Monitoring of Pathogens Carried by Imported Flies and Cockroaches at Shenzhen Ports
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection, Composition Ratio, and Statistical Analysis of Origins
2.1.1. Sample Collection
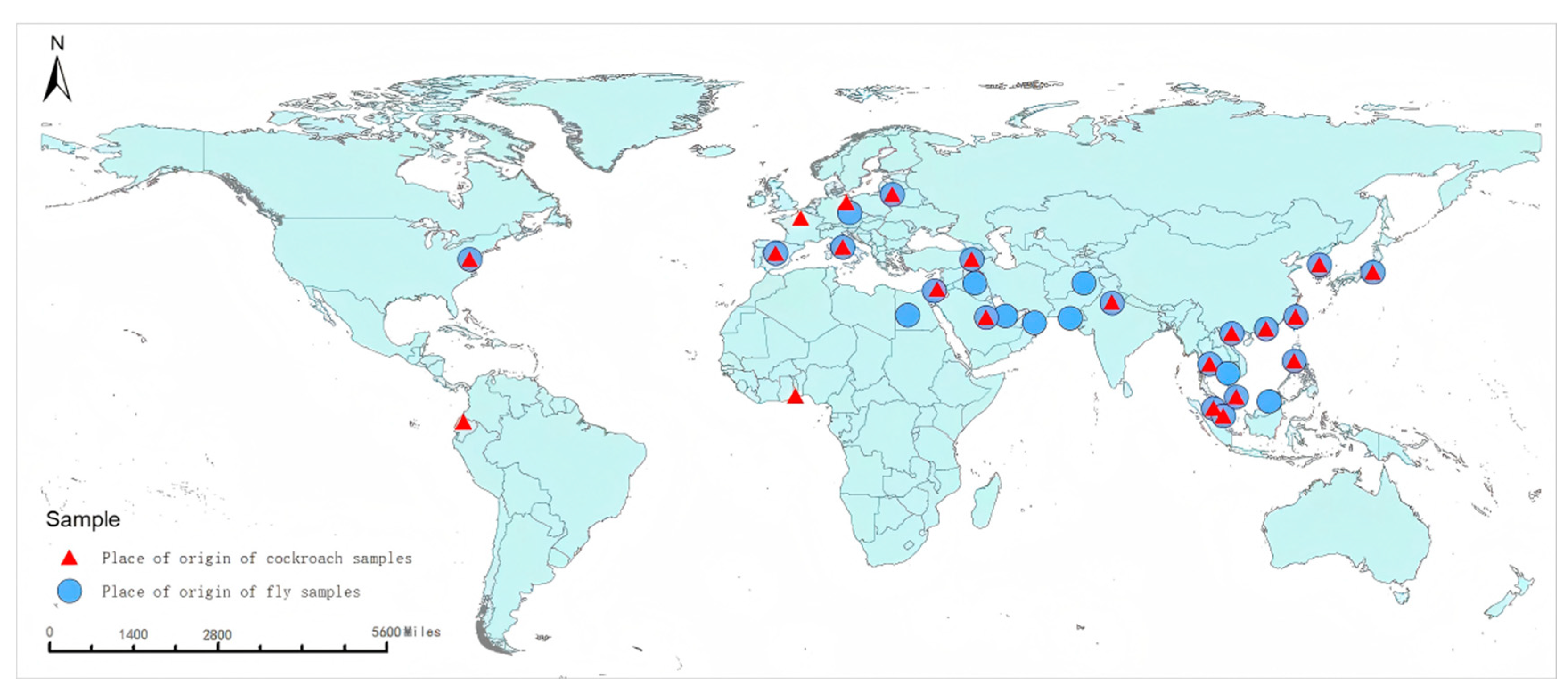
2.1.2. Composition Ratio and Origin Distribution
2.2. Main Reagents and Instruments
2.3. DNA Extraction
2.4. Quantitative Real-Time PCR Detection of Pathogens
2.5. Nucleic Acid Quality Control
2.6. Library Construction and Metagenomic Sequencing
2.7. Raw Data Processing and Bioinformatics Analysis
3. Results
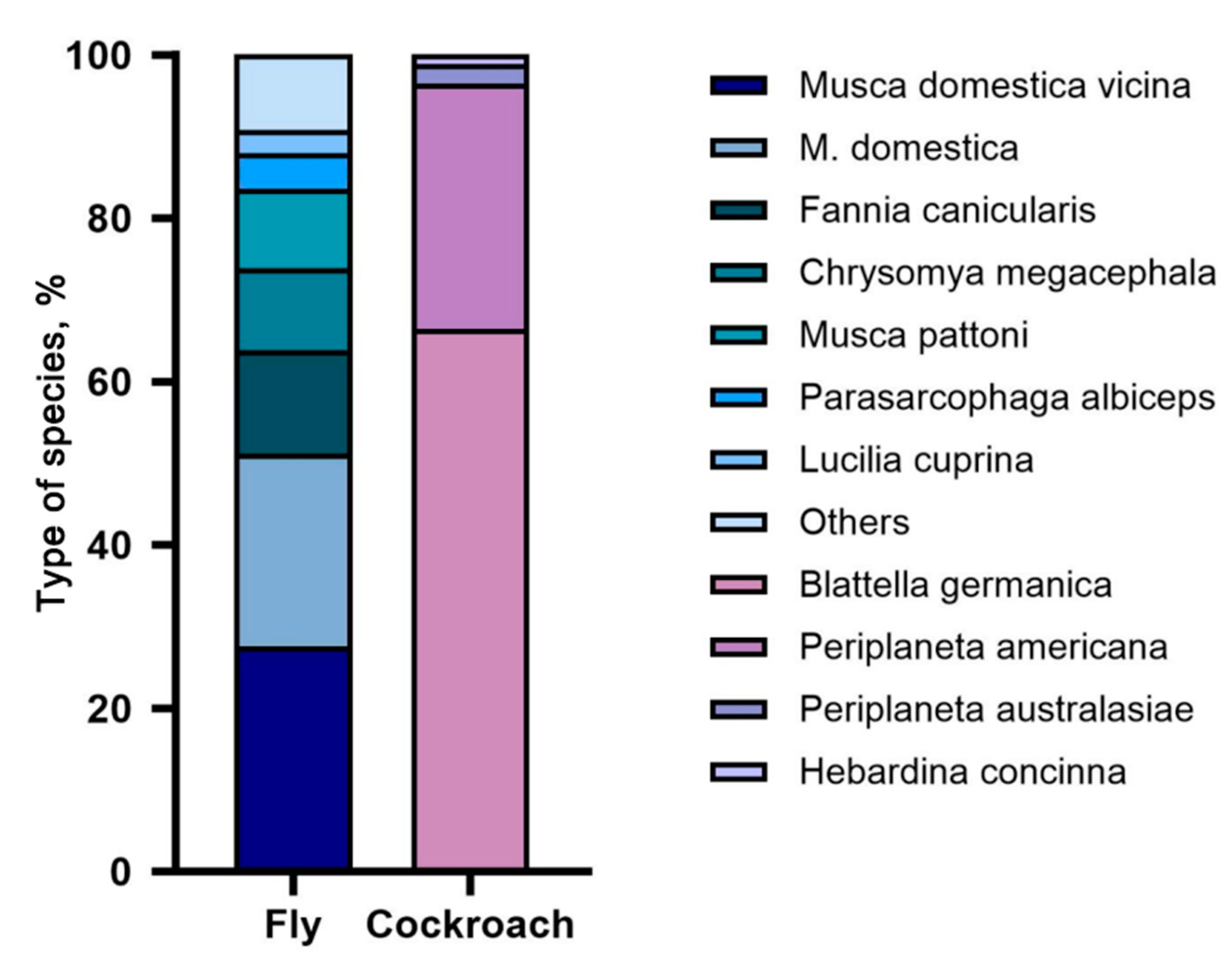
3.1. Analysis of Composition Ratio and Origin Distribution of Imported Vectors
3.2. Pathogen Detection Status
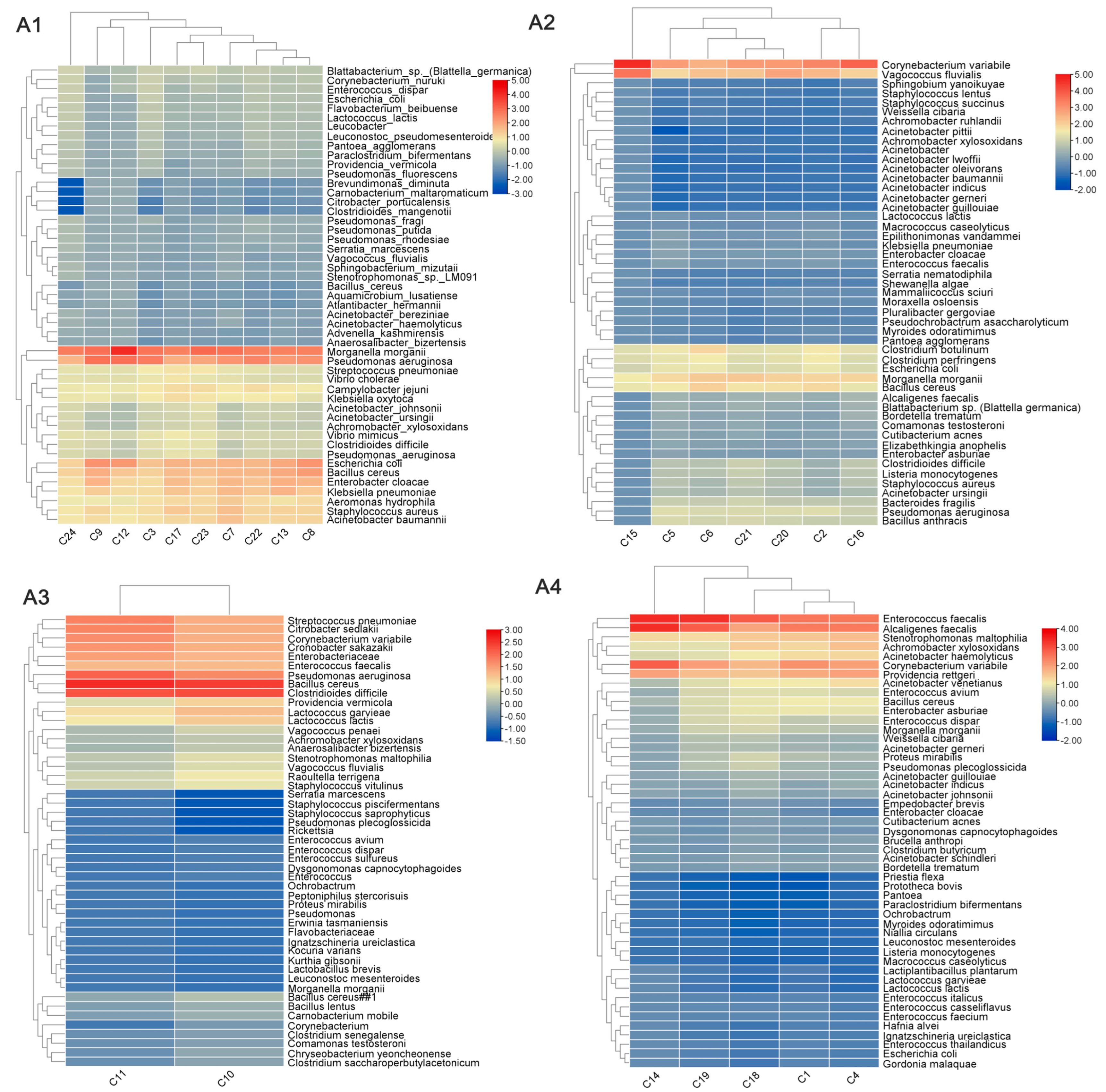
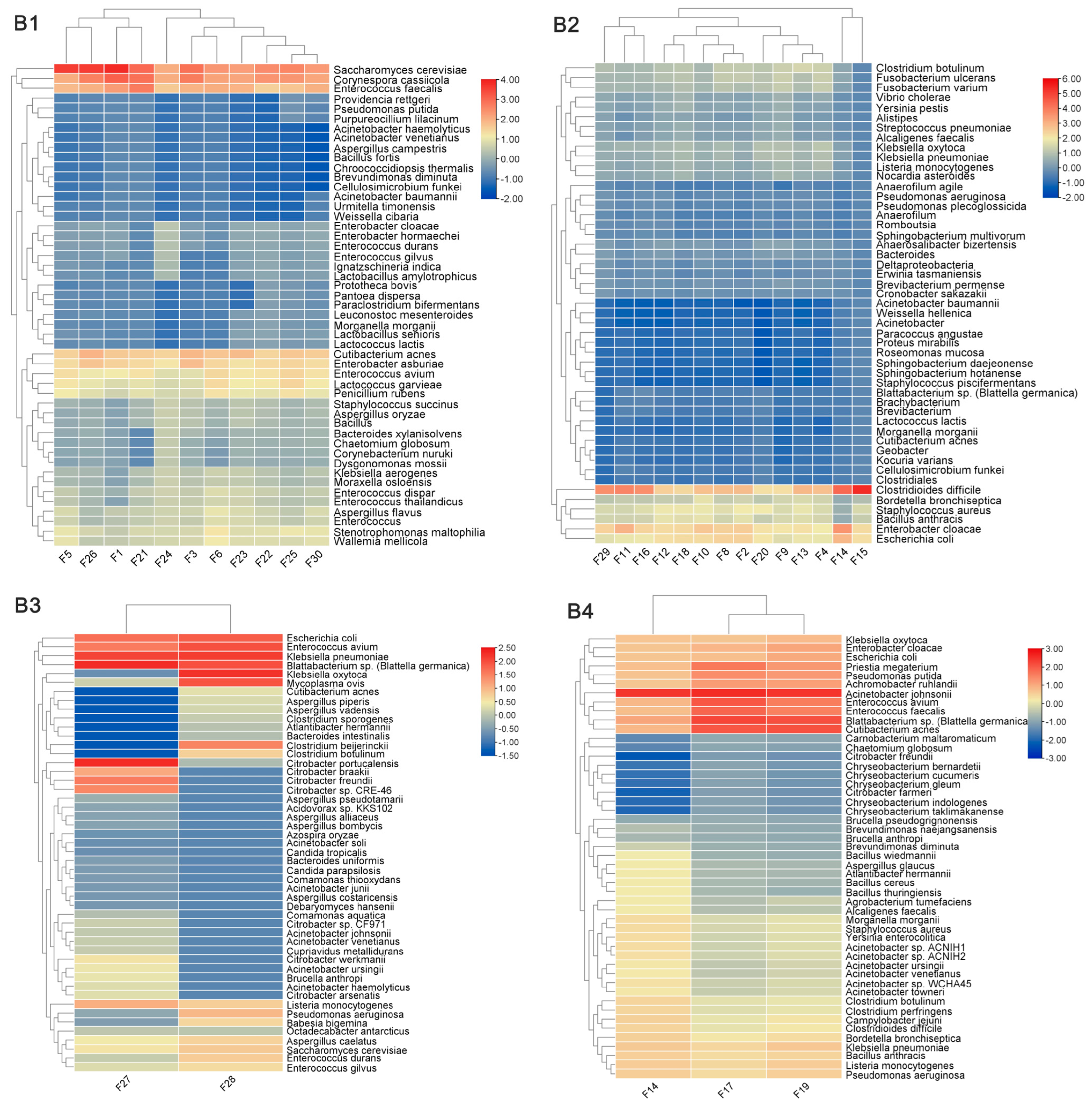
3.3. Differences in the Composition of Pathogens Carried by Imported Flies and Cockroaches from Different Regions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VBD | Vector-borne disease; |
| qPCR | Polymerase chain reaction. |
References
- Wilson, A.L.; Courtenay, O.; Kelly-Hope, L.A.; Scott, T.W.; Takken, W.; Torr, S.J.; Lindsay, S.W. The Importance of Vector Control for the Control and Elimination of Vector-Borne Diseases. PLoS Negl. Trop. Dis. 2020, 14, e0007831. [Google Scholar] [CrossRef]
- Golding, N.; Wilson, A.L.; Moyes, C.L.; Cano, J.; Pigott, D.M.; Velayudhan, R.; Brooker, S.J.; Smith, D.L.; Hay, S.I.; Lindsay, S.W. Integrating Vector Control Across Diseases. BMC Med. 2015, 13, 249. [Google Scholar] [CrossRef]
- Wylezich, C.; Belka, A.; Hanke, D.; Beer, M.; Blome, S.; Höper, D. Metagenomics for Broad and Improved Parasite Detection: A Proof-of-Concept Study Using Swine Faecal Samples. Int. J. Parasitol. 2019, 49, 769–777. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, Y.; Feng, L.; Lin, C.; Ye, C.; Liu, J.; Li, H.; Hao, L.; Liu, H. Intestinal Pathogens Detected Within Cockroach Species within Different Food-Related Environments in Pudong, China. Sci. Rep. 2024, 14, 1947. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, J.; Liu, Q.; Li, T.; Luo, M.; Gong, Z. Practice of Integrated Vector Surveillance of Arthropod Vectors, Pathogens and Reservoir Hosts to Monitor the Occurrence of Tropical Vector-Borne Diseases in 2020 in Zhejiang Province, China. Front. Vet. Sci. 2022, 9, 1003550. [Google Scholar] [CrossRef] [PubMed]
- Arenas, M.; Araujo, N.M.; Branco, C.; Castelhano, N.; Castro-Nallar, E.; Pérez-Losada, M. Mutation and Recombination in Pathogen Evolution: Relevance, Methods and Controversies. Infect. Genet. Evol. 2018, 63, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, A.; Nitsche, A.; Kohl, C. Viral Metagenomics on Blood-Feeding Arthropods as a Tool for Human Disease Surveillance. Int. J. Mol. Sci. 2016, 17, 1743. [Google Scholar] [CrossRef] [PubMed]
- Balaraman, V.; Drolet, B.S.; Mitzel, D.N.; Wilson, W.C.; Owens, J.; Gaudreault, N.N.; Meekins, D.A.; Bold, D.; Trujillo, J.D.; Noronha, L.E.; et al. Mechanical Transmission of SARS-CoV-2 by House Flies. Parasit. Vectors 2021, 14, 214. [Google Scholar] [CrossRef]
- Hoffmann, C.; Stockhausen, M.; Merkel, K.; Calvignac-Spencer, S.; Leendertz, F.H. Assessing the Feasibility of Fly Based Surveillance of Wildlife Infectious Diseases. Sci. Rep. 2016, 6, 37952. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Sun, S.; Huang, Y.; Gao, Q.; Xie, X.; Wang, P.; Li, J.; Liang, L.; He, X.; Jiang, Y.; et al. Metagenomic Analysis Revealed the Potential Role of Gut Microbiome in Gout. npj Biofilms Microbiomes 2021, 7, 66. [Google Scholar] [CrossRef]
- Sayers, E.W.; Agarwala, R.; Bolton, E.E.; Brister, J.R.; Canese, K.; Clark, K.; Connor, R.; Fiorini, N.; Funk, K.; Hefferon, T.; et al. Database Resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2019, 47, D23–D28. [Google Scholar] [CrossRef]
- China National Health Commission of the People’s Republic of China. List of Human Infectious Pathogenic Microorganisms. China. 2023. Available online: http://www.nhc.gov.cn/qjjys/s7948/202308/b6b51d792d394fbea175e4c8094dc87e.shtml (accessed on 6 September 2024).
- Li, Q.; Liu, M.; Liu, T.; Tong, Y.; Zhang, Y. An Evolutionary Game Study of Cockroach Control Strategies in Residential Households. Sci. Rep. 2023, 13, 7342. [Google Scholar] [CrossRef]
- Chivian, D.; Jungbluth, S.P.; Dehal, P.S.; Wood-Charlson, E.M.; Canon, R.S.; Allen, B.H.; Clark, M.M.; Gu, T.; Land, M.L.; Price, G.A.; et al. Metagenome-Assembled Genome Extraction and Analysis from Microbiomes Using KBase. Nat. Protoc. 2023, 18, 208–238. [Google Scholar] [CrossRef]
- Banerjee, S.; Maiti, T.K.; Roy, R.N. Enzyme Producing Insect Gut Microbes: An Unexplored Biotechnological Aspect. Crit. Rev. Biotechnol. 2022, 42, 384–402. [Google Scholar] [CrossRef] [PubMed]
- Popoff, M.R.; Brüggemann, H. Regulatory Networks Controlling Neurotoxin Synthesis in Clostridium botulinum and Clostridium tetani. Toxins 2022, 14, 364. [Google Scholar] [CrossRef]
- Tuipulotu, D.E.; Mathur, A.; Ngo, C.; Man, S.M. Bacillus cereus: Epidemiology, Virulence Factors, and Host-Pathogen Interactions. Trends Microbiol. 2021, 29, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Yekani, M.; Rezaee, M.A.; Beheshtirouy, S.; Baghi, H.B.; Bazmani, A.; Farzinazar, A.; Memar, M.Y.; Sóki, J. Carbapenem Resistance in Bacteroides fragilis: A Review of Molecular Mechanisms. Anaerobe 2022, 76, 102606. [Google Scholar] [CrossRef]
- Miguelena Chamorro, B.; De Luca, K.; Swaminathan, G.; Longet, S.; Mundt, E.; Paul, S. Bordetella bronchiseptica and Bordetella pertussis: Similarities and Differences in Infection, Immuno-Modulation, and Vaccine Considerations. Clin. Microbiol. Rev. 2023, 36, e0016422. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Song, F.L.; Yang, Y.; Gao, Y.F.; Ci, Y.; Cheng, X.L.; Nie, C.; Liu, L.J.; Zhang, X.L.; Wang, J. Epidemiological and Molecular Study on Tick-Borne Pathogens in Argun Port Area Near the Chinese-Russian Border. Vector Borne Zoonotic Dis. 2023, 23, 447–457. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, Y.; Godson, D.L.; Fernando, C.; Alexander, T.W.; Hill, J.E. Assessment of Metagenomic Sequencing and qPCR for Detection of Influenza D Virus in Bovine Respiratory Tract Samples. Viruses 2020, 12, 814. [Google Scholar] [CrossRef] [PubMed]
- Sarcina, L.; Macchia, E.; Loconsole, G.; D’Attoma, G.; Bollella, P.; Catacchio, M.; Leonetti, F.; Di Franco, C.; Elicio, V.; Scamarcio, G.; et al. Fast and Reliable Electronic Assay of a Xylella fastidiosa Single Bacterium in Infected Plants Sap. Adv. Sci. 2022, 9, e2203900. [Google Scholar] [CrossRef] [PubMed]
- Dembélé, P.; Cissoko, M.; Diarra, A.Z.; Doumbia, L.; Koné, A.; Magassa, M.H.; Mehadji, M.; Thera, M.A.; Ranque, S. Evaluation of the Performance of Rapid Diagnostic Tests for Malaria Diagnosis and Mapping of Different Plasmodium Species in Mali. Int. J. Environ. Res. Public Health 2024, 21, 228. [Google Scholar] [CrossRef]
- Pan, J.; Zeng, M.; Zhao, M.; Huang, L. Research Progress on the Detection Methods of Porcine Reproductive and Respiratory Syndrome Virus. Front. Microbiol. 2023, 14, 1097905. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.E.; Lu, J.; Langmead, B. Improved Metagenomic Analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef]
- Carr, V.R.; Shkoporov, A.; Hill, C.; Mullany, P.; Moyes, D.L. Probing the Mobilome: Discoveries in the Dynamic Microbiome. Trends Microbiol. 2021, 29, 158–170. [Google Scholar] [CrossRef]
- Liu, Y.X.; Qin, Y.; Chen, T.; Lu, M.; Qian, X.; Guo, X.; Bai, Y. A Practical Guide to Amplicon and Metagenomic Analysis of Microbiome Data. Protein Cell 2021, 12, 315–330. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, G.; Lau, H.C.; Yu, J. Metagenomic Sequencing for Microbial DNA in Human Samples: Emerging Technological Advances. Int. J. Mol. Sci. 2022, 23, 2181. [Google Scholar] [CrossRef]
- Elbait, D.G.; Daou, M.; Abuoudah, M.; Elmekawy, A.; Hasan, S.W.; Everett, D.B.; Alsafar, H.; Henschel, A.; Yousef, A.F. Comparison of qPCR and metagenomic sequencing methods for quantifying antibiotic resistance genes in wastewater. PLoS ONE 2024, 19, e0298325. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.; Martínez, D.; Páez-Triana, L.; Luna, N.; De Las Salas, J.L.; Hernández, C.; Flórez, A.Z.; Muñoz, M.; Ramírez, J.D. Characterizing Viral Species in Mosquitoes (Culicidae) in the Colombian Orinoco: Insights from a Preliminary Metagenomic Study. Sci. Rep. 2023, 13, 22081. [Google Scholar] [CrossRef]
- He, X.; Yin, Q.; Zhou, L.; Meng, L.; Hu, W.; Li, F.; Li, Y.; Han, K.; Zhang, S.; Fu, S.; et al. Metagenomic Sequencing Reveals Viral Abundance and Diversity in Mosquitoes from the Shaanxi-Gansu-Ningxia Region, China. PLoS Negl. Trop. Dis. 2021, 15, e0009381. [Google Scholar] [CrossRef]
| Species | Vibrio cholerae | Salmonella | Shigella | Escherichia coli O157 | Staphylococcus aureus |
|---|---|---|---|---|---|
| Blattella germanica | 1/113 (0.88) | 0/113 (0.00) | 1/113 (0.88) | 0/113 (0.00) | 10/113 (8.85) |
| Periplaneta australasiae | 0/4 (0.00) | 0/4 (0.00) | 0/4 (0.00) | 0/4 (0.00) | 0/4 (0.00) |
| Periplaneta americana | 0/51 (0.00) | 0/51 (0.00) | 0/51 (0.00) | 0/51 (0.00) | 1/51 (1.96) |
| Hebardina concinna | 0/2 (0.00) | 0/2 (0.00) | 0/2 (0.00) | 0/2 (0.00) | 0/2 (0.00) |
| Total | 1/170 (0.59) | 0/170 (0.00) | 1/170 (0.59) | 0/170 (0.00) | 11/170 (6.47) |
| Species | Vibrio cholerae | Salmonella | Shigella | Escherichia coli O157 | Staphylococcus aureus |
|---|---|---|---|---|---|
| Parasarcophaga albiceps | 1/15 (6.67) | 0/15 (0.00) | 0/15 (0.00) | 0/15 (0.00) | 1/15 (6.67) |
| Fannia leucosticta | 0/8 (0.00) | 0/8 (0.00) | 0/8 (0.00) | 0/8 (0.00) | 0/8 (0.00) |
| Chrysomya megacephala | 0/35 (0.00) | 0/35 (0.00) | 0/35 (0.00) | 0/35 (0.00) | 2/35 (5.71) |
| Anthomyia illocata | 0/8 (0.00) | 0/8 (0.00) | 0/8 (0.00) | 0/8 (0.00) | 0/8 (0.00) |
| Musca domestica vicina | 0/96 (0.00) | 0/96 (0.00) | 0/96 (0.00) | 2/96 (2.08) | 4/96 (4.17) |
| M. domestica | 0/82 (0.00) | 0/82 (0.00) | 0/82 (0.00) | 2/96 (2.08) | 6/96 (6.25) |
| Sarcophagidae | 0/2 (0.00) | 0/2 (0.00) | 0/2 (0.00) | 0/2 (0.00) | 0/2 (0.00) |
| Musca sorbens | 0/1 (0.00) | 0/1 (0.00) | 0/1 (0.00) | 0/1 (0.00) | 0/1 (0.00) |
| Lucilia sericata | 0/6 (0.00) | 0/6 (0.00) | 0/6 (0.00) | 0/6 (0.00) | 0/6 (0.00) |
| Lucilia cuprina | 0/10 (0.00) | 0/10 (0.00) | 0/10 (0.00) | 0/10 (0.00) | 1/10 (10.00) |
| Musca pattoni | 0/34 (0.00) | 0/34 (0.00) | 0/34 (0.00) | 0/34 (0.00) | 1/34 (2.94) |
| Fannia canicularis | 1/44 (2.27) | 0/44 (0.00) | 0/44 (0.00) | 0/44 (0.00) | 0/44 (0.00) |
| Neomyia timorensis | 0/6 (0.00) | 0/6 (0.00) | 0/6 (0.00) | 0/6 (0.00) | 0/6 (0.00) |
| Syrphidae | 0/1 (0.00) | 0/1 (0.00) | 0/1 (0.00) | 0/1 (0.00) | 0/1 (0.00) |
| Total | 2/348 (0.57) | 0/348 (0.00) | 0/348 (0.00) | 4/348 (1.15) | 15/348 (4.31) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Zhao, C.; Liu, G.; Guo, L.; Zhang, R.; Yan, J.; He, J.; Guo, C. Monitoring of Pathogens Carried by Imported Flies and Cockroaches at Shenzhen Ports. Trop. Med. Infect. Dis. 2025, 10, 57. https://doi.org/10.3390/tropicalmed10020057
Zhang S, Zhao C, Liu G, Guo L, Zhang R, Yan J, He J, Guo C. Monitoring of Pathogens Carried by Imported Flies and Cockroaches at Shenzhen Ports. Tropical Medicine and Infectious Disease. 2025; 10(2):57. https://doi.org/10.3390/tropicalmed10020057
Chicago/Turabian StyleZhang, Siqi, Chunzhong Zhao, Guoping Liu, Liwei Guo, Ran Zhang, Junyu Yan, Jianan He, and Cheng Guo. 2025. "Monitoring of Pathogens Carried by Imported Flies and Cockroaches at Shenzhen Ports" Tropical Medicine and Infectious Disease 10, no. 2: 57. https://doi.org/10.3390/tropicalmed10020057
APA StyleZhang, S., Zhao, C., Liu, G., Guo, L., Zhang, R., Yan, J., He, J., & Guo, C. (2025). Monitoring of Pathogens Carried by Imported Flies and Cockroaches at Shenzhen Ports. Tropical Medicine and Infectious Disease, 10(2), 57. https://doi.org/10.3390/tropicalmed10020057





