Progression from Type 2 Macular Neovascularization to Fibrovascular Pigment Epithelial Detachment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
3. Results
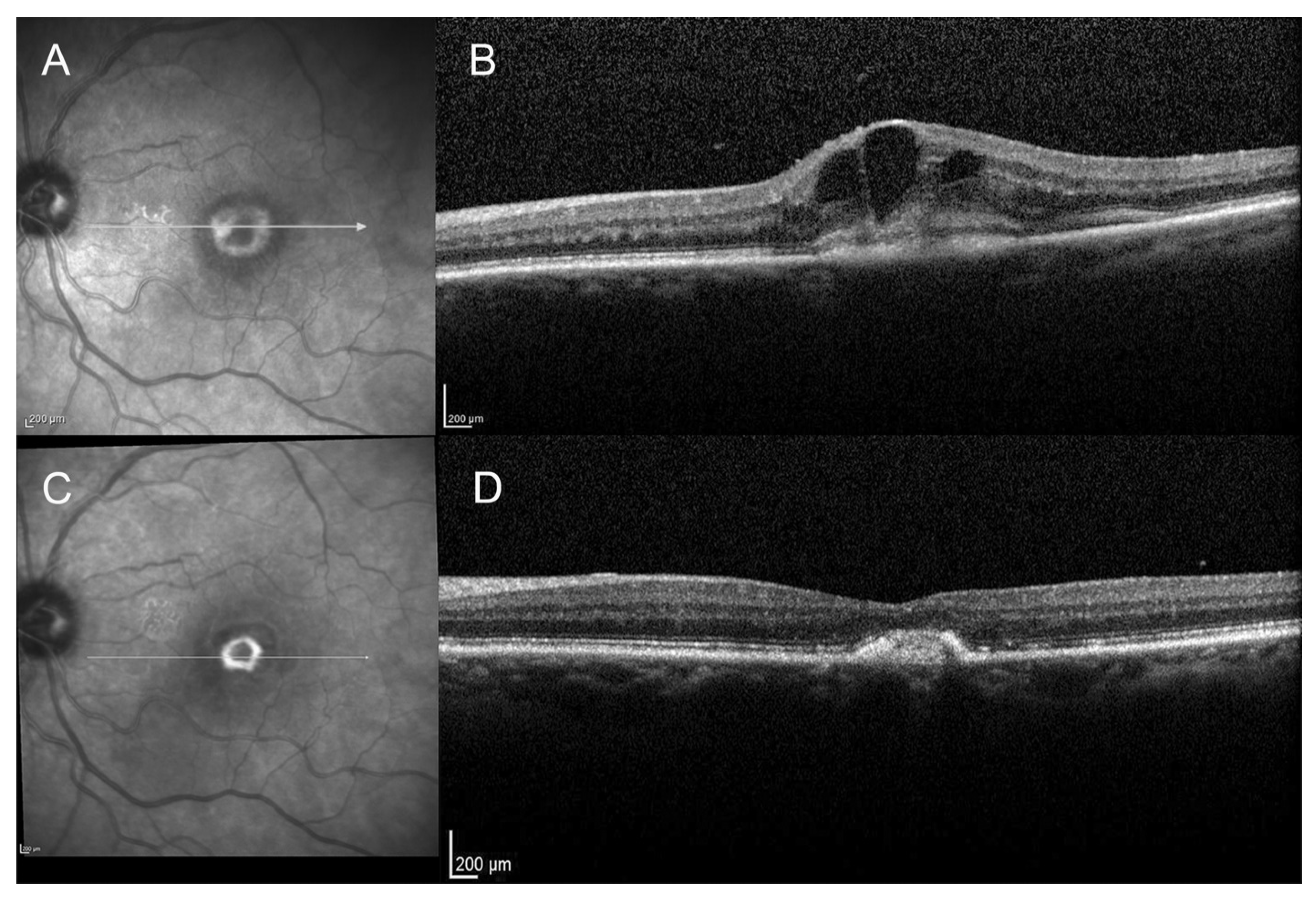
3.1. Study Population
3.2. Visual Acuity
3.3. Anatomical Outcome
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Bourne, R.R.; Stevens, G.A.; White, R.A.; Smith, J.L.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; et al. Causes of vision loss worldwide, 1990–2010: A systematic analysis. Lancet Glob. Health 2013, 1, 339–349. [Google Scholar] [CrossRef] [Green Version]
- Bressler, N.M. Age-Related Macular Degeneration Is the Leading Cause of Blindness. JAMA 2004, 291, 1900–1901. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, P.J.; Shapiro, H.; Tuomi, L.; Webster, M.; Elledge, J.; Blodi, B. Characteristics of Patients Losing Vision after 2 Years of Monthly Dosing in the Phase III Ranibizumab Clinical Trials. Ophthalmology 2011, 118, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Plyukhova, A.A.; Budzinskaya, M.V.; Starostin, K.M.; Rejdak, R.; Bucolo, C.; Reibaldi, M.; Toro, M.D. Comparative Safety of Bevacizumab, Ranibizumab, and Aflibercept for Treatment of Neovascular Age-Related Macular Degeneration (AMD): A Systematic Review and Network Meta-Analysis of Direct Comparative Studies. J. Clin. Med. 2020, 9, 1522. [Google Scholar] [CrossRef] [PubMed]
- Reibaldi, M.; Russo, A.; Avitabile, T.; Uva, M.G.; Franco, L.; Longo, A.; Toro, M.D.; Cennamo, G.; Mariotti, C.; Neri, P.; et al. Treatment of persistent serous retinal detachment in vogt–koyanagi–harada syndrome with intravitreal bevacizumab during the systemic steroid treatment. Retina 2014, 34, 490–496. [Google Scholar] [CrossRef]
- Yousef, Y.A.; Elrimawi, A.H.; Nazzal, R.M.; Qaroot, A.F.; Alaref, A.H.; Mohammad, M.; Abureesh, O.; Rejdak, R.; Nowomiejska, K.; Avitabile, T.; et al. Coats’ disease: Characteristics, management, outcome, and scleral external drainage with anterior chamber maintainer for stage 3b disease. Medicine 2020, 99, e19623. [Google Scholar] [CrossRef] [PubMed]
- Elfalah, M.; AlRyalat, S.A.; Toro, M.D.; Rejdak, R.; Zweifel, S.; Nazzal, R.; Abu-Ameerh, M.; Ababneh, O.; Gharaibeh, A.; Sharif, Z.; et al. Delayed Intravitreal Anti-VEGF Therapy for Patients During the COVID-19 Lockdown: An Ethical Endeavor. Clin. Ophthalmol. 2021, 15, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Ceravolo, I.; Oliverio, G.W.; Alibrandi, A.; Bhatti, A.; Trombetta, L.; Rejdak, R.; Toro, M.D.; Trombetta, C.J. The Application of Structural Retinal Biomarkers to Evaluate the Effect of Intravitreal Ranibizumab and Dexamethasone Intravitreal Implant on Treatment of Diabetic Macular Edema. Diagnostics 2020, 10, 413. [Google Scholar] [CrossRef] [PubMed]
- Macular Photocoagulation Study Group. Subfoveal neovascular lesions in age-related macular degeneration. Guidelines for evaluation and treatment in the macular photocoagulation study group. Arch. Ophthalmol. 1991, 109, 1242–1457. [Google Scholar] [CrossRef]
- Grossniklaus, H.; Gass, J. Clinicopathologic correlations of surgically excised type 1 and type 2 submacular choroidal neovascular membranes. Am. J. Ophthalmol. 1998, 126, 59–69. [Google Scholar] [CrossRef]
- Freund, K.B.; Zweifel, S.A.; Engelbert, M. Do We Need a New Classification for Choroidal Neovascularization in Age-Related Macular Degeneration? Retina 2010, 30, 1333–1349. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.Y.; Creuzot-Garcher, C.; Darmon, J.; Desmettre, T.; Korobelnik, J.F.; Levrat, F.; Quentel, G.; Palies, S.; Sanchez, A.; de Gendre, A.S.; et al. Types of choroidal neovascularisation in newly diagnosed ex-udative age-related macular degeneration. Br. J. Ophthalmol. 2007, 91, 1173–1176. [Google Scholar] [CrossRef]
- El Ameen, A.; Cohen, S.Y.; Semoun, O.; Miere, A.; Srour, M.; Maftouhi, M.Q.E.; Oubraham, H.; Blanco-Garavito, R.; Querques, G.; Souied, E.H. Type 2 Neovascularization Secondary to Age-Related Macular De-generation Imaged by Optical Coherence Tomography Angiography. Retina 2015, 35, 2212–2218. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Ferreira, A.; Hughes, R.; Carter, G.; Mitchell, P. Epidemiology and Disease Burden of Pathologic Myopia and Myopic Choroidal Neovascularization: An Evidence-Based Systematic Review. Am. J. Ophthalmol. 2014, 157, 9–25.e12. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Ohno-Matsui, K.; Shimada, N.; Moriyama, M.; Kojima, A.; Hayashi, W.; Yasuzumi, K.; Nagaoka, N.; Saka, N.; Yoshida, T.; et al. Long-term pattern of progression of myopic maculopathy: A natural history study. Ophthalmology 2010, 117, 1595–1611. [Google Scholar] [CrossRef] [PubMed]
- Willis, J.R.; Vitale, S.; Morse, L.; Parke, D.W., II; Rich, W.L.; Lum, F.; Cantrell, R.A. The Prevalence of Myopic Choroidal Neovascularization in the United States: Analysis of the IRIS((R)) Data Registry and NHANES. Ophthalmology 2016, 123, 1771–1782. [Google Scholar] [CrossRef] [PubMed]
- Tilleul, J.; Mimoun, G.; Querques, G.; Puche, N.; Zerbib, J.; Lalloum, F.; Srour, M.; Souied, E.H. Intravitreal ranibizumab for choroidal neovascularization in angioid streaks: Four-Year Follow-up. Retina 2016, 36, 483–491. [Google Scholar] [CrossRef]
- Connor, P.; Juergens, J.L.; Perry, H.O.; Hollenhorst, R.W.; Edwards, J.E. Pseudoxanthoma elasticum and angioid streaks. Am. J. Med. 1961, 30, 537–543. [Google Scholar] [CrossRef]
- Coscas, F.; Querques, G.; Forte, R.; Terrada, C.; Coscas, G.; Souied, E.H. Combined fluorescein angiography and spectral-domain optical coherence tomography imaging of classic choroidal neovascularization secondary to age-related macular degeneration before and after intravitreal ranibizumab injections. Retina 2012, 32, 1069–1076. [Google Scholar] [CrossRef]
- Coscas, F.; Coscas, G.; Souied, E.; Tick, S.; Soubrane, G. Optical coherence tomography identification of occult choroidal neovas-cularization in age-related macular degeneration. Am. J. Ophthalmol. 2007, 144, 592–599. [Google Scholar] [CrossRef]
- Querques, G.; Srour, M.; Massamba, N.; Georges, A.; Ben Moussa, N.; Rafaeli, O.; Souied, E.H. Functional Characterization and Multimodal Imaging of Treatment-Naïve “Quiescent” Choroidal Neovascularization. Investig. Opthalmology Vis. Sci. 2013, 54, 6886–6892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolz-Marco, R.; Phasukkijwatana, N.; Sarraf, D.; Freund, K.B. Regression of type 2 neovascularization into a type 1 pattern after intravitreal anti-vascular endothelial growth factor therapy for neovascular age-related macular degeneration. Retina 2017, 37, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Semoun, O.; Guigui, B.; Tick, S.; Coscas, G.; Soubrane, G.; Souied, E.H. Infrared features of classic choroidal neovascularisation in exudative age-related macular degeneration. Br. J. Ophthalmol. 2008, 93, 182–185. [Google Scholar] [CrossRef]
- Stern, J.; Eveleth, D.; Masula, J.; Temple, S. Slow progression of exudative age related macular degeneration associated with hypertrophy of the retinal pigment epithelium. F1000Research 2014, 3, 293. [Google Scholar] [CrossRef] [Green Version]
- Stern, J.; Temple, S. Retinal pigment epithelial cell proliferation. Exp. Biol. Med. 2015, 240, 1079–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mimoun, G.; Ebran, J.-M.; Grenet, T.; Donati, A.; Cohen, S.-Y.; Ponthieux, A. Ranibizumab for choroidal neovascularization secondary to pseudoxanthoma elasticum: 4-year results from the PIXEL study in France. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1651–1660. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.; Chakravarthy, U.; Klein, R.; Mitchell, P.; Zlateva, G.; Buggage, R.; Fahrbach, K.; Probst, C.; Sledge, I. The natural history and prognosis of neovascular age-related macular degeneration: A systematic review of the literature and meta-analysis. Ophthalmology 2008, 115, 116–126. [Google Scholar] [CrossRef]
- Pauleikhoff, D. Neovascular age-related macular degeneration: Natural History and Treatment Outcomes. Retina 2005, 25, 1065–1084. [Google Scholar] [CrossRef]
- Daniel, E.; Toth, C.A.; Grunwald, J.E.; Jaffe, G.J.; Martin, D.F.; Fine, S.L.; Huang, J.; Ying, G.-S.; Hagstrom, S.A.; Winter, K.; et al. Risk of Scar in the Comparison of Age-related Macular Degeneration Treatments Trials. Ophthalmology 2014, 121, 656–666. [Google Scholar] [CrossRef] [Green Version]
- Daniel, E.; Ying, G.-S.; Kim, B.J.; Toth, C.A.; Ferris, F.; Martin, D.F.; Grunwald, J.E.; Jaffe, G.J.; Dunaief, J.L.; Pan, W.; et al. Five-Year Follow-up of Nonfibrotic Scars in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2019, 126, 743–751. [Google Scholar] [CrossRef]
- Stroeva, O.G.; Mitashov, V.I. Retinal Pigment Epithelium: Proliferation and Differentiation during Development and Regeneration. Adv. Clin. Chem. 1983, 83, 221–293. [Google Scholar] [CrossRef]
- Anderson, D.H.; Stern, W.H.; Fisher, S.K.; Erickson, P.A.; Borgula, G.A. The onset of pigment epithelial proliferation after retinal detach-ment. Investig. Ophthalmol. Vis. Sci. 1981, 21, 10–16. [Google Scholar]
- Querques, G.; Rousseau, A.; Forte, R.; Scemama, C.; Caillaux, V.; Querques, L.; Souied, E.H. Longitudinal anatomical response of retinal–choroidal anastomosis to anti–vascular endothelial growth factor therapy. Retina 2012, 32, 458–467. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
LE, H.M.; Mimoun, G.; Cohen, S.Y.; Jung, C.; Semoun, O.; Souied, E.H. Progression from Type 2 Macular Neovascularization to Fibrovascular Pigment Epithelial Detachment. Vision 2021, 5, 16. https://doi.org/10.3390/vision5020016
LE HM, Mimoun G, Cohen SY, Jung C, Semoun O, Souied EH. Progression from Type 2 Macular Neovascularization to Fibrovascular Pigment Epithelial Detachment. Vision. 2021; 5(2):16. https://doi.org/10.3390/vision5020016
Chicago/Turabian StyleLE, Hoang Mai, Gérard Mimoun, Salomon Y. Cohen, Camille Jung, Oudy Semoun, and Eric H. Souied. 2021. "Progression from Type 2 Macular Neovascularization to Fibrovascular Pigment Epithelial Detachment" Vision 5, no. 2: 16. https://doi.org/10.3390/vision5020016
APA StyleLE, H. M., Mimoun, G., Cohen, S. Y., Jung, C., Semoun, O., & Souied, E. H. (2021). Progression from Type 2 Macular Neovascularization to Fibrovascular Pigment Epithelial Detachment. Vision, 5(2), 16. https://doi.org/10.3390/vision5020016



