Visual Field Loss: Integrating Overlayed Information to Increase the Effective Field of View
Abstract
1. Introduction
1.1. Restitution, Compensation, Substitution
1.2. Augmented Reality and the Future
1.3. The Current Experiment
2. Methodology
2.1. Participants
2.2. Apparatus and Stimuli
- i.
- a white fixation cross: this was shifted 10 to the left or right of the centre of the screen, depending on group. The fixation cross was 2 and the fixation window for the eye tracker was 3.3. The large fixation window allowed participants to remain fixated and view the augmentation window (when presented) without losing fixation. Eye tracking was used for fixation monitoring only, stimuli were only present when fixation was met. When fixation was lost, the stimulus disappeared.
- ii.
- a black target line that was 0.3 thick and one of 22 lengths, categorised as short (6.7, 8.0, 9.3, 10.7, 12.0, 13.3, 14.7, 16.0), medium (17.3, 18.7, 20.0, 21.3, 22.7, 24.0, 25.3) or long (26.7, 28.0, 29.3, 30.7, 32.0, 33.3, 34.7). Each participant was presented with only 16 of the 22 line lengths, which was randomised between participants, but held constant for all conditions for each individual. This provided us with three broad categories of line length (short, medium and long) whilst still allowing variation within and between participants [52].
- iii.
- a black reference line with a fixed length of 42.8 and 0.3 thick, that was presented 6 below the target line and centred on the screen. The reference line played an important role in the augmentation condition, but did not directly form a part of the bisection task.
- iv.
- a simulated scotoma consisting of an overlaying mid grey oval with a horizontal radius of 13 and a vertical radius of 6.67, with its edges smoothed by a Gaussian luminance profile with a standard deviation of 18.1 arc minutes. The scotoma was placed left or right of the fixation cross, depending on the group, but not gaze contingent.
- v.
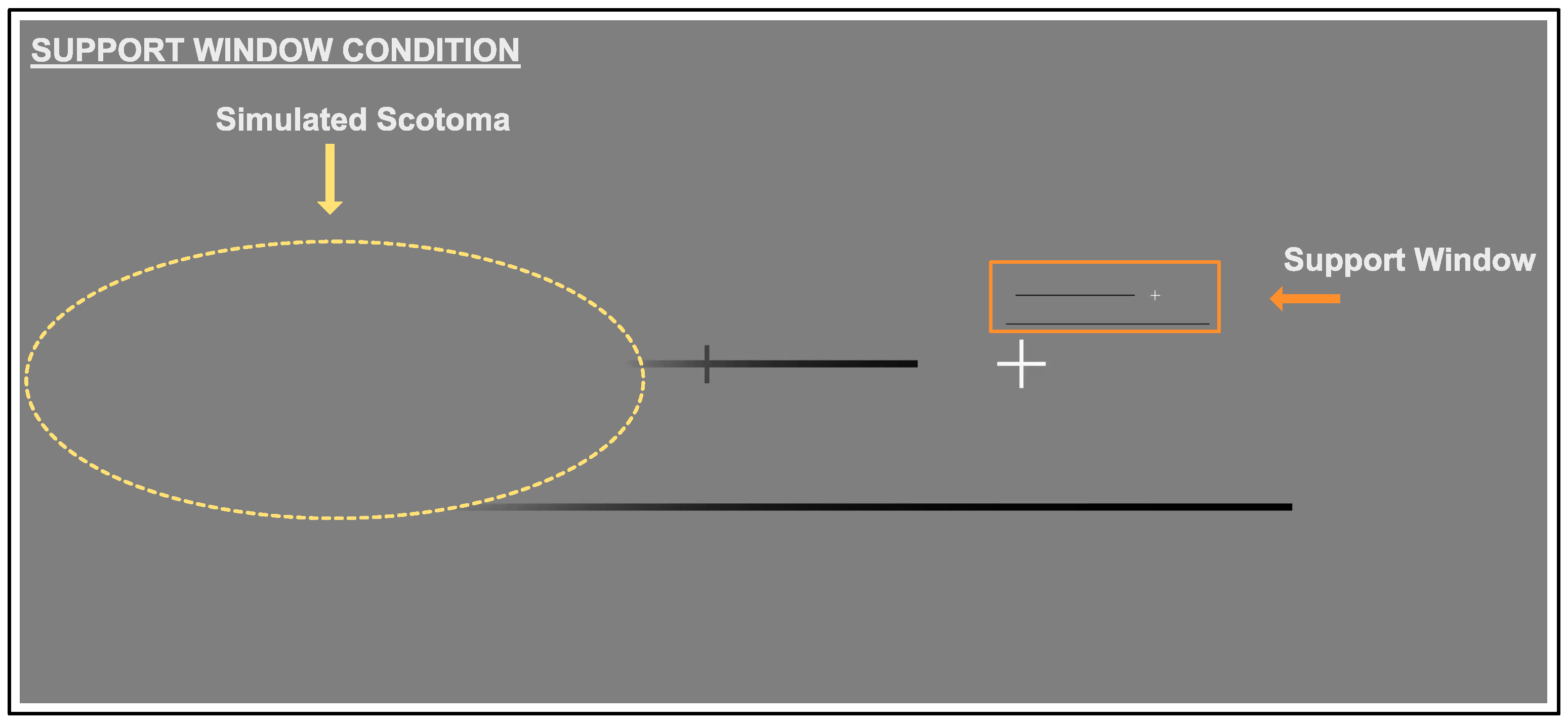
- the support window that was created with the same aspect ratio as the screen. The support window was 512 × 216 px (), and the base of the window was positioned 1 above the fixation cross. A scaled down copy of the whole of the screen, with the exception of the cursor, was presented in the support window. The fixation cross, target line, and reference line were all replicated in the support window.
2.3. Procedure
2.3.1. Baseline Condition
2.3.2. Scotoma Condition
2.3.3. Support Window Condition
3. Results
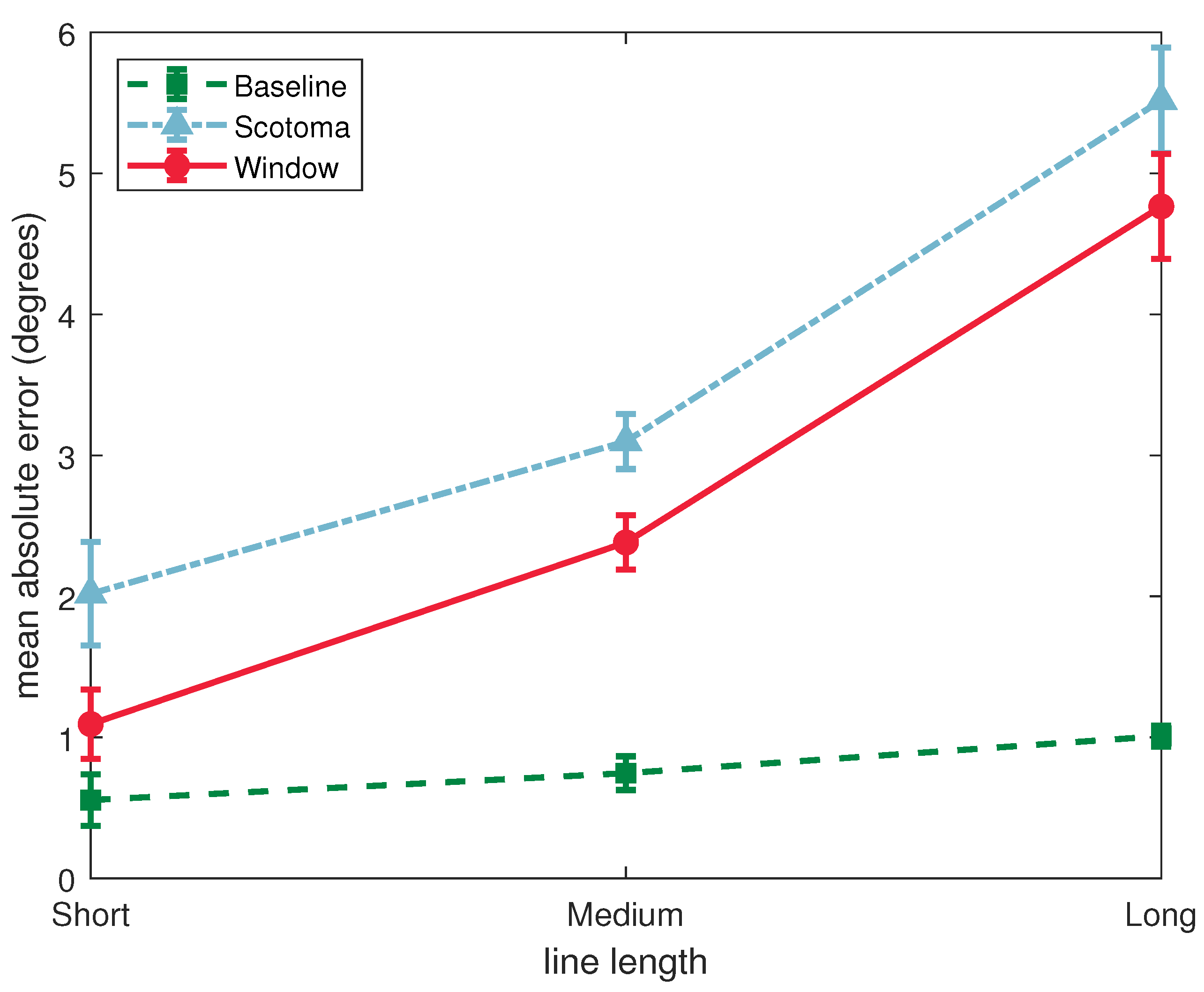
3.1. Absolute Deviation
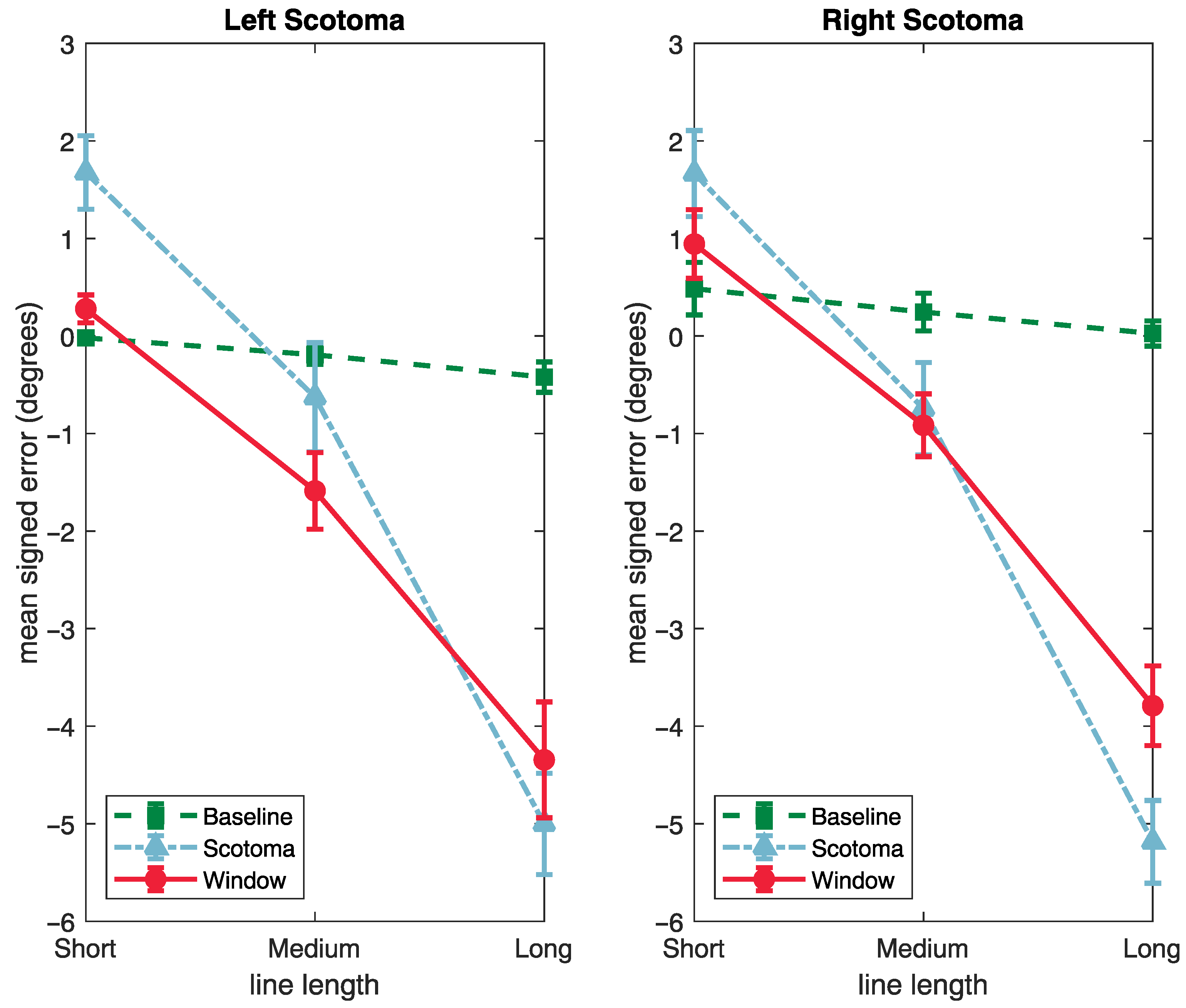
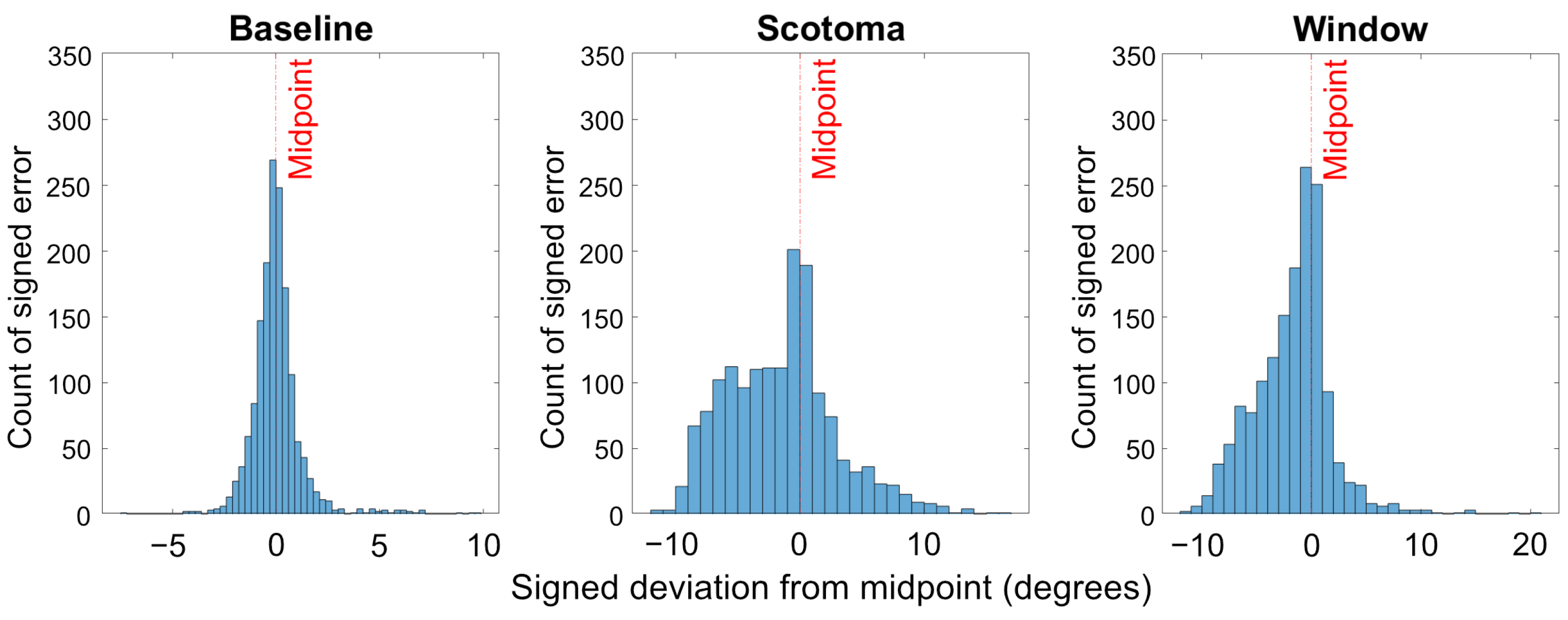
3.2. Signed Deviation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AR | Augmented Reality |
| VR | Virtual Reality |
| VFL | Visual Field Loss |
References
- Pollock, A.; Hazelton, C.; Rowe, F.J.; Jonuscheit, S.; Kernohan, A.; Angilley, J.; Henderson, C.A.; Langhorne, P.; Campbell, P. Interventions for visual field defects in people with stroke. Cochrane Database Syst. Rev. 2019, 5, CD008388. [Google Scholar] [CrossRef] [PubMed]
- Urbanski, M.; Coubard, O.A.; Bourlon, C. Visualizing the blind brain: Brain imaging of visual field defects from early recovery to rehabilitation techniques. Front. Integr. Neurosci. 2014, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Rowe, F.J.; Hepworth, L.R.; Howard, C.; Hanna, K.L.; Cheyne, C.P.; Currie, J. High incidence and prevalence of visual problems after acute stroke: An epidemiology study with implications for service delivery. PLoS ONE 2019, 14, e0213035. [Google Scholar] [CrossRef] [PubMed]
- Pollock, A.; Hazelton, C.; Henderson, C.A.; Angilley, J.; Dhillon, B.; Langhorne, P.; Livingstone, K.; Munro, F.A.; Orr, H.; Rowe, F.J.; et al. Interventions for disorders of eye movement in patients with stroke. Cochrane Database Syst. Rev. 2011, 10, CD008389. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A.; Shinton, R.A. Improving outcome in stroke patients with visual problems. Age Ageing 2006, 35, 560–565. [Google Scholar] [CrossRef]
- Rowe, F.; Brand, D.; Jackson, C.A.; Price, A.; Walker, L.; Harrison, S.; Eccleston, C.; Scott, C.; Akerman, N.; Dodridge, C.; et al. Visual impairment following stroke: Do stroke patients require vision assessment? Age Ageing 2009, 38, 188–193. [Google Scholar] [CrossRef]
- Hepworth, L.; Rowe, F.J.; Walker, M.; Rockliffe, J.; Noonan, C.; Howard, C.; Currie, J. Post-stroke Visual Impairment: A Systematic Literature Review of Types and Recovery of Visual Conditions. Ophthalmol. Res. Int. J. 2016, 5, 1–43. [Google Scholar] [CrossRef]
- Rowe, F.J.; Wright, D.; Brand, D.; Jackson, C.; Harrison, S.; Maan, T.; Scott, C.; Vogwell, L.; Peel, S.; Akerman, N.; et al. A prospective profile of visual field loss following stroke: Prevalence, type, rehabilitation, and outcome. BioMed Res. Int. 2013, 2013, 719096. [Google Scholar] [CrossRef]
- Spector, R.H. Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Butterworths: Carlisle, UK, 1990; Chapter 116. [Google Scholar]
- Larson, A.M.; Loschky, L.C. The contributions of central versus peripheral vision to scene gist recognition. J. Vis. 2009, 9, 6. [Google Scholar] [CrossRef]
- Neville, H.J.; Lawson, D. Attention to central and peripheral visual space in a movement detection task: An event-related potential and behavioral study. I. Normal hearing adults. Brain Res. 1987, 405, 253–267. [Google Scholar] [CrossRef]
- Lee, H.W.; Legge, G.E.; Ortiz, A. Is word recognition different in central and peripheral vision? Vis. Res. 2003, 43, 2837–2846. [Google Scholar] [CrossRef]
- Zihl, J. Visual scanning behavior in patients with homonymous hemianopia. Neuropsychologia 1995, 33, 287–303. [Google Scholar] [CrossRef]
- McKean-Cowdin, R.; Varma, R.; Wu, J.; Hays, R.D.; Azen, S.P.; Los Angeles Latino Eye Study Group. Severity of visual field loss and health-related quality of life. Am. J. Ophthalmol. 2007, 143, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, H.; Julkunen, L. Treatment of visual field defects after stroke. Adv. Clin. Neurosci. Rehabil. 2004, 3, 17–18. [Google Scholar]
- Kerkhoff, G. Neurovisual rehabilitation: Recent developments and future directions. J. Neurol. Neurosurg. Psychiatry 2000, 68, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Romano, J.G.; Schulz, P.; Kenkel, S.; Todd, D.P. Visual field changes after a rehabilitation intervention: Vision restoration therapy. J. Neurol. Sci. 2008, 273, 70–74. [Google Scholar] [CrossRef]
- Sahraie, A.; Trevethan, C.T.; MacLeod, M.J.; Murray, A.D.; Olson, J.A.; Weiskrantz, L. Increased sensitivity after repeated stimulation of residual spatial channels in blindsight. Proc. Natl. Acad. Sci. USA 2006, 103, 14971–14976. [Google Scholar] [CrossRef]
- Huxlin, K.R.; Martin, T.; Kelly, K.; Riley, M.; Friedman, D.I.; Burgin, W.S.; Hayhoe, M. Perceptual relearning of complex visual motion after V1 damage in humans. J. Neurosci. 2009, 29, 3981–3991. [Google Scholar] [CrossRef]
- McFadzean, R.M. NovaVision: Vision restoration therapy. Curr. Opin. Ophthalmol. 2006, 17, 498–503. [Google Scholar] [CrossRef]
- Aimola, L.; Lane, A.R.; Smith, D.T.; Kerkhoff, G.; Ford, G.A.; Schenk, T. Efficacy and feasibility of home-based training for individuals with homonymous visual field defects. Neurorehabilit. Neural Repair 2014, 28, 207–218. [Google Scholar] [CrossRef]
- Roth, T.; Sokolov, A.; Messias, A.; Roth, P.; Weller, M.; Trauzettel-Klosinski, S. Comparing explorative saccade and flicker training in hemianopia: A randomized controlled study. Neurology 2009, 72, 324–331. [Google Scholar] [CrossRef] [PubMed]
- de Haan, G.A.; Melis-Dankers, B.J.; Brouwer, W.H.; Tucha, O.; Heutink, J. The effects of compensatory scanning training on mobility in patients with homonymous visual field defects: A randomized controlled trial. PLoS ONE 2015, 10, e0134459. [Google Scholar] [CrossRef] [PubMed]
- Spitzyna, G.; Wise, R.; McDonald, S.; Plant, G.; Kidd, D.; Crewes, H.; Leff, A. Optokinetic therapy improves text reading in patients with hemianopic alexia: A controlled trial. Neurology 2007, 68, 1922–1930. [Google Scholar] [CrossRef] [PubMed]
- Rowe, F.; Barton, P.; Bedson, E.; Breen, R.; Conroy, E.; Cwiklinski, E.; Dodridge, C.; Drummond, A.; Garcia-Finana, M.; Howard, C.; et al. A randomised controlled trial to compare the clinical and cost-effectiveness of prism glasses, visual search training and standard care in patients with hemianopia following stroke: A protocol. BMJ Open 2014, 4, e005885. [Google Scholar] [CrossRef] [PubMed]
- Rowe, F.; Conroy, E.; Barton, P.; Bedson, E.; Cwiklinski, E.; Dodridge, E.; Drummond, A.; Garcia-Finana, M.; Howard, C.; Johnson, S.; et al. A randomised controlled trial of treatment for post-stroke homonymous hemianopia: Screening and recruitment. Neuro-Ophthalmology 2016, 40, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rowe, F.; Conroy, E.J.; Bedson, E.; Cwiklinski, E.; Drummond, A.; García-Fiñana, M.; Howard, C.; Pollock, A.; Shipman, T.; Dodridge, C.; et al. A pilot randomized controlled trial comparing effectiveness of prism glasses, visual search training and standard care in hemianopia. Acta Neurol. Scand. 2017, 136, 310–321. [Google Scholar] [CrossRef]
- Keller, I.; Lefin-Rank, G. Improvement of visual search after audiovisual exploration training in hemianopic patients. Neurorehabilit. Neural Repair 2010, 24, 666–673. [Google Scholar] [CrossRef]
- Schuett, S.; Heywood, C.A.; Kentridge, R.W.; Dauner, R.; Zihl, J. Rehabilitation of reading and visual exploration in visual field disorders: Transfer or specificity? Brain 2012, 135, 912–921. [Google Scholar] [CrossRef]
- Mödden, C.; Behrens, M.; Damke, I.; Eilers, N.; Kastrup, A.; Hildebrandt, H. A randomized controlled trial comparing 2 interventions for visual field loss with standard occupational therapy during inpatient stroke rehabilitation. Neurorehabilit. Neural Repair 2012, 26, 463–469. [Google Scholar] [CrossRef]
- Peli, E. Field expansion for homonymous hemianopia by optically induced peripheral exotropia. Optom. Vis. Sci. 2000, 77, 453–464. [Google Scholar] [CrossRef]
- Rossi, P.W.; Kheyfets, S.; Reding, M.J. Fresnel prisms improve visual perception in stroke patients with homonymous hemianopia or unilateral visual neglect. Neurology 1990, 40, 1597. [Google Scholar] [CrossRef] [PubMed]
- Bowers, A.R.; Keeney, K.; Peli, E. Randomized crossover clinical trial of real and sham peripheral prism glasses for hemianopia. JAMA Ophthalmol. 2014, 132, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, W.; Reding, M. Full-field prisms for hemi-field visual impairments following stroke-a controlled trial. In Neurology; Little Brown Co: Boston, MA, USA, 1994; Volume 44, pp. A312–A313. [Google Scholar]
- Szlyk, J.P.; Seiple, W.; Stelmack, J.; McMahon, T. Use of prisms for navigation and driving in hemianopic patients. Ophthalmic Physiol. Opt. 2005, 25, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Hanna, K.; Rowe, F. Clinical versus evidence-based rehabilitation options for post-stroke visual impairment. Neuro-Ophthalmology 2017, 41, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Acesight—Low Vision Electronic Glasses. Available online: https://www.acesight.com/ (accessed on 28 June 2022).
- eSight Electronic Eyewear for the Visually Impaired. Available online: https://esighteyewear.com/gb (accessed on 28 June 2022).
- Irisvision Our Vision Is to Improve Yours. Available online: https://irisvision.com/ (accessed on 28 September 2022).
- Kennedy, W.L.; Rosten, J.G.; Young, L.M.; Ciuffreda, K.J.; Levin, M.I. A field expander for patients with retinitis pigmentosa: A clinical study. Am. J. Optom. Physiol. Opt. 1977, 54, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Hoeft, W.W.; Feinbloom, W.; Brilliant, R.; Gordon, R.; Hollander, C.; Newman, J.; Novak, E.; Rosenthal, B.; Voss, E. Amorphic lenses: A mobility aid for patients with retinitis pigmentosa. Am. J. Optom. Physiol. Opt. 1985, 62, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Lowe, J.; Drasdo, N. Using a binocular field expander on a wide-field search task. Optom. Vis. Sci. 1992, 69, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Bowers, A.R.; Luo, G.; Rensing, N.M.; Peli, E. Evaluation of a prototype minified augmented-view device for patients with impaired night vision. Ophthalmic Physiol. Opt. 2004, 24, 296–312. [Google Scholar] [CrossRef]
- Luo, G.; Peli, E. Use of an augmented-vision device for visual search by patients with tunnel vision. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4152–4159. [Google Scholar] [CrossRef]
- Sperber, C.; Karnath, H.O. Diagnostic validity of line bisection in the acute phase of stroke. Neuropsychologia 2016, 82, 200–204. [Google Scholar] [CrossRef]
- Mitra, A.R.; Abegg, M.; Viswanathan, J.; Barton, J.J. Line bisection in simulated homonymous hemianopia. Neuropsychologia 2010, 48, 1742–1749. [Google Scholar] [CrossRef] [PubMed]
- Zihl, J.; Sämann, P.; Schenk, T.; Schuett, S.; Dauner, R. On the origin of line bisection error in hemianopia. Neuropsychologia 2009, 47, 2417–2426. [Google Scholar] [CrossRef] [PubMed]
- Brainard, D.H. The psychophysics toolbox. Spat. Vis. 1997, 10, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, M.; Brainard, D.; Pelli, D.; Ingling, A.; Murray, R.; Broussard, C.; Cornelissen, F. What is new in Psychtoolbox-3. Perception 2007, 36, 1. [Google Scholar]
- Pelli, D.G. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spat. Vis. 1997, 10, 437–442. [Google Scholar] [CrossRef]
- Cornelissen, F.W.; Peters, E.M.; Palmer, J. The Eyelink Toolbox: Eye tracking with MATLAB and the Psychophysics Toolbox. Behav. Res. Methods Instrum. Comput. 2002, 34, 613–617. [Google Scholar] [CrossRef]
- Keefe, B.; Elsby, M.; Watt, S. Visually guided grasping: Using a small stimulus set can lead to overestimation of the effectiveness of depth cues. J. Vis. 2008, 8, 302. [Google Scholar] [CrossRef]
- Jewell, G.; McCourt, M.E. Pseudoneglect: A review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia 2000, 38, 93–110. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asher, J.M.; Hibbard, P.B. Visual Field Loss: Integrating Overlayed Information to Increase the Effective Field of View. Vision 2022, 6, 67. https://doi.org/10.3390/vision6040067
Asher JM, Hibbard PB. Visual Field Loss: Integrating Overlayed Information to Increase the Effective Field of View. Vision. 2022; 6(4):67. https://doi.org/10.3390/vision6040067
Chicago/Turabian StyleAsher, Jordi M., and Paul B. Hibbard. 2022. "Visual Field Loss: Integrating Overlayed Information to Increase the Effective Field of View" Vision 6, no. 4: 67. https://doi.org/10.3390/vision6040067
APA StyleAsher, J. M., & Hibbard, P. B. (2022). Visual Field Loss: Integrating Overlayed Information to Increase the Effective Field of View. Vision, 6(4), 67. https://doi.org/10.3390/vision6040067





