function, and Programming of Adult Learning and Memory. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Zapatero-Caballero, H.; Sanchez-Franco, F.; Fernandez-Mendez, C.; García-San Frutos, M.; Botella-Cubells, L.M.; Fernandez-Vazquez, G. Gonadotropin-Releasing Hormone Receptor Gene Expression during Pubertal Development of Female Rats. Biol. Reprod. 2004, 70, 348–355. [Google Scholar] [CrossRef]
- Pignatelli, D.; Xiao, F.; Gouveia, A.M.; Ferreira, J.G.; Vinson, G.P. Adrenarche in the Rat. J. Endocrinol. 2006, 191, 301–308. [Google Scholar] [CrossRef]
- Zapatero-Caballero, H.; Sanchez-Franco, F.; Guerra-Perez, N.; Fernandez-Mendez, C.; Fernandez-Vazquez, G. Gonadotropin-Releasing Hormone Receptor Gene Expression during Pubertal Development of Male Rats. Biol. Reprod. 2003, 68, 1764–1770. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yuan, R.; Meng, Q.; Nautiyal, J.; Flurkey, K.; Tsaih, S.W.; Krier, R.; Parker, M.G.; Harrison, D.E.; Paigen, B. Genetic Coregulation of Age of Female Sexual Maturation and Lifespan through Circulating IGF1 among Inbred Mouse Strains. Proc. Natl. Acad. Sci. USA 2012, 109, 8224–8229. [Google Scholar] [CrossRef]
- Bell, M.R. Comparing Postnatal Development of Gonadal Hormones and Associated Social Behaviors in Rats, Mice, and Humans. Endocrinology 2018, 159, 2596–2613. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S.; Dushay, J.; Flier, S.N.; Prabakaran, D.; Flier, J.S. Leptin Accelerates the Onset of Puberty in Normal Female Mice. J. Clin. Investig. 1997, 99, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Selmanoff, M.K.; Goldman, B.D.; Ginsburg, B.E. Developmental Changes in Serum Luteinizing Hormone, Follicle Stimulating Hormone and Androgen Levels in Males of Two Inbred Mouse Strains. Endocrinology 1977, 100, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.M.; Sisk, C.L. Pubertal Hormones, the Adolescent Brain, and the Maturation of Social Behaviors: Lessons from the Syrian Hamster. Mol. Cell. Endocrinol. 2006, 254–255, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Juraska, J.M.; Sisk, C.L.; DonCarlos, L.L. Sexual Differentiation of the Adolescent Rodent Brain: Hormonal Influences and Developmental Mechanisms. Horm. Behav. 2013, 64, 203–210. [Google Scholar] [CrossRef]
- Schulz, K.M.; Sisk, C.L. The Organizing Actions of Adolescent Gonadal Steroid Hormones on Brain and Behavioral Development. Neurosci. Biobehav. Rev. 2016, 70, 148–158. [Google Scholar] [CrossRef]
- Brock, O.; Baum, M.J.; Bakker, J. The Development of Female Sexual Behavior Requires Prepubertal Estradiol. J. Neurosci. 2011, 31, 5574–5578. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.M.; Richardson, H.N.; Zehr, J.L.; Osetek, A.J.; Menard, T.A.; Sisk, C.L. Gonadal Hormones Masculinize and Defeminize Reproductive Behaviors during Puberty in the Male Syrian Hamster. Horm. Behav. 2004, 45, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.M.; Menard, T.A.; Smith, D.A.; Albers, H.E.; Sisk, C.L. Testicular Hormone Exposure during Adolescence Organizes Flank-Marking Behavior and Vasopressin Receptor Binding in the Lateral Septum. Horm. Behav. 2006, 50, 477–483. [Google Scholar] [CrossRef]
- Shrenker, P.; Maxson, S.C.; Ginsburg, B.E. The Role of Postnatal Testosterone in the Development of Sexually Dimorphic Behaviors in DBA/1Bg Mice. Physiol. Behav. 1985, 35, 757–762. [Google Scholar] [CrossRef]
- Bloch, G.J.; Mills, R.; Gale, S. Prepubertal Testosterone Treatment of Female Rats: Defeminization of Behavioral and Endocrine Function in Adulthood. Neurosci. Biobehav. Rev. 1995, 19, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Bloch, G.J.; Mills, R. Prepubertal Testosterone Treatment of Neonatally Gonadectomized Male Rats: Defeminization and Masculinization of Behavioral and Endocrine Function in Adulthood. Neurosci. Biobehav. Rev. 1995, 19, 187–200. [Google Scholar] [CrossRef]
- Kercmar, J.; Snoj, T.; Tobet, S.A.; Majdic, G. Gonadectomy Prior to Puberty Decreases Normal Parental Behavior in Adult Mice. Horm. Behav. 2014, 66, 667–673. [Google Scholar] [CrossRef]
- Primus, R.J.; Kellogg, C.K. Gonadal Hormones during Puberty Organize Environment-Related Social Interaction in the Male Rat. Horm. Behav. 1990, 24, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Cooke, B.M.; Woolley, C.S. Effects of Prepubertal Gonadectomy on a Male-Typical Behavior and Excitatory Synaptic Transmission in the Amygdala. Dev. Neurobiol. 2009, 69, 141–152. [Google Scholar] [CrossRef]
- Ahmed, E.I.; Zehr, J.L.; Schulz, K.M.; Lorenz, B.H.; DonCarlos, L.L.; Sisk, C.L. Pubertal Hormones Modulate the Addition of New Cells to Sexually Dimorphic Brain Regions. Nat. Neurosci. 2008, 11, 995–997. [Google Scholar] [CrossRef] [PubMed]
- Markham, J.A.; Morris, J.R.; Juraska, J.M. Neuron Number Decreases in the Rat Ventral, but Not Dorsal, Medial Prefrontal Cortex between Adolescence and Adulthood. Neuroscience 2007, 144, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.T.; Schneider, A.; Doncarlos, L.L.; Breedlove, S.M.; Jordan, C.L. Astrocytes in the Rat Medial Amygdala Are Responsive to Adult Androgens. J. Comp. Neurol. 2012, 520, 2531–2544. [Google Scholar] [CrossRef]
- Reid, S.N.M.; Juraska, J.M. Sex Differences in the Gross Size of the Rat Neocortex. J. Comp. Neurol. 1992, 321, 442–447. [Google Scholar] [CrossRef]
- Reid, S.N.M.; Juraska, J.M. Sex Differences in the Number of Synaptic Junctions in the Binocular Area of the Rat Visual Cortex. J. Comp. Neurol. 1995, 352, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, J.L.; Sodhi, J.; Juraska, J.M. Ovarian Hormones after Postnatal Day 20 Reduce Neuron Number in the Rat Primary Visual Cortex. J. Neurobiol. 2002, 52, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Antonio Muñoz-Cueto, J.; Miguel García-Segura, L.; Ruiz-Marcos, A. Developmental Sex Differences and Effect of Ovariectomy on the Number of Cortical Pyramidal Cell Dendritic Spines. Brain Res. 1990, 515, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Willing, J.; Juraska, J.M. The Timing of Neuronal Loss across Adolescence in the Medial Prefrontal Cortex of Male and Female Rats. Neuroscience 2015, 301, 268–275. [Google Scholar] [CrossRef]
- Koss, W.A.; Lloyd, M.M.; Sadowski, R.N.; Wise, L.M.; Juraska, J.M. Gonadectomy before Puberty Increases the Number of Neurons and Glia in the Medial Prefrontal Cortex of Female, but Not Male, Rats. Dev. Psychobiol. 2015, 57, 305–312. [Google Scholar] [CrossRef]
- Bell, H.C.; McCaffrey, D.R.; Forgie, M.L.; Kolb, B.; Pellis, S.M. The Role of the Medial Prefrontal Cortex in the Play Fighting of Rats. Behav. Neurosci. 2009, 123, 1158–1168. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, J.; Zhu, H.; Zhang, Q.; Lin, Z.; Hu, H. Bidirectional Control of Social Hierarchy by Synaptic Efficacy in Medial Prefrontal Cortex. Science 2011, 334, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.L. Trajectories of Brain Development: Point of Vulnerability or Window of Opportunity? Neurosci. Biobehav. Rev. 2003, 27, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain Development in Rodents and Humans: Identifying Benchmarks of Maturation and Vulnerability to Injury across Species. Prog. Neurobiol. 2013, 106–107, 1–16. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M.; Wright, C.L. Convergence of Sex Differences and the Neuroimmune System in Autism Spectrum Disorder. Biol. Psychiatry 2017, 81, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Chini, M.; Hanganu-Opatz, I.L. Prefrontal Cortex Development in Health and Disease: Lessons from Rodents and Humans. Trends Neurosci. 2021, 44, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Amateur, S.K.; McCarthy, M.M. Sexual Differentiation of Astrocyte Morphology in the Developing Rat Preoptic Area. J. Neuroendocrinol. 2002, 14, 904–910. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Sholar, P.W.; Bilbo, S.D. Sex Differences in Microglial Colonization of the Developing Rat Brain. J. Neurochem. 2012, 120, 948–963. [Google Scholar] [CrossRef]
- Mong, J.A.; Kurzweil, R.L.; Davis, A.M.; Rocca, M.S.; McCarthy, M.M. Evidence for Sexual Differentiation of Glia in Rat Brain. Horm. Behav. 1996, 30, 553–562. [Google Scholar] [CrossRef]
- Mong, J.A.; Glaser, E.; McCarthy, M.M. Gonadal Steroids Promote Glial Differentiation and Alter Neuronal Morphology in the Developing Hypothalamus in a Regionally Specific Manner. J. Neurosci. 1999, 19, 1464–1472. [Google Scholar] [CrossRef]
- Bilbo, S.D.; Smith, S.H.; Schwarz, J.M. A Lifespan Approach to Neuroinflammatory and Cognitive Disorders: A Critical Role for Glia. J. Neuroimmune Pharmacol. 2012, 7, 24–41. [Google Scholar] [CrossRef]
- Clipperton-Allen, A.E.; Zhang, A.; Cohen, O.S.; Page, D.T. Environmental Enrichment Rescues Social Behavioral Deficits and Synaptic Abnormalities in Pten Haploinsufficient Mice. Genes 2021, 12, 1366. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, S.; Urruela, M.; Devine, D.P. Effects of Environmental Enrichment on Repetitive Behaviors in the Btbr T+tf/j Mouse Model of Autism. Autism Res. 2013, 6, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Mouton, P.R.; Long, J.M.; Lei, D.L.; Howard, V.; Jucker, M.; Calhoun, M.E.; Ingram, D.K. Age and Gender Effects on Microglia and Astrocyte Numbers in Brains of Mice. Brain Res. 2002, 956, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Guneykaya, D.; Ivanov, A.; Hernandez, D.P.; Haage, V.; Wojtas, B.; Meyer, N.; Maricos, M.; Jordan, P.; Buonfiglioli, A.; Gielniewski, B.; et al. Transcriptional and Translational Differences of Microglia from Male and Female Brains. Cell Rep. 2018, 24, 2773–2783.e6. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Perry, V.H. Microglial Physiology: Unique Stimuli, Specialized Responses. Annu. Rev. Immunol. 2009, 27, 119–145. [Google Scholar] [CrossRef] [PubMed]
- Depino, A.M.A.M.; Earl, C.; Kaczmarczyk, E.; Ferrari, C.; Besedovsky, H.; Del Rey, A.; Pitossi, F.J.F.J.; Oertel, W.H.W.H.; Rey, A.; Pitossi, F.J.F.J.; et al. Microglial Activation with Atypical Proinflammatory Cytokine Expression in a Rat Model of Parkinson’s Disease. Eur. J. Neurosci. 2003, 18, 2731–2742. [Google Scholar] [CrossRef]
- Pott Godoy, M.C.; Tarelli, R.; Ferrari, C.C.; Sarchi, M.I.; Pitossi, F.J. Central and Systemic IL-1 Exacerbates Neurodegeneration and Motor Symptoms in a Model of Parkinson’s Disease. Brain 2008, 131, 1880–1894. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Gelosa, P.; Castiglioni, L.; Cimino, M.; Rizzi, N.; Pepe, G.; Lolli, F.; Marcello, E.; Sironi, L.; Vegeto, E.; et al. Sex-Specific Features of Microglia from Adult Mice. Cell Rep. 2018, 23, 3501–3511. [Google Scholar] [CrossRef]
- Vegeto, E.; Bonincontro, C.; Pollio, G.; Sala, A.; Viappiani, S.; Nardi, F.; Brusadelli, A.; Viviani, B.; Ciana, P.; Maggi, A. Estrogen Prevents the Lipopolysaccharide-Induced Inflammatory Response in Microglia. J. Neurosci. 2001, 21, 1809–1818. [Google Scholar] [CrossRef]
- Williamson, L.L.; Chao, A.; Bilbo, S.D. Environmental Enrichment Alters Glial Antigen Expression and Neuroimmune Function in the Adult Rat Hippocampus. Brain. Behav. Immun. 2012, 26, 500–510. [Google Scholar] [CrossRef]
- van den Berg, H. Evaluating the Validity of Animal Models of Mental Disorder: From Modeling Syndromes to Modeling Endophenotypes. Hist. Philos. Life Sci. 2022, 44, 1–26. [Google Scholar] [CrossRef]
- Seyhan, A.A. Lost in Translation: The Valley of Death across Preclinical and Clinical Divide–Identification of Problems and Overcoming Obstacles. Transl. Med. Commun. 2019, 4, 18. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).