Optimal Stimulus Properties for Steady-State Visually Evoked Potential Brain–Computer Interfaces: A Scoping Review
Abstract
:1. Introduction
- Active BCI, where the user actively generates the brain signals that should be classified, for example, using motor imagery (MI). These are usually based on event-related synchronization/desynchronization (ERS/ERD).
- Reactive BCI, where a stimulus is presented and the user’s response signal is measured. These signal responses can be, for example, visually evoked potentials (VEPs), steady-state visually evoked potentials (SSVEPs), which are evoked by looking at specific frequencies, or P300 waves, which are event-related potentials stimulated in the process of decision making.
- Passive BCI, where no effort on the part of the user is required. In this type of BCI, the mental state of the user is monitored automatically, for example, whether the mental state is in attention or relaxation mode.
- Which parameters, based on the literature, have an influence on SSVEP BCI performance?
- What are the optimal configurations of the parameters?
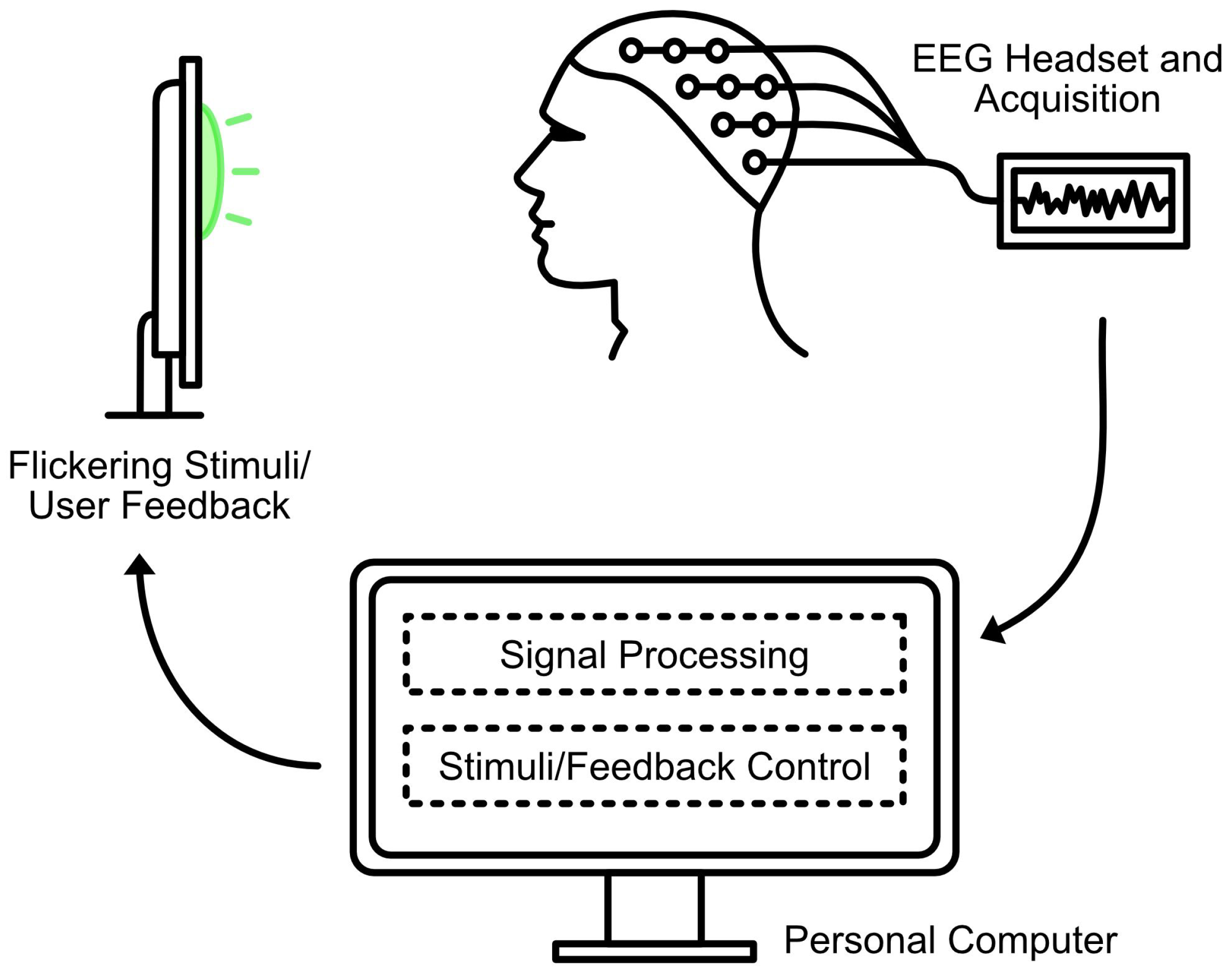
2. Materials and Methods
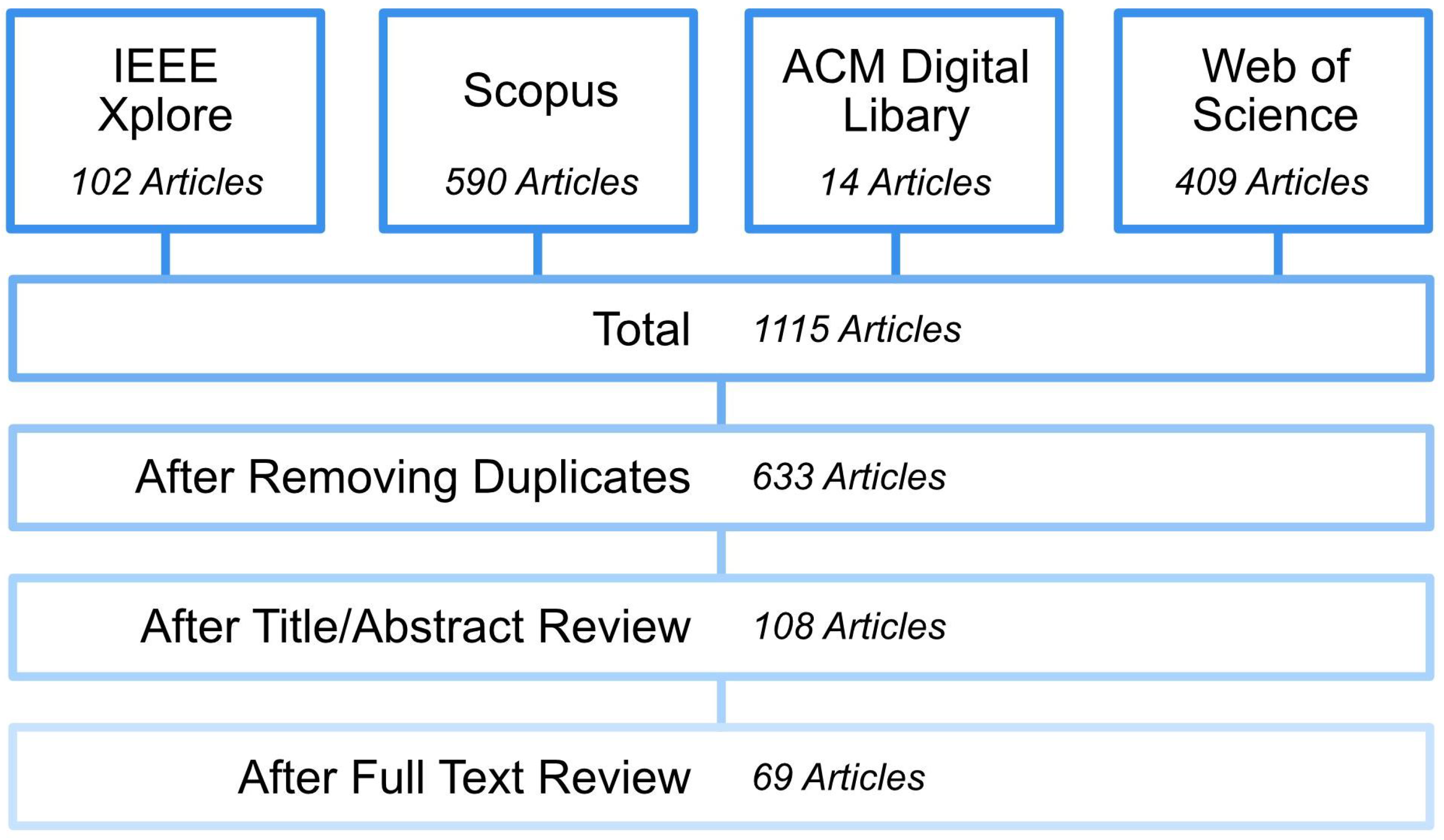
Literature Search and Inclusion Criteria
3. Quantitative Results
3.1. Proposals vs. Evaluations
3.2. BCI and Experimental Setups
3.2.1. Number of Targets
3.2.2. Electrodes Used for Evaluation
3.2.3. Participants
3.3. Dependent Variables
3.4. Stimuli Categorization
3.4.1. Stimulus Type
3.4.2. Device
4. Qualitative Results
4.1. Evaluated Stimuli Factors
4.1.1. Stimulus Type
4.1.2. Device
4.1.3. Frequency
4.1.4. Wave Parameters
4.1.5. Luminance
4.1.6. Color
4.1.7. Size and Shape
4.1.8. Viewing Distance
4.1.9. Dimensions
4.1.10. Fixation Point
5. Discussion
5.1. Study Setup
5.1.1. Number of Targets
5.1.2. Electrodes
5.1.3. Participants
5.1.4. Dependent Variables
5.2. Optimal Stimuli Factors
5.2.1. Size, Viewing Distance and Shape
5.2.2. Luminance and Device
5.2.3. Color
5.2.4. Stimulus Type
5.2.5. Frequency
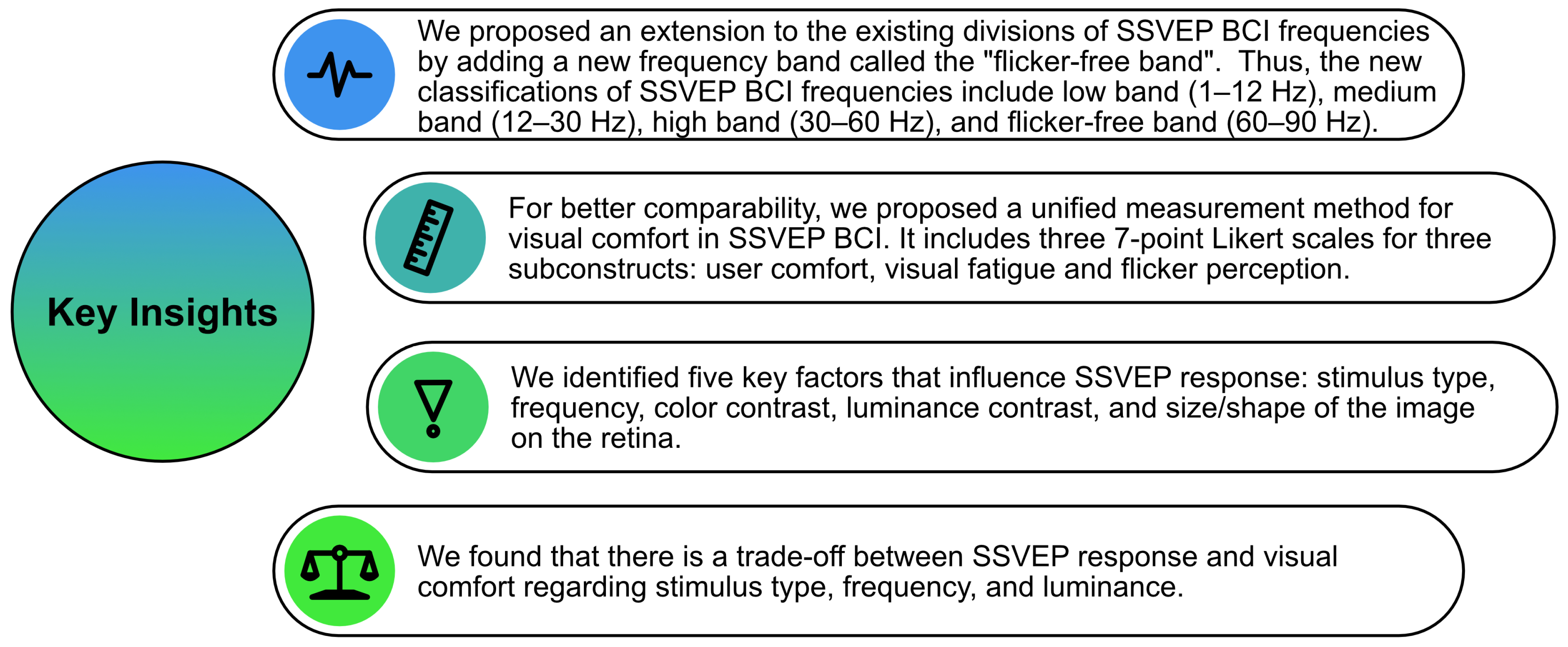
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AR | Augmented reality |
| BCI | Brain–computer interface(s) |
| CFF | Critical flicker frequency |
| CRT | Cathode-ray tube |
| ECoG | Electrocorticography |
| EEG | Electroencephalography |
| ERS/ERD | Event-related synchronization/desynchronization |
| fMRI | Functional magnetic resonance imaging |
| fNIRS | Functional near-infrared spectroscopy |
| HMD | Head-mounted displays |
| ITR | Information transfer rate |
| K-pathway | Koniocellular pathway |
| LCD | Liquid–crystal displays |
| LED | Light-emitting diodes |
| LGN | Lateral geniculate nucleus |
| M-pathway | Magnocellular pathway |
| MEG | Magnetoencephalography |
| MI | Motor imagery |
| P-pathway | Parvocellular pathway |
| PSD | Power of spectral density |
| RVS | Repetitive visual stimulus |
| SNR | Signal-to-noise ratio |
| SSMVEP | Steady-state motion visually evoked potential |
| SSVEP | Steady-state visually evoked potential |
| SSVER | Steady-state visually evoked response |
| VEP | Visually evoked potential |
| VR | Virtual reality |
Appendix A. Search Strings
Appendix A.1. IEEE Xplore
Appendix A.2. Scopus
Appendix A.3. ACM Digital Library
Appendix A.4. Web of Science
Appendix B. Data Charting of the Reviewed Articles
| Study | Device | Evaluated Factors | Stimulus Types | Frequencies (Hz) | Number of Targets | Number of Participants |
|---|---|---|---|---|---|---|
| Byczuk et al. [24] | LED | Color | Flicker | 7–47 Hz | 1 | 21 |
| Floriano et al. [42] | LED | Color | Flicker | 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65 Hz | 3 | 12 |
| Vahid et al. [33] | LED | Color | Flicker | 27, 28, 29, 30 Hz | 1 | 16 |
| Jukiewicz and Cysewska-Sobusiak [27] | LED | Color, frequency, waveform | Flicker | 8–48 Hz (1 Hz steps) | 1 | 8 |
| Wu et al. [22] | CRT, LED, LCD | Device | Flicker | 4.6, 10.8, 16.1 Hz | 1 | 10 |
| Lee et al. [64] | LED | Duty cycle | Flicker | 13.16 Hz | 6 | 30, 6 |
| Diez et al. [47] | LED | Frequency | Flicker | 37, 38, 39, 40 Hz | 4 | 6 |
| Ajami et al. [61] | LED | Frequency | Flicker | 6, 8, 12, 16, 20, 24, 28, 30, 31.1, 32.1, 32, 33.2, 34.2, 35, 36, 36.2, 37.3, 38.3, 39.4, 40, 41, 42, 43, 44, 56, 60 Hz | 5 | 5 |
| Tello et al. [14] | LED | Frequency, color | Flicker | 8, 11, 13, 15 Hz | 1 | 20 |
| Chien et al. [28] | LED | Frequency, color | Flicker | 32, 40 Hz | 1 | 10 |
| Sakurada et al. [35] | LED | Frequency, luminance | Flicker | 30–70 Hz (5 Hz steps), 41, 43, 45, 61, 63, 65 Hz | 1, 3 | 12 |
| Chang et al. [65] | LED | Proposal (coding, amplitude, modulation) | Flicker, amplitude modulation | 9–12 Hz, 40–43 Hz (1 Hz steps) | 6 | 12, 9 |
| Shyu et al. [63] | LED | Proposal (coding, dual-frequency) | Flicker | 16.4, 19.1, 17.5, 20.2 Hz | 6 | 7 |
| Dreyer et al. [32] | LED | Proposal (coding, frequency modulation) | Flicker | 71, 74, 77, 100 Hz | 1 | 14 |
| Dreyer et al. [26] | LED | Proposal (coding, frequency modulation) | Flicker, frequency modulation | 10–100 Hz (10 Hz steps) | 1 | 12, 25 |
| Zhang et al. [30] | LED, LCD | Proposal (coding, multimodal) | Flicker, ring-shaped pattern-reversal checkerboard | 5, 8, 12, 13, 13.3, 14, 15, 16, 17, 17.1, 20 Hz | 1 | 10 |
| Study | Device | Evaluated Factors | Stimulus Types | Frequencies (Hz) | Number of Targets | Number of Participants |
|---|---|---|---|---|---|---|
| Kapeller et al. [48] | LCD | Background | Flicker | 8.57, 10, 12, 15 Hz | 4 | 4 |
| Shu et al. [40] | LCD | Background | Flicker | 8.33, 9.37, 12.5 Hz | 3 | 10 |
| Singla et al. [38] | LCD | Color | Flicker | 7, 9, 11, 13 Hz | 2 | 20 |
| Chu et al. [29] | LCD | Color | Flicker | 10 Hz | 1 | 15 |
| Sato et al. [74] | LCD | Color | Flicker | 4, 5, 6, 7.5, 10, 12, 15, 20, 30 Hz | 9 | 6 |
| Du and Zhao [57] | LCD, AR | Color, device | Flicker | 7.5, 8.57, 10, 12 Hz | 4 | 10 |
| Yan et al. [41] | Screen | Color, luminance | Ring-shaped checkerboard pattern reversal | 11, 16, 18 Hz | 3 | 9 |
| Wu et al. [22] | CRT, LED, LCD | Device | Flicker | 4.6, 10.8, 16.1 Hz | 1 | 10 |
| Wang et al. [67] | AR, LCD | Device | Flicker | 8, 9, 10, 11, 12, 13 Hz | 6 | 4 |
| Wang et al. [60] | AR, LCD | Device | Flicker | 7.4, 9.4, 10.1, 11.3 Hz | 4 | 12, 6 |
| Si-Mohammed et al. [43] | AR, LCD | Device, distance, size | Flicker | 10, 12, 15 Hz | 3 | 13, 15, 42, 24 |
| Park et al. [54] | AR, LCD | Device, stimulus type | Pattern-reversal checkerboard, flicker, grow-shrink | 7.5, 8.57, 10, 12 Hz | 4 | 20 |
| Wilson and Palaniappan [82] | LCD | Duty cycle | Flicker | 6.66, 7.5, 8.57, 10, 12 Hz | 35 | 5 |
| Jiang et al. [58] | LCD | Frequency | Pattern-reversal checkerboard | 15, 17, 20, 24, 30, 40, 60, 120 Hz | 4 | 17 |
| Ladouce et al. [37] | LCD | Frequency, amplitude | Flicker | 8–30 Hz (2 Hz steps), 32, 34, 36, 38, 40, 42, 44, 46, 54, 56, 58, 60, some above 60 Hz | 1, 9 | 12, 12, 6 |
| Xu et al. [19] | LCD | Frequency, background | Flicker | 8, 8.67, 9.33, 10, 24, 26, 28, 30 Hz | 4 | 10 |
| Gerloff and Schilling [25] | LCD | Frequency, color | Pattern-reversal checkerboard | 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 Hz | 1 | 7 |
| Duart et al. [31] | LCD | Frequency, color | Flicker | 5, 12, 30 Hz | 1 | 42 |
| Siribunyaphat and Punsawad [71] | LCD | Frequency, stimulus type | Pattern-reversal checkerboard, QR | 6.5, 7, 13, 14, 17 Hz | 8 | 12 |
| Chen et al. [36] | LCD | Frequency, waveform | Flicker | 6–40 Hz (2 Hz steps) | 1, 9 | 12 |
| Mukesh et al. [21] | LCD | Proposal (coding, dual-frequency) | Pattern-reversal checkerboard | 6, 7, 12, 13, 14 Hz | 1 | 15 |
| Yan and Xu [73] | LCD | Proposal (coding, dual-type) | Flicker, rotation | 7, 8, 9, 10, 11 Hz | 9 | 10 |
| Chen et al. [69] | LCD | Proposal (coding, multimodal) | Flicker, color-change | 0.5, 1, 10, 13, 15 Hz | 8 | 10, 8 |
| Chen et al. [72] | LCD | Proposal (coding, multimodal) | Flicker, color-change, luminance-change | 0.5, 1, 1.5, 2.14, 2.5, 3, 3.75, 5, 7.5, 15 Hz | 9 | 12 |
| Li et al. [80] | LCD | Proposal (coding, multimodal) | Flicker, grow–shrink | 30 Hz, 0.2–3.4 Hz (0.2 Hz steps) | 16 | 13, 12 |
| Li et al. [76] | LCD | Proposal (coding, multimodal) | Flicker, sliding shape | 0, 0.2, 0.4, 0.6 Hz, 8–12 Hz (0.5 Hz steps) | 9 | 17, 12 |
| Zhang et al. [30] | LED, LCD | Proposal (coding, multimodal) | Flicker, ring-shaped pattern-reversal checkerboard | 5, 8, 12, 13, 13.3, 14, 15, 16, 17, 17.1, 20 Hz | 1 | 10 |
| Lopez-Gordo et al. [46] | CRT | Proposal (coding, phase) | Pattern-reversal checkerboard | 16 Hz | 4 | 10 |
| Maymandi et al. [81] | LCD–LED hybrid | Proposal (device) | Flicker | 34–49.5 Hz (0.5 Hz steps) | 32 | 5 |
| Punsawad and Wongsawat [51] | LCD | Proposal (stimulus type) | Sliding shape | 7 Hz | 4 | 7 |
| Han et al. [20] | LCD | Proposal (stimulus type) | Contracting ring-shaped pattern-reversal checkerboard | 8.6, 12, 15 Hz | 1, 40 | 8 |
| Rekrut et al. [77] | LCD | Proposal (stimulus type) | Pattern-reversal checkerboard, sliding shape, grow–shrink, rotation, spinning icon, checkerboard pattern reversal | 7.5, 10, 13 Hz | 10 | 18 |
| Niu et al. [18] | LCD | Shape | Flicker | 8.57, 10, 12, 15 Hz | 4 | 20 |
| Wen et al. [17] | LCD | Shape, color | Flicker | 8, 9, 10, 11, 12 Hz | 1 | 24 |
| Ming et al. [78] | LCD | Shape, luminance | Flicker | 9.25–14.75 Hz (0.5 Hz steps) | 12 | 28 |
| Chai et al. [66] | LCD | Shape, stimulus type | Flicker, grow–shrink, Newton rings | 8, 9, 10, 11, 12, 13, 14, 15 Hz | 6 | 8 |
| Ng et al. [49] | LCD | Size | Flicker | 11.6, 13.6, 16.1, 18.3, 21.4, 23.3 Hz | 4 | 7 |
| Duszyk et al. [50] | LCD/LED Hybrid | Size, color, shape, fixation point | Flicker | 14, 17, 25, 30 Hz | 4 | 20 |
| Lopez-Gordo et al. [23] | CRT | Spatial frequency | Pattern-reversal checkerboard | 14–18 Hz | 1 | 6 |
| Waytowich et al. [52] | LCD | Spatial frequency | Pattern-reversal checkerboard | 6, 6.66, 7.5, 8.571 Hz | 4 | 11, 10 |
| Hwang et al. [34] | LCD | Stimulus type | Dual frequency checkerboard | 6, 6.66, 7.5, 8.57 Hz | 1, 12 | 11, 10 |
| Zhang et al. [53] | LCD | Stimulus type | Flicker, ring-shaped pattern-reversal checkerboard, action video | 8.57, 10, 12, 15 Hz | 4 | 10 |
| Stawicki and Volosyak [55] | LCD | Stimulus type | Flicker, sliding shape spinning icon, ring-shaped pattern-reversal checkerboard, ring-shaped arc inverse pulsation, ring-shaped arc inverse rotational oscillation | 7.06, 7.50, 8.0, 8.57 Hz | 4 | 9 |
| Bisht et al. [44] | LCD | Stimulus type | Flicker, rotation | 3, 7.5, 10 Hz | 3 | 10 |
| Ming et al. [75] | LCD | Stimulus type, spatial frequency | Flicker, checkerboard flicker with white and black background | 11, 13, 15, 38, 40, 42 Hz | 9 | 30 |
| Kwon et al. [59] | LCD | Stimulus type, waveform | Flicker, checkerboard pattern reversal, grow–shrink | 6, 6.67, 7.5, 10 Hz | 4 | 20 |
| Oralhan and Tokmakci [79] | LCD | Waveform, duty cycle | Flicker | 6, 12, 15 Hz | 16 | 6 |
| Peguero et al. [62] | LCD | Waveform, stimulus type | Flicker, pattern-reversal checkerboard | 8.5714, 10.9091, 15, 20, 24 Hz | 5 | 27 |
| Study | Device | Evaluated Factors | Stimulus Types | Frequencies (Hz) | Number of Targets | Number of Participants |
|---|---|---|---|---|---|---|
| Ravi et al. [56] | AR | Background | Flicker, ring-shaped pattern-reversal checkerboard | 8, 10, 12, 15 Hz | 4 | 26 |
| Du and Zhao [57] | AR, LCD | Color, device | Flicker | 7.5, 8.57, 10, 12 Hz | 4 | 10 |
| Wang et al. [67] | AR, LCD | Device | Flicker | 8, 9, 10, 11, 12, 13 Hz | 6 | 4 |
| Wang et al. [60] | AR, LCD | Device | Flicker | 7.4, 9.4, 10.1, 11.3 Hz | 4 | 12, 6 |
| Si-Mohammed et al. [43] | AR, LCD | Device, distance, size | Flicker | 10, 12, 15 Hz | 3 | 13, 15, 42, 24 |
| Park et al. [54] | AR, LCD | Device, stimulus type | pattern-reversal checkerboard, flicker, grow–shrink | 7.5, 8.57, 10, 12 Hz | 4 | 20 |
| Zehra et al. [16] | AR | Dimension, stimulus type | Flicker, grow–shrink | 12, 13, 14, 15, 16 Hz | 5 | 12 |
| Kramberger et al. [70] | VR | Proposal (coding, phase) | Flicker | 16 Hz | 8 | 10 |
| Hsu et al. [68] | AR | Proposal (coding, phase-approximation) | Flicker | 19, 21, 23, 25, 27, 29 Hz | 6 | 20 |
| Bi et al. [39] | Head up display | Proposal (device) | Pattern-reversal checkerboard | 12, 13 Hz | 2 | 4 |
| Zhu et al. [45] | VR | Shape, color, dimension, frequency | Flicker | 9, 11, 13 Hz | 3 | 10 |
| Choi et al. [15] | VR | Stimulus type | Pattern-reversal checkerboard, grow–shrink | 6, 7.5, 9, 10 Hz | 4 | 14 |
Appendix C. Proposed Measuring Method for Visual Comfort

References
- Nam, C.S.; Nijholt, A.; Lotte, F. Brain–Computer Interfaces Handbook: Technological and Theoretical Advances; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Vidal, J.J. Toward direct brain–computer communication. Annu. Rev. Biophys. Bioeng. 1973, 2, 157–180. [Google Scholar] [CrossRef] [PubMed]
- Wolpaw, J.R.; Birbaumer, N.; Mcfarland, D.J.; Pfurtscheller, G.; Vaughan, T.M. Brain-computer interfaces for communication and control. Clin. Neurophysiol. 2002, 113, 767–791. [Google Scholar] [CrossRef] [PubMed]
- Zavala, S.P.; López, J.L.M.; Chicaiza, K.O.; Yoo, S.G. Review of Steady State Visually Evoked Potential Brain-Computer Interface Applications: Technological Analysis and Classification. ARPN J. Eng. Appl. Sci. 2020, 15, 659–678. [Google Scholar]
- Lal, T.; Hinterberger, T.; Widman, G.; Schröder, M.; Hill, N.; Rosenstiel, W.; Elger, C.; Birbaumer, N.; Schölkopf, B. Methods towards invasive human brain computer interfaces. Adv. Neural Inf. Process. Syst. 2004, 17, 1–8. [Google Scholar]
- Ríos-Lago, M. Functional magnetic resonance and neuropsychology: Basic concepts. Radiologia 2008, 50, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Bunce, S.C.; Izzetoglu, M.; Izzetoglu, K.; Onaral, B.; Pourrezaei, K. Functional near-infrared spectroscopy. IEEE Eng. Med. Biol. Mag. 2006, 25, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, M.; Hari, R.; Ilmoniemi, R.J.; Knuutila, J.; Lounasmaa, O.V. Magnetoencephalography—Theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev. Mod. Phys. 1993, 65, 413. [Google Scholar] [CrossRef]
- Berger, H. Über das elektroenkephalogramm des menschen. Arch. Psychiatr. Nervenkrankh. 1929, 87, 527–570. [Google Scholar] [CrossRef]
- Kumar, M.K.; Parameshachari, B.; Prabu, S.; liberata Ullo, S. Comparative analysis to identify efficient technique for interfacing BCI system. IOP Conf. Ser. Mater. Sci. Eng. 2020, 925, 012062. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.; Hong, B.; Jia, C.; Gao, S. Brain-computer interfaces based on visual evoked potentials. IEEE Eng. Med. Biol. Mag. 2008, 27, 64–71. [Google Scholar] [CrossRef]
- Zhu, D.; Bieger, J.; Molina, G.G.; Aarts, R.M. A survey of stimulation methods used in SSVEP-based BCIs. Comput. Intell. Neurosci. 2010, 2010, 702357. [Google Scholar] [CrossRef] [PubMed]
- Byczuk, M.; Poryzała, P.; Materka, A. On Possibility of Stimulus Parameter Selection for SSVEP-Based Brain-Computer Interface; Czachórski, T., Kozielski, S., Stańczyk, U., Eds.; Man-Machine Interactions 2. Advances in Intelligent and Soft Computing; Springer: Berlin/Heidelberg, Germany, 2011; Volume 103. [Google Scholar]
- Tello, R.J.M.G.; Müller, S.M.T.; Ferreira, A.; Bastos, T.F. Comparison of the influence of stimuli color on steady-state visual evoked potentials. Rev. Bras. Eng. Biomed. 2015, 31, 218–231. [Google Scholar] [CrossRef]
- Choi, K.M.; Park, S.; Im, C.H.; Baek, H.J. Comparison of visual stimuli for steady-state visual evoked potential-based brain–computer interfaces in virtual reality environment in terms of classification accuracy and visual comfort. Comput. Intell. Neurosci. 2019, 2019, 9680697. [Google Scholar] [CrossRef] [PubMed]
- Zehra, S.R.; Mu, J.; Syiem, B.V.; Burkitt, A.N.; Grayden, D.B. Evaluation of optimal stimuli for SSVEP-based augmented reality brain–computer interfaces. IEEE Access 2023, 11, 87305–87315. [Google Scholar] [CrossRef]
- Wen, D.; Jiang, M.; Jiao, W.; Wan, X.; Lan, X.; Zhou, Y. The Design Method of SSVEP Stimulus Source based on Overlooking Map. Assoc. Comput. Mach. 2022, 11, 459–464. [Google Scholar]
- Niu, Y.; Zhou, Z.; Li, Z.; Wang, J.; Wu, J.; Yang, W.; Xue, C. Improving SSVEP-BCI System Interaction Efficiency: Design Recommendations for Shape of Visual Stimuli and Number of Auxiliary Stimuli. Int. J. Hum.-Comput. Interact. 2023, 1–22. [Google Scholar] [CrossRef]
- Xu, H.; Hsu, S.H.; Nakanishi, M.; Lin, Y.; Jung, T.P.; Cauwenberghs, G. Stimulus Design for Visual Evoked Potential Based Brain-Computer Interfaces. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 2545–2551. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Xu, G.; Xie, J.; Chen, C.; Zhang, S. Highly Interactive Brain-Computer Interface Based on Flicker-Free Steady-State Motion Visual Evoked Potential. Sci. Rep. 2018, 8, 5835. [Google Scholar] [CrossRef]
- Mukesh, T.M.S.; Jaganathan, V.; Reddy, M.R. A novel multiple frequency stimulation method for steady state VEP based brain computer interfaces. Physiol. Meas. 2006, 27, 61–71. [Google Scholar] [CrossRef]
- Wu, Z.; Lai, Y.; Xia, Y.; Wu, D.; Yao, D. Stimulator selection in SSVEP-based BCI. Med. Eng. Phys. 2008, 30, 1079–1088. [Google Scholar] [CrossRef]
- Lopez-Gordo, M.A.; Prieto, A.; Pelayo, F.; Morillas, C. Customized stimulation enhances performance of independent binary SSVEP-BCIs. Clin. Neurophysiol. 2011, 122, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Byczuk, M.; Poryzala, P.; Materka, A. On diversity within operators’ EEG responses to LED-produced alternate stimulus in SSVEP BCI. Bull. Pol. Acad. Sci. Tech. Sci. 2012, 60, 447–453. [Google Scholar] [CrossRef]
- Gerloff, M.; Schilling, M. Subject response variability in terms of colour and frequency of capacitive SSVEP measurements. Biomed. Tech. 2012, 57, 95–98. [Google Scholar] [CrossRef]
- Dreyer, A.M.; Herrmann, C.S. Frequency-modulated steady-state visual evoked potentials: A new stimulation method for brain–computer interfaces. J. Neurosci. Methods 2015, 241, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jukiewicz, M.; Cysewska-Sobusiak, A. Stimuli design for SSVEP-based brain computer-interface. Int. J. Electron. Telecommun. 2016, 62, 109–113. [Google Scholar] [CrossRef]
- Chien, Y.Y.; Lin, F.C.; Zao, J.K.; Chou, C.C.; Huang, Y.P.; Kuo, H.Y.; Wang, Y.; Jung, T.P.; Shieh, H.P.D. Polychromatic SSVEP stimuli with subtle flickering adapted to brain-display interactions. J. Neural Eng. 2017, 14, 016018. [Google Scholar] [CrossRef]
- Chu, L.; Fernández-Vargas, J.; Kita, K.; Yu, W. Influence of Stimulus Color on Steady State Visual Evoked Potentials; Springer: Berlin, Germany, 2017; Volume 531, pp. 499–509. [Google Scholar]
- Zhang, X.; Xu, G.; Xie, J.; Zhang, X. Brain response to luminance-based and motion-based stimulation using intermodulation frequencies. PLoS ONE 2017, 12, e0188073. [Google Scholar]
- Duart, X.; Quiles, E.; Suay, F.; Chio, N.; García, E.; Morant, F. Evaluating the effect of stimuli color and frequency on SSVEP. Sensors 2021, 21, 117. [Google Scholar] [CrossRef]
- Dreyer, A.M.; Heikkinen, B.L.; Herrmann, C.S. The Influence of the Modulation Index on Frequency-Modulated Steady-State Visual Evoked Potentials. Front. Hum. Neurosci. 2022, 16, 859519. [Google Scholar] [CrossRef]
- Vahid, F.; Behboodi, M.; Mahnam, A. Bichromatic visual stimulus with subharmonic response to achieve a high-accuracy SSVEP BCI system with low eye irritation. Biomed. Signal Process. Control. 2023, 83, 104629. [Google Scholar] [CrossRef]
- Hwang, H.J.; Kim, D.H.; Han, C.H.; Im, C.H. A new dual-frequency stimulation method to increase the number of visual stimuli for multi-class SSVEP-based brain–computer interface (BCI). Brain Res. 2013, 1515, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Sakurada, T.; Kawase, T.; Komatsu, T.; Kansaku, K. Use of high-frequency visual stimuli above the critical flicker frequency in a SSVEP-based BMI. Clin. Neurophysiol. 2015, 126, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Y.; Zhang, S.; Xu, S.; Gao, X. Effects of stimulation frequency and stimulation waveform on steady-state visual evoked potentials using a computer monitor. J. Neural Eng. 2019, 16, 066007. [Google Scholar] [CrossRef] [PubMed]
- Ladouce, S.; Darmet, L.; Tresols, J.J.T.; Velut, S.; Ferraro, G.; Dehais, F. Improving user experience of SSVEP BCI through low amplitude depth and high frequency stimuli design. Sci. Rep. 2022, 12, 8865. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.; Khosla, A.; Jha, R. Influence of stimuli colour in SSVEP-based BCI wheelchair control using support vector machines. J. Med. Eng. Technol. 2014, 38, 125–134. [Google Scholar] [CrossRef]
- Bi, L.; Fan, X.A.; Jie, K.; Teng, T.; Ding, H.; Liu, Y. Using a head-up display-based steady-state visually evoked potential brain–computer interface to control a simulated vehicle. IEEE Trans. Intell. Transp. Syst. 2014, 15, 959–966. [Google Scholar] [CrossRef]
- Shu, X.; Yao, L.; Meng, J.; Sheng, X.; Zhu, X. Visual stimulus background effects on SSVEP-Based BCI towards a practical Robot car control. Int. J. Humanoid Robot. 2015, 12, 1550014. [Google Scholar] [CrossRef]
- Yan, W.; Xu, G.; Li, M.; Xie, J.; Han, C.; Zhang, S.; Luo, A.; Chen, C. Steady-State Motion Visual Evoked Potential (SSMVEP) based on equal luminance colored enhancement. PLoS ONE 2017, 12, e0169642. [Google Scholar] [CrossRef]
- Floriano, A.; Diez, P.F.; Bastos-Filho, T.F. Evaluating the influence of chromatic and luminance stimuli on SSVEPs from behind-the-ears and occipital areas. Sensors 2018, 18, 615. [Google Scholar] [CrossRef]
- Si-Mohammed, H.; Petit, J.; Jeunet, C.; Argelaguet, F.; Spindler, F.; Evain, A.; Roussel, N.; Casiez, G.; Lecuyer, A. Towards BCI-Based Interfaces for Augmented Reality: Feasibility, Design and Evaluation. IEEE Trans. Vis. Comput. Graph. 2020, 26, 1608–1621. [Google Scholar] [CrossRef] [PubMed]
- Bisht, A.; Srivastava, S.; Purushothaman, G. A new 360° rotating type stimuli for improved SSVEP based brain computer interface. Biomed. Signal Process. Control. 2020, 57, 101778. [Google Scholar] [CrossRef]
- Zhu, S.; Yang, J.; Ding, P.; Wang, F.; Gong, A.; Fu, Y. Optimization of SSVEP-BCI Virtual Reality Stereo Stimulation Parameters Based on Knowledge Graph. Brain Sci. 2023, 13, 710. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gordo, M.A.; Prieto, A.; Pelayo, F.; Morillas, C. Use of phase in brain–computer interfaces based on steady-state visual evoked potentials. Neural Process. Lett. 2010, 32, 1–9. [Google Scholar] [CrossRef]
- Diez, P.F.; Mut, V.A.; Perona, E.M.A.; Leber, E.L. Asynchronous BCI control using high-frequency SSVEP. J. Neuroeng. Rehabil. 2011, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Kapeller, C.; Hintermüller, C.; Guger, C. Usability of Video-Overlaying SSVEP Based BCIs. In Proceedings of the 3rd Augmented Human International Conference, Megève, France, 8–9 March 2012; p. 162. [Google Scholar]
- Ng, K.B.; Bradley, A.P.; Cunnington, R. Stimulus specificity of a steady-state visual-evoked potential-based brain–computer interface. J. Neural Eng. 2012, 9, 036008. [Google Scholar] [CrossRef] [PubMed]
- Duszyk, A.; Bierzyńska, M.; Radzikowska, Z.; Milanowski, P.; Suffczyński, R.K.P.; Michalska, M.; Labecki, M.; Zwoliński, P.; Durka, P. Towards an optimization of stimulus parameters for brain–computer interfaces based on steady state visual evoked potentials. PLoS ONE 2014, 9, e112099. [Google Scholar] [CrossRef] [PubMed]
- Punsawad, Y.; Wongsawat, Y. Enhancement of steady-state visual evoked potential-based brain–computer interface systems via a steady-state motion visual stimulus modality. IEEJ Trans. Electr. Electron. Eng. 2017, 12, S89–S94. [Google Scholar] [CrossRef]
- Waytowich, N.R.; Yamani, Y.; Krusienski, D.J. Optimization of Checkerboard Spatial Frequencies for Steady-State Visual Evoked Potential Brain-Computer Interfaces. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 557–565. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, G.; Ravi, A.; Yan, W.; Jiang, N. Fusing Frontal and Occipital EEG Features to Detect “brain Switch” by Utilizing Convolutional Neural Network. IEEE Access 2019, 7, 82817–82825. [Google Scholar] [CrossRef]
- Park, S.; Cha, H.S.; Im, C.H. Development of an Online Home Appliance Control System Using Augmented Reality and an SSVEP-Based Brain-Computer Interface. IEEE Access 2019, 7, 163604–163614. [Google Scholar] [CrossRef]
- Stawicki, P.; Volosyak, I. Comparison of modern highly interactive flicker-free steady state motion visual evoked potentials for practical brain–computer interfaces. Brain Sci. 2020, 10, 686. [Google Scholar] [CrossRef] [PubMed]
- Ravi, A.; Lu, J.; Pearce, S.; Jiang, N. Enhanced System Robustness of Asynchronous BCI in Augmented Reality Using Steady-State Motion Visual Evoked Potential. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhao, X. Visual stimulus color effect on SSVEP-BCI in augmented reality. Biomed. Signal Process. Control. 2022, 78, 103906. [Google Scholar] [CrossRef]
- Jiang, L.; Pei, W.; Wang, Y. A User-Friendly SSVEP-Based BCI Using Imperceptible Phase-Coded Flickers at 60 Hz. China Commun. 2022, 19, 1–14. [Google Scholar] [CrossRef]
- Kwon, J.; Hwang, J.; Nam, H.; Im, C.H. Novel hybrid visual stimuli incorporating periodic motions into conventional flickering or pattern-reversal visual stimuli for steady-state visual evoked potential-based brain–computer interfaces. Front. Neuroinform. 2022, 16, 997068. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wen, Y.; Bi, J.; Li, H.; Sun, J. A portable SSVEP-BCI system for rehabilitation exoskeleton in augmented reality environment. Biomed. Signal Process. Control. 2023, 83, 104664. [Google Scholar] [CrossRef]
- Ajami, S.; Mahnam, A.; Abootalebi, V. Development of a practical high frequency brain–computer interface based on steady-state visual evoked potentials using a single channel of EEG. Biocybern. Biomed. Eng. 2018, 38, 106–114. [Google Scholar] [CrossRef]
- Peguero, J.D.C.; Hernández-Rojas, L.G.; Mendoza-Montoya, O.; Caraza, R.; Antelis, J.M. SSVEP detection assessment by combining visual stimuli paradigms and no-training detection methods. Front. Neurosci. 2023, 17, 1142892. [Google Scholar] [CrossRef]
- Shyu, K.K.; Lee, P.L.; Liu, Y.J.; Sie, J.J. Dual-frequency steady-state visual evoked potential for brain computer interface. Neurosci. Lett. 2010, 483, 28–31. [Google Scholar] [CrossRef]
- Lee, P.L.; Yeh, C.L.; Cheng, J.Y.S.; Yang, C.Y.; Lan, G.Y. An SSVEP-based BCI using high duty-cycle visual flicker. IEEE Trans. Biomed. Eng. 2011, 58, 3350–3359. [Google Scholar] [CrossRef]
- Chang, M.H.; Baek, H.J.; Lee, S.M.; Park, K.S. An amplitude-modulated visual stimulation for reducing eye fatigue in SSVEP-based brain–computer interfaces. Clin. Neurophysiol. 2014, 125, 1380–1391. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.; Zhang, Z.; Guan, K.; Liu, G.; Niu, H. A radial zoom motion-based paradigm for steady state motion visual evoked potentials. Front. Hum. Neurosci. 2019, 13, 127. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, K.; Zhang, X.; Wang, J.; Wei, R. Research on the Application of Augmented Reality in SSVEP-BCI. Assoc. Comput. Mach. 2020, 4, 505–509. [Google Scholar]
- Hsu, H.T.; Shyu, K.K.; Hsu, C.C.; Lee, L.H.; Lee, P.L. Phase-Approaching Stimulation Sequence for SSVEP-Based BCI: A Practical Use in VR/AR HMD. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 2754–2764. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, Z.; Gao, S.; Gao, X. Brain-computer interface based on intermodulation frequency. J. Neural Eng. 2013, 10, 066009. [Google Scholar] [CrossRef] [PubMed]
- Kramberger, I.; Kacic, Z.; Donaj, G. Binocular Phase-Coded Visual Stimuli for SSVEP-Based BCI. IEEE Access 2019, 7, 48912–48922. [Google Scholar] [CrossRef]
- Siribunyaphat, N.; Punsawad, Y. Steady-State Visual Evoked Potential-Based Brain–Computer Interface Using a Novel Visual Stimulus with Quick Response (QR) Code Pattern. Sensors 2022, 22, 1439. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Zhang, S.; Gao, S.; Hu, Y.; Gao, X. A novel stimulation method for multi-class SSVEP-BCI using intermodulation frequencies. J. Neural Eng. 2017, 14, 026013. [Google Scholar] [CrossRef]
- Yan, W.; Xu, G. Brain-computer interface method based on light-flashing and motion hybrid coding. Cogn. Neurodynamics 2020, 14, 697–708. [Google Scholar] [CrossRef]
- Sato, Y.; Kitamura, Y.; Hirata, T.; Bao, Y. Investigation of visual stimulus signals using hue change for ssvep. Appl. Sci. 2021, 11, 1045. [Google Scholar] [CrossRef]
- Ming, G.; Pei, W.; Chen, H.; Gao, X.; Wang, Y. Optimizing spatial properties of a new checkerboard-like visual stimulus for user-friendly SSVEP-based BCIs. J. Neural Eng. 2021, 18, 056046. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, N.; Gao, X.; Ma, R.; Dong, J.; Chen, X.; Cui, H. A Novel SSVEP Brain-Computer Interface System Based on Simultaneous Modulation of Luminance and Motion. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Rekrut, M.; Jungbluth, T.; Alexandersson, J.; Krüger, A. Spinning Icons: Introducing a Novel SSVEP-BCI Paradigm Based on Rotation. Assoc. Comput. Mach. 2021, 4, 234–243. [Google Scholar]
- Ming, G.; Zhong, H.; Pei, W.; Gao, X.; Wang, Y. A new grid stimulus with subtle flicker perception for user-friendly SSVEP-based BCIs. J. Neural Eng. 2023, 20, 026010. [Google Scholar] [CrossRef] [PubMed]
- Oralhan, Z.; Tokmakçi, M. The Effect of Duty Cycle and Brightness Variation of Visual Stimuli on SSVEP in Brain Computer Interface Systems. IETE J. Res. 2016, 62, 795–803. [Google Scholar] [CrossRef]
- Li, M.; Chen, X.; Cui, H. A High-Frequency SSVEP-BCI System Based on Simultaneous Modulation of Luminance and Motion Using Intermodulation Frequencies. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Maymandi, H.; Benitez, J.L.P.; Gallegos-Funes, F.; Benitez, J.A.P. A novel monitor for practical brain–computer interface applications based on visual evoked potential. Brain-Comput. Interfaces 2021, 8, 1–13. [Google Scholar] [CrossRef]
- Wilson, J.J.; Palaniappan, R. On the stimulus duty cycle in steady state visual evoked potential. Int. J. Knowl.-Based Intell. Eng. Syst. 2014, 18, 73–79. [Google Scholar] [CrossRef]
- Hart, S.G.; Staveland, L.E. Development of NASA-TLX (Task Load Index): Results of empirical and theoretical research. In Advances in Psychology; Elsevier: Amsterdam, The Netherlands, 1988; Volume 52, pp. 139–183. [Google Scholar]
- Schrepp, M.; Thomaschewski, J.; Hinderks, A. Construction of a benchmark for the user experience questionnaire (UEQ). Int. J. Interact. Multimed. Artif. Intell. 2017, 4, 40–44. [Google Scholar] [CrossRef]
- Cheng, M.; Gao, X.; Gao, S.; Xu, D. Design and implementation of a brain–computer interface with high transfer rates. IEEE Trans. Biomed. Eng. 2002, 49, 1181–1186. [Google Scholar] [CrossRef]
- Herrmann, C.S. Human EEG responses to 1–100 Hz flicker: Resonance phenomena in visual cortex and their potential correlation to cognitive phenomena. Exp. Brain Res. 2001, 137, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Regan, D. Steady-state evoked potentials. JOSA 1977, 67, 1475–1489. [Google Scholar] [CrossRef] [PubMed]
- Eisen-Enosh, A.; Farah, N.; Burgansky-Eliash, Z.; Polat, U.; Mandel, Y. Evaluation of critical flicker-fusion frequency measurement methods for the investigation of visual temporal resolution. Sci. Rep. 2017, 7, 15621. [Google Scholar] [CrossRef] [PubMed]
- Holcombe, A.O. Seeing slow and seeing fast: Two limits on perception. Trends Cogn. Sci. 2009, 13, 216–621. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.; Gerd, A.; Graeser, P.; Lueth, T.; Graeser, A. High Frequency Steady-State Visual Evoked Potentials: An Empirical Study on Re-test Stability for Brain-Computer Interface Usage. In Proceedings of the 3rd International Conference on Computer-Human Interaction Research and Applications, Vienna, Austria, 20–21 September 2019. [Google Scholar]
- López, J.L.M.; Ramírez, J.C.C.; Yoo, S.G. Study of the Influences of Stimuli Characteristics in the Implementation of Steady State Visual Evoked Potentials Based Brain Computer Interface Systems. Springer Sci. Bus. Media Dtschl. GmbH 2021, 12855, 302–317. [Google Scholar]
- Wu, C.H.; Lakany, H. The effect of the viewing distance of stimulus on SSVEP response for use in brain–computer interfaces. In Proceedings of the 2013 IEEE International Conference on Systems, Man, and Cybernetics, Manchester, UK, 13–16 October 2013; pp. 1840–1845. [Google Scholar]
- Mouli, S.; Palaniappan, R. Eliciting Higher SSVEP Response from LED Visual Stimulus with Varying Luminosity Levels. In Proceedings of the 2016 International Conference for Students on Applied Engineering (ICSAE), Newcastle Upon Tyne, UK, 20–21 October 2016. [Google Scholar]
- Wu, C.H.; Lakany, H. Evaluation of the feasibility of a novel distance adaptable Steady- State Visual Evoked Potential based Brain-Computer Interface. In Proceedings of the 2015 7th International IEEE/EMBS Conference on Neural Engineering (NER), Montpellier, France, 22–24 April 2015. [Google Scholar]
- Kwak, N.S.; Won, D.O.; Kim, K.T.; Park, H.J.; Lee, S.W. Analysis of Steady State Visual Evoked Potentials based on Viewing Distance Changes for Brain–Machine Interface Speller. In Proceedings of the 2016 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Budapest, Hungary, 9–12 October 2016. [Google Scholar]
- Garcia, D.E.; Zheng, K.W.; Liu, Y.; Tao, Y.S.; Mann, S. Painting with the Eye: Understanding the Visual Field of the Human Eye with SSVEP. In Proceedings of the 2020 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Toronto, ON, Canada, 11–14 October 2020. [Google Scholar]
- Chen, X.; Liu, B.; Wang, Y.; Gao, X. A Spectrally-Dense Encoding Method for Designing a High-Speed SSVEP-BCI with 120 Stimuli. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 2764–2772. [Google Scholar] [CrossRef] [PubMed]
- Vialatte, F.B.; Maurice, M.; Dauwels, J.; Cichocki, A. Steady-state visually evoked potentials: Focus on essential paradigms and future perspectives. Prog. Neurobiol. 2010, 90, 418–438. [Google Scholar] [CrossRef]
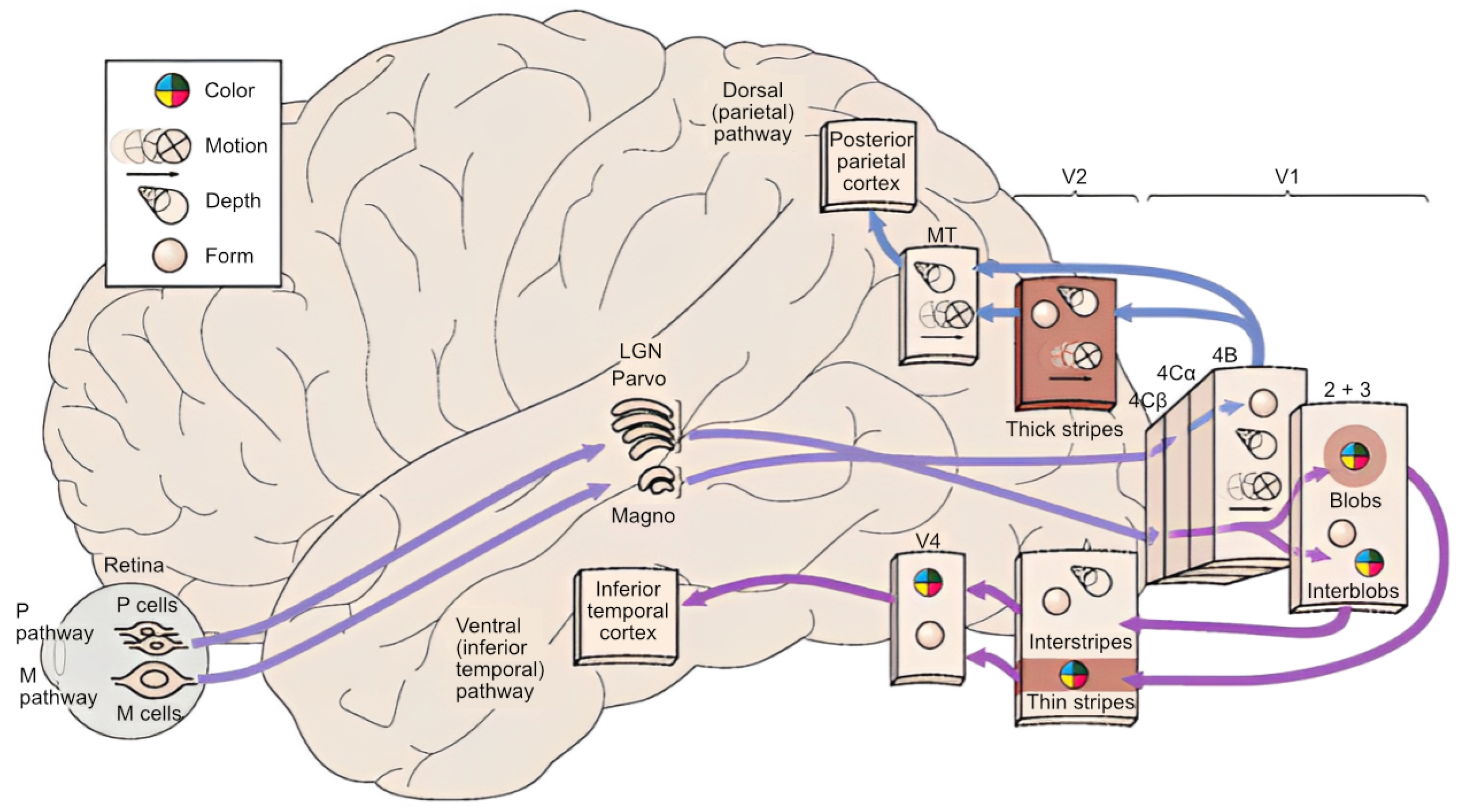
- Kaplan, E. The M, P, and K Pathways of the Primate Visual System; MIT Press: Cambridge, MA, USA, 2022; pp. 481–493. [Google Scholar]
- Kandel, E.R.; Schwartz, J.H.; Jessell, T.M.; Siegelbaum, S.; Hudspeth, A.J.; Mack, S. Principles of Neural Science; McGraw-Hill: New York, NY, USA, 2000; Volume 4. [Google Scholar]
- Berga, D. Understanding Eye Movements: Psychophysics and a Model of Primary Visual Cortex. Ph.D. Thesis, Universitat Autònoma de Barcelona, Bellaterra, Spain, 2019. [Google Scholar]
- Dow, B.M.; Snyder, A.Z.; Vautin, R.G.; Bauer, R. Magnification Factor and Receptive Field Size in Foveal Striate Cortex of the Monkey. Exp. Brain Res. 1981, 44, 213–228. [Google Scholar] [CrossRef]
- Busch, N.A.; Debener, S.; Kranczioch, C.; Engel, A.K.; Herrmann, C.S. Size matters: Effects of stimulus size, duration and eccentricity on the visual gamma-band response. Clin. Neurophysiol. 2004, 115, 1810–1820. [Google Scholar] [CrossRef]
- Campbell, F.; Maffei, L. Electrophysiological evidence for the existence of orientation and size detectors in the human visual system. J. Physiol. 1970, 207, 635–652. [Google Scholar] [CrossRef]
- Albrecht, D.G.; Hamilton, D.B. Striate Cortex of Monkey and Cat: Contrast Response Function. J. Neurophysiol. 1982, 48, 217–237. [Google Scholar] [CrossRef] [PubMed]
- Rols, G.; Tallon-Baudry, C.; Girard, P.; Bertrand, O.; Bullier, J. Cortical mapping of gamma oscillations in areas V1 and V4 of the macaque monkey. Vis. Neurosci. 2001, 18, 527–540. [Google Scholar] [CrossRef] [PubMed]

| Number of Targets | Number of Articles | Articles |
|---|---|---|
| 1 | 20 | [14,17,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37] |
| 2 | 2 | [38,39] |
| 3 | 7 | [35,40,41,42,43,44,45] |
| 4 | 18 | [15,18,19,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60] |
| 5 | 3 | [16,61,62] |
| 6 | 6 | [63,64,65,66,67,68] |
| 8 | 3 | [69,70,71] |
| 9 | 7 | [36,37,72,73,74,75,76] |
| 10 | 1 | [77] |
| 12 | 2 | [34,78] |
| 16 | 2 | [79,80] |
| 32 | 1 | [81] |
| 35 | 1 | [82] |
| 40 | 1 | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reitelbach, C.; Oyibo, K. Optimal Stimulus Properties for Steady-State Visually Evoked Potential Brain–Computer Interfaces: A Scoping Review. Multimodal Technol. Interact. 2024, 8, 6. https://doi.org/10.3390/mti8020006
Reitelbach C, Oyibo K. Optimal Stimulus Properties for Steady-State Visually Evoked Potential Brain–Computer Interfaces: A Scoping Review. Multimodal Technologies and Interaction. 2024; 8(2):6. https://doi.org/10.3390/mti8020006
Chicago/Turabian StyleReitelbach, Clemens, and Kiemute Oyibo. 2024. "Optimal Stimulus Properties for Steady-State Visually Evoked Potential Brain–Computer Interfaces: A Scoping Review" Multimodal Technologies and Interaction 8, no. 2: 6. https://doi.org/10.3390/mti8020006






