A Novel Method for Detection and Progress Assessment of Visual Distortion Caused by Macular Disorder: A Central Serous Chorioretinopathy (CSR) Case Study
Abstract
:1. Introduction
2. Related Works
3. CSR VD Changes
3.1. Effect of CSR in the Visual Field
3.2. Spherical Shape CSR Modeling
4. Proposed Method and Platform
4.1. Hardware Development
4.2. Software Development
4.2.1. NGRID VD Test and SVG VD Test Editor
4.2.2. Admin Interface
4.3. Heatmap Generator
4.4. Other Programs
5. Implementation and VD Test Results
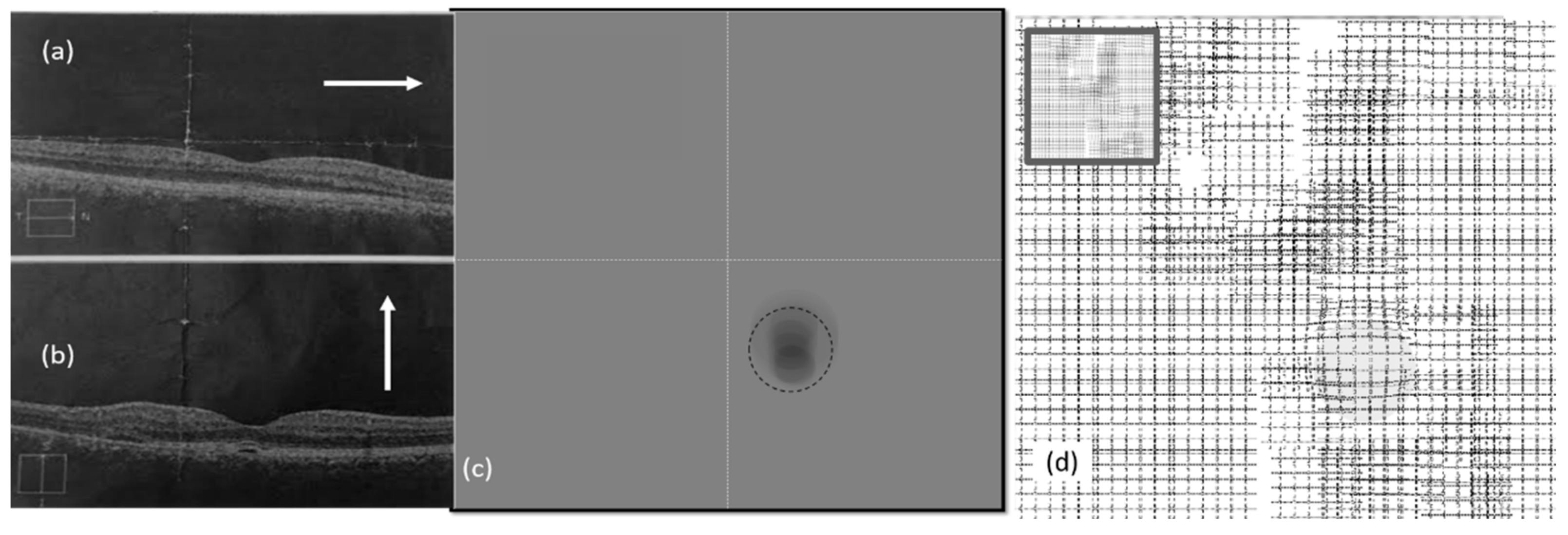
5.1. Simulation Results of CSR Modeling
5.2. Visual Distortion Test
5.3. Estimation of Visual Distortion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Friedman, D.S.; O’Colmain, B.J.; Munoz, B.; Tomany, S.C.; McCarty, C.; De Jong, P.T.; Nemesure, B.; Mitchell, P.; Kempen, J. The Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch. Ophthalmol. 2004, 122, 564–572. [Google Scholar] [PubMed] [Green Version]
- Ji, Y.; Li, M.; Zhang, X.; Peng, Y.; Wen, F. Poor sleep quality is the risk factor for central serous chorioretinopathy. J. Ophthalmol. 2018, 2018, 9450297. [Google Scholar] [CrossRef] [PubMed]
- Peiris, T.J.; El Rami, H.E.; Sun, J.K. Central serous chorioretinopathy associated with steroid enema. Retin. Cases Brief Rep. 2018. [Google Scholar] [CrossRef] [PubMed]
- Amsler, M. Earliest symptoms of diseases of the macula. Br. J. Ophthalmol. 1953, 37, 521. [Google Scholar] [CrossRef] [Green Version]
- Schuchard, R.A. Validity and interpretation of Amsler grid reports. Arch. Ophthalmol. 1993, 111, 776–780. [Google Scholar] [CrossRef]
- Isaac, D.L.C.; Ávila, M.P.D.; Cialdini, A.P. Comparison of the original Amsler grid with the preferential hyperacuity perimeter for detecting choroidal neovascularization in age-related macular degeneration. Arq. Bras. Oftalmol. 2007, 70, 771–776. [Google Scholar] [CrossRef] [Green Version]
- Loewenstein, A.; Pollack, A.; Schachat, A. Results of a multicentered, masked clinical trial to evaluate the macular computerized psychophysical test (MCPT) for detection of age-related macular degeneration (AMD). Investig. Ophtalmol. Vis. Sci. 2002, 43, 1213. [Google Scholar]
- Crossland, M.; Rubin, G. The Amsler chart: Absence of evidence is not evidence of absence. Br. J. Ophthalmol. 2007, 91, 391–393. [Google Scholar] [CrossRef]
- Kohn, W.; Klingshirn, J.A. Characterization and Correction of Macular Distortion. U.S. Patent 8,708,495, 29 April 2014. [Google Scholar]
- Wang, M.; Munch, I.C.; Hasler, P.W.; Prünte, C.; Larsen, M. Central serous chorioretinopathy. Acta Ophthalmol. 2008, 86, 126–145. [Google Scholar] [CrossRef]
- Kim, J.; Lee, W. Stochastic decision making for adaptive crowdsourcing in medical big-data platforms. IEEE Trans. Syst. Man Cybern. Syst. 2015, 45, 1471–1476. [Google Scholar] [CrossRef]
- Siuly, S.; Zhang, Y. Medical big data: Neurological diseases diagnosis through medical data analysis. Data Sci. Eng. 2016, 1, 54–64. [Google Scholar] [CrossRef] [Green Version]
- Yao, Q.; Tian, Y.; Li, P.-F.; Tian, L.-L.; Qian, Y.-M.; Li, J.-S. Design and development of a medical big data processing system based on hadoop. J. Med. Syst. 2015, 39, 23. [Google Scholar] [CrossRef] [PubMed]
- Besharse, J.C.; Bok, D. The Retina and Its Disorders; Academic Press: San Diego, CA, USA, 2011; ISBN 978-0-12-382198-0. [Google Scholar]
- Palanker, D. Metamorphopsia Testing and Related Methods. U.S. Patent 9/462,941; WO2014022850A1, 11 October 2014. [Google Scholar]
- Lakshminarayanan, V.; Enoch, J.M. Vernier acuity and aging. Int. Ophthalmol. 1995, 19, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Kaas, J.H.; Krubitzer, L.A.; Chino, Y.M.; Langston, A.L.; Polley, E.H.; Blair, N. Reorganization of retinotopic cortical maps in adult mammals after lesions of the retina. Science 1990, 248, 229–231. [Google Scholar] [CrossRef] [Green Version]
- Nowomiejska, K.; Oleszczuk, A.; Brzozowska, A.; Grzybowski, A.; Ksiazek, K.; Maciejewski, R.; Ksiazek, P.; Juenemann, A.; Rejdak, R. M-charts as a tool for quantifying metamorphopsia in age-related macular degeneration treated with the bevacizumab injections. BMC Ophthalmol. 2013, 13, 13. [Google Scholar] [CrossRef] [Green Version]
- Inami Ltd. Quantitatable Metamorphopsia Chart. Available online: http://www.inami.co.jp/english/surgical_instruments/innovations/kdm1 (accessed on 31 October 2015).
- Chew, E.Y.; Clemons, T.E.; Bressler, S.B.; Elman, M.J.; Danis, R.P.; Domalpally, A.; Heier, J.S.; Kim, J.E.; Garfinkel, R. Randomized Trial of a Home monitoring system for early detection of choroidal neovascularization home monitoring of the eye (HOME) study. Ophthalmology 2014, 121, 535–544. [Google Scholar] [CrossRef] [Green Version]
- Vazquez, N.P.; Knox, P.C. Assessment of visual distortions in age-related macular degeneration: emergence of new approaches. Br. Ir. Orthopt. J. 2015, 12, 9. [Google Scholar] [CrossRef] [Green Version]
- Trevino, R. Recent progress in macular function self-assessment. Ophthalmic Physiol. Opt. 2008, 28, 183–192. [Google Scholar] [CrossRef]
- Robison, C.D.; Jivrajka, R.V.; Bababeygy, S.R.; Fink, W.; Sadun, A.A.; Sebag, J. Distinguishing wet from dry age-related macular degeneration using three-dimensional computer-automated threshold Amsler grid testing. Br. J. Ophthalmol. 2011, 95, 1419–1423. [Google Scholar] [CrossRef]
- Fitts, P.M. The information capacity of the human motor system in controlling the amplitude of movement. J. Exp. Psychol. 1954, 47, 381. [Google Scholar] [CrossRef] [Green Version]
- Grenga, P.L.; Fragiotta, S.; Meduri, A.; Lupo, S.; Marenco, M.; Vingolo, E.M. Fixation stability measurements in patients with neovascular age-related macular degeneration treated with ranibizumab. Can. J. Ophthalmol. Can. Ophtalmol. 2013, 48, 394–399. [Google Scholar] [CrossRef]
- Mohaghegh, N.; Munidasa, S.; Ziho, X.; Owen, Q.; Magierowski, S.; Ghafar-Zadeh, E. Age-Related Macular Degeneration Diagnostic Tools: Hardware and Software Development. In Proceedings of the 2018 IEEE 61st International Midwest Symposium on Circuits and Systems (MWSCAS), Windsor, ON, Canada, 5–8 August 2018; pp. 721–724. [Google Scholar]
- Kaufman, A. Rendering, Visualization and Rasterization Hardware; Springer Science & Business Media: New York, NY, USA, 1993; ISBN 978-3-540-56787-5. [Google Scholar]











| Program | Language | Input/Process/Output |
|---|---|---|
| VD Test | C++ and C# | Consumes stored VD tests patterns from online admin interface and produces raw test results stored in the cloud data center cluster. |
| SVG VD Test Editor | HTML5, CSS3 and Javascript | Facilitates creating various SVGs that compose patterns used in tests. The outputs are stored in database |
| Admin Interface | Scala, Java, HTML5/CSS3 and Javascript | An online website that allows physicians and admins to login and manage various patients and control a different aspect of the VD test. |
| Heatmap Generator and Analyzer | R, Python, C#, C++, OpenCV, HTML5, CSS3 and Javascript | Consumes raw VD test results along with various rasterization and image processing techniques to produce heatmap results for a test that a patient has completed. |
| Database | SQL and NoSQL | A highly available database within datacenter that keeps all test data, hashed credentials and test results. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohaghegh, N.; Magierowski, S.; Ghafar-Zadeh, E. A Novel Method for Detection and Progress Assessment of Visual Distortion Caused by Macular Disorder: A Central Serous Chorioretinopathy (CSR) Case Study. Vision 2019, 3, 68. https://doi.org/10.3390/vision3040068
Mohaghegh N, Magierowski S, Ghafar-Zadeh E. A Novel Method for Detection and Progress Assessment of Visual Distortion Caused by Macular Disorder: A Central Serous Chorioretinopathy (CSR) Case Study. Vision. 2019; 3(4):68. https://doi.org/10.3390/vision3040068
Chicago/Turabian StyleMohaghegh, Navid, Sebastian Magierowski, and Ebrahim Ghafar-Zadeh. 2019. "A Novel Method for Detection and Progress Assessment of Visual Distortion Caused by Macular Disorder: A Central Serous Chorioretinopathy (CSR) Case Study" Vision 3, no. 4: 68. https://doi.org/10.3390/vision3040068
APA StyleMohaghegh, N., Magierowski, S., & Ghafar-Zadeh, E. (2019). A Novel Method for Detection and Progress Assessment of Visual Distortion Caused by Macular Disorder: A Central Serous Chorioretinopathy (CSR) Case Study. Vision, 3(4), 68. https://doi.org/10.3390/vision3040068






