Mapping Disease Transmission Risk of Nipah Virus in South and Southeast Asia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Model Covariates
2.4. Model Calibration
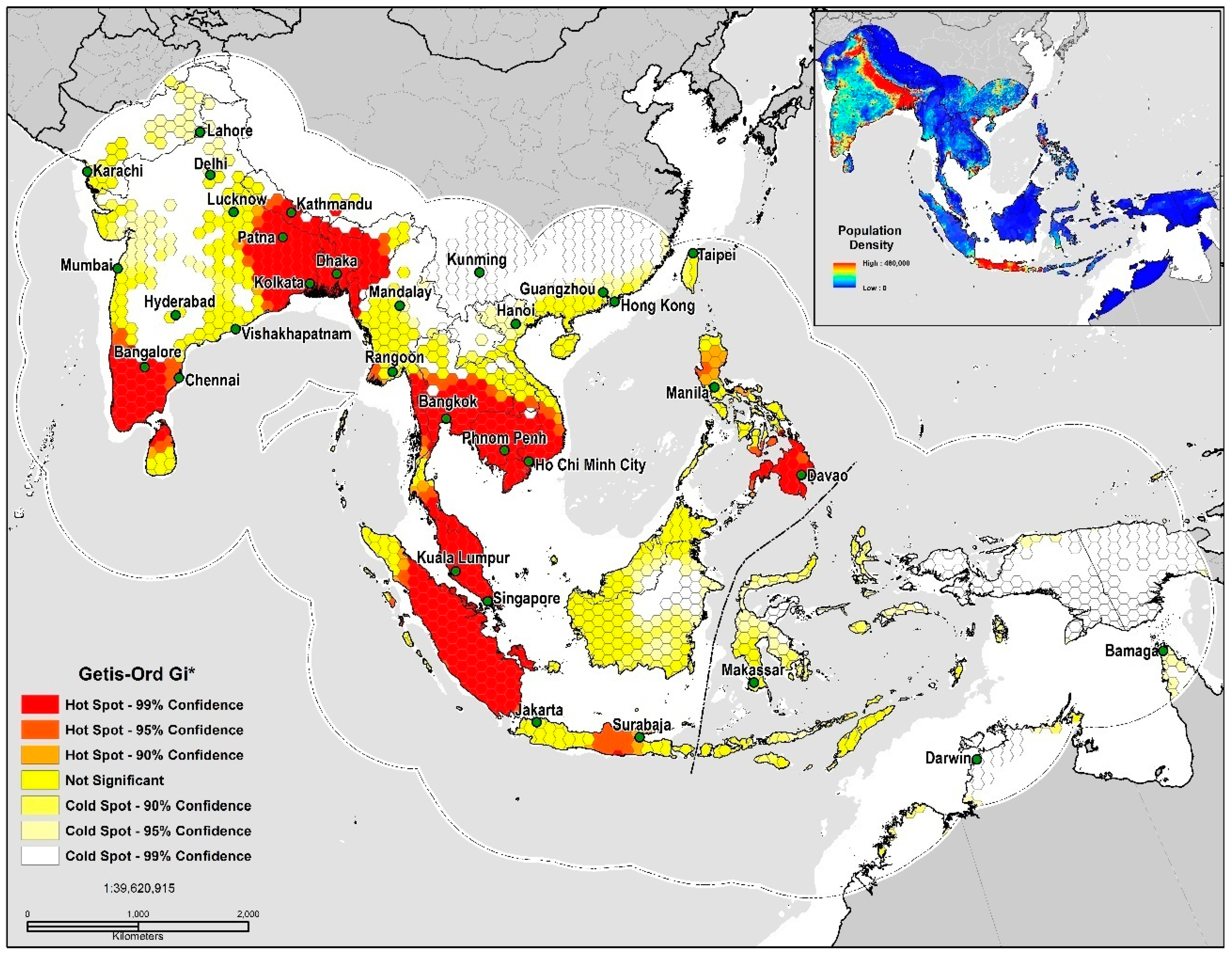
2.5. Geospatial Analysis
2.6. Measuring Niche Overlap
3. Results
Geospatial and Niche Overlap Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Woolhouse, M.E.J.; Gowtage-Sequeria, S. Host range and emerging and reemerging pathogens. Emerg. Infect. Dis. 2005, 11, 1842–1847. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.; Rogers, R.; Selvey, L.; Selleck, P.; Hyatt, A.; Gould, A.; Gleeson, L.; Hooper, P.; Westbury, H. A novel morbillivirus pneumonia of horses and its transmission to humans. Emerg. Infect. Dis. 1995, 1, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-F.F.; Michalski, W.P.; Yu, M.; Pritchard, L.I.; Crameri, G.; Shiell, B.; Eaton, B.T. A novel P/V/C gene in a new member of the Paramyxoviridae family, which causes lethal infection in humans, horses, and other animals. J. Virol. 1998, 72, 1482–1490. [Google Scholar] [PubMed]
- Montgomery, J.M.; Hossain, M.J.; Gurley, E.; Carroll, D.S.; Croisier, A.; Bertherat, E.; Asgari, N.; Formenty, P.; Keeler, N.; Comer, J.; et al. Risk factors for Nipah virus encephalitis in Bangladesh. Emerg. Infect. Dis. 2008, 14, 1526–1532. [Google Scholar] [CrossRef] [PubMed]
- Simons, R.R.L.; Gale, P.; Horigan, V.; Snary, E.L.; Breed, A.C. Potential for introduction of bat-borne zoonotic viruses into the EU: A review. Viruses 2014, 6, 2084–2121. [Google Scholar] [CrossRef] [PubMed]
- Field, H.; Young, P.; Yob, J.M.; Mills, J.; Hall, L.; Mackenzie, J. The natural history of Hendra and Nipah viruses. Microbes Infect. 2001, 3, 307–314. [Google Scholar] [CrossRef]
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Emerging infectious diseases of wildlife—threats to biodiversity and human health. Science 2000, 287, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Wacharapluesadee, S.; Samseeneam, P.; Phermpool, M.; Kaewpom, T.; Rodpan, A.; Maneeorn, P.; Srongmongkol, P.; Kanchanasaka, B.; Hemachudha, T. Molecular characterization of Nipah virus from Pteropus hypomelanus in southern Thailand. Virol. J. 2016, 13, 53. [Google Scholar] [CrossRef] [PubMed]
- Mohd Nor, M.N.; Gan, C.H.; Ong, B.L. Nipah virus infection of pigs in Peninsular Malaysia. Rev. Sci. Tech. 2000, 19, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.B.; Bellini, W.J.; Rota, P.A.; Harcourt, B.H.; Tamim, A.; Lam, S.K.; Ksiazek, T.G.; Rollin, P.E.; Zaki, S.R.; Shieh, W.-J.; et al. Nipah virus: A recently emergent deadly paramyxovirus. Science 2000, 288, 1432–1435. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.B. Nipah virus outbreak in Malaysia. J. Clin. Virol. 2003, 26, 265–275. [Google Scholar] [CrossRef]
- Wacharapluesadee, S.; Boongird, K.; Wanghongsa, S.; Ratanasetyuth, N.; Supavonwong, P.; Saengsen, D.; Gongal, G.N.; Hemachudha, T. A longitudinal study of the prevalence of Nipah virus in Pteropus lylei bats in Thailand: Evidence for seasonal preference in disease transmission. Vector Borne Zoonotic Dis. 2010, 10, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Ching, P.K.G.; de Los Reyes, V.C.; Sucaldito, M.N.; Tayag, E.; Columna-Vingno, A.B.; Malbas, F.F.; Bolo, G.C.; Sejvar, J.J.; Eagles, D.; Playford, G.; et al. Outbreak of henipavirus infection, Philippines, 2014. Emerg. Infect. Dis. 2015, 21, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Thibault, P.A.; Watkinson, R.E.; Moreira-Soto, A.; Drexler, J.F.; Lee, B. Zoonotic potential of emerging Paramyxoviruses: Knowns and unknowns. Adv. Virus Res. 2017, 98, 1–55. [Google Scholar] [PubMed]
- Hsu, V.P.; Hossain, M.J.; Parashar, U.D.; Ali, M.M.; Ksiazek, T.G.; Kuzmin, I.; Niezgoda, M.; Rupprecht, C.; Bresee, J.; Breiman, R.F. Nipah virus encephalitis re-emergence, Bangladesh. Emerg. Infect. Dis. 2004, 10, 2082–2087. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Nipah Virus Outbreaks in the WHO South-East Asia Region. 2012. Available online: http://www.searo.who.int/entity/emerging_diseases/links/nipah_virus_outbreaks_sear/en/# (accessed on 4 February 2018).
- Bishop, K.A.; Broder, C.C. Hendra and Nipah Viruses: Lethal Zoonotic Paramyxoviruses; American Society for Microbiology: Washington, DC, USA, 2008. [Google Scholar]
- Openshaw, J.J.; Hegde, S.; Sazzad, H.M.S.; Khan, S.U.; Hossain, M.J.; Epstein, J.H.; Daszak, P.; Gurley, E.S.; Luby, S.P. Bat hunting and bat–human interactions in Bangladeshi villages: Implications for zoonotic disease transmission and bat conservation. Transbound. Emerg. Dis. 2017, 64, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Blattner, E.B. The Palms of British India and Ceylon; Periodical Experts Book Agency: Delhi, India, 1978. [Google Scholar]
- Middleton, D.J.; Morrissy, C.J.; van der Heide, B.M.; Russell, G.M.; Braun, M.A.; Westbury, H.A.; Halpin, K.; Daniels, P.W. Experimental Nipah virus infection in pteropid bats (Pteropus poliocephalus). J. Comp. Pathol. 2007, 136, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Halpin, K.; Hyatt, A.D.; Fogarty, R.; Middleton, D.; Bingham, J.; Epstein, J.H.; Rahman, S.A.; Hughes, T.; Smith, C.; Field, H.E.; et al. Pteropid bats are confirmed as the reservoir hosts of henipaviruses: A comprehensive experimental study of virus transmission. Am. J. Trop. Med. Hyg. 2011, 85, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Munshi-South, J.; Wilkinson, G.S. Bats and birds: Exceptional longevity despite high metabolic rates. Ageing Res. Rev. 2010, 9, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Eby, P. Seasonal movements of grey-headed flying-foxes, Pteropus poliocephalus (Chiroptera : Pteropodidae), from two maternity camps in northern New South Wales. Wildl. Res. 1991, 18, 547–559. [Google Scholar] [CrossRef]
- Epstein, J.H.; Olival, K.J.; Pulliam, J.R.C.; Smith, C.; Westrum, J.; Hughes, T.; Dobson, A.P.; Zubaid, A.; Rahman, S.A.; Basir, M.M.; et al. Pteropus vampyrus, a hunted migratory species with a multinational home-range and a need for regional management. J. Appl. Ecol. 2009, 46, 991–1002. [Google Scholar] [CrossRef]
- Breed, A.C.; Field, H.E.; Smith, C.S.; Edmonston, J.; Meers, J. Bats without borders: Long-distance movements and implications for disease risk management. Ecohealth 2010, 7, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Preventing emerging infectious diseases: A strategy for the 21st Century. Recomm. Rep. 1998, 47, 1–15. [Google Scholar]
- World Health Organization (WHO). WHO Target Product Profile for Lassa Virus Vaccine. 2017. Available online: http://www.who.int/blueprint/priority-diseases/key-action/Nipah_virus_vaccineTPP.pdf?ua=1 (accessed on 4 February 2018).
- Harcourt, B.H.; Lowe, L.; Tamim, A.; Liu, X.; Bankamp, B.; Bowden, N.; Rollin, P.E.; Comer, J.A.; Gsiazek, T.G.; Hosssain, M.J.; et al. Genetic characterization of Nipah virus, Bangladesh, 2004. Emerg. Infect. Dis. 2005, 11, 1594–1597. [Google Scholar] [CrossRef] [PubMed]
- Eaton, B.T.; Broder, C.C.; Middleton, D.; Wang, L.-F.F. Hendra and Nipah viruses: Different and dangerous. Nat. Rev. Microbiol. 2006, 4, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Pallister, J.; Middleton, D.; Broder, C. Henipavirus vaccine development. J. Bioterror. Biodef. 2011, 1, 1–8. [Google Scholar] [CrossRef]
- Harit, A.K.; Ichhpujani, R.L.; Gupta, S.; Gill, K.S.; Lal, S.; Ganguly, N.K.; Agarwal, S.P. Nipah/Hendra virus outbreak in Siliguri, West Bengal, India in 2001. Indian J. Med. Res. 2006, 123, 553–560. [Google Scholar] [PubMed]
- Luby, S.P.; Hossain, M.J.; Gurley, E.S.; Ahmed, B.N.; Banu, S.; Khan, S.U.; Homaira, N.; Rota, P.A.; Rollin, P.E.; Comer, J.A.; et al. Recurrent zoonotic transmission of Nipah virus into humans, Bangladesh, 2001–2007. Emerg. Infect. Dis. 2009, 15, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Hossain, M.J.; Sultana, S.; Homaira, N.; Khan, S.U.; Rahman, M.; Gurley, E.S.; Rollin, P.E.; Lo, M.K.; Comer, J.A.; et al. Date palm sap linked to Nipah virus outbreak in Bangladesh, 2008. Vector-Borne Zoonotic Dis. 2012, 12, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Sazzad, H.M.S.; Hossain, M.J.; Gurley, E.S.; Ameen, K.M.H.; Parveen, S.; Islam, M.S.; Faruque, L.I.; Podder, G.; Banu, S.S.; Lo, M.K.; et al. Nipah virus infection outbreak with nosocomial and corpse-to-human transmission, Bangladesh. Emerg. Infect. Dis. 2013, 19, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.U.; Hossain, J.; Gurley, E.S.; Nahar, N.; Sultana, R.; Luby, S.P. Use of infrared camera to understand bats’ access to date palm sap: Implications for preventing Nipah virus transmission. Ecohealth 2011, 7, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Sazzad, H.M.S.; Hossain, M.J.; Islam, M.S.; Parveen, S.; Husain, M.; Banu, S.S.; Podder, G.; Afroj, S.; Rollin, P.E.; et al. Evolving epidemiology of Nipah virus infection in Bangladesh: Evidence from outbreaks during 2010–2011. Epidemiol. Infect. 2016, 144, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Jeyapraba, L. Roosting ecology of Pteropus giganteus (Brunnich, 1782) Indian flying fox and threats for their survival. Int. J. Comput. Res. Dev. 2016, 1, 102–105. [Google Scholar]
- Hahn, M.B. Infectious Disease Ecology. In Green Infrastructure and Public Health; Coutts, C., Ed.; Routledge: Abington-on-Thames, UK, 2016. [Google Scholar]
- Walsh, M.G.; Haseeb, M. The landscape configuration of zoonotic transmission of Ebola virus disease in West and Central Africa: Interaction between population density and vegetation cover. PeerJ 2015, 3, e735. [Google Scholar] [CrossRef] [PubMed]
- Plowright, R.K.; Eby, P.; Hudson, P.J.; Smith, I.L.; Westcott, D.; Bryden, W.L.; Middleton, D.; Reid, P.A.; McFarlane, R.A.; Martin, G.; et al. Ecological dynamics of emerging bat virus spillover. Proc. R. Soc. B Biol. Sci. 2014, 282. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.T. Mapping risk of Nipah virus transmission across Asia and across Bangladesh. Asia Pac. J. Public Health 2013, 27, NP824–NP832. [Google Scholar] [CrossRef] [PubMed]
- Hann, M.B.; Epstein, J.H.; Gurley, E.S.; Islam, M.S.; Luby, S.P.; Daszak, P.; Patz, J.A. Roosting behaviour and habitat selection of Pteropus giganteus reveals potential links to Nipah virus e. J. Appl. Ecol. 2014, 51, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.G. Mapping the risk of Nipah virus spillover into human populations in South and Southeast Asia. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Bourn, D.; Cresencio, R.; Gealone, M.; Molina, J.; Morales, R.; Wint, W. Disease risk modelling and mapping in the Philippines. In Proceedings of the International Conference on Emerging Vector-Borne Diseases, Poster of EAHMI I Activities, Le Corum, Montpellier, France, 10–12 May 2010. [Google Scholar]
- Escobar, L.E.; Craft, M.E. Advances and limitations of disease biogeography using ecological niche modeling. Front. Microbiol. 2016, 7, 1174. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.G.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.N.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife 2015, 4, e08347. [Google Scholar] [CrossRef] [PubMed]
- Brady, O.J.; Golding, N.; Pigott, D.M.; Kraemer, M.U.G.; Messina, J.P.; Reiner, R.C.; Scott, T.W.; Smith, D.L.; Gething, P.W.; Hay, S.I. Global temperature constraints on Aedes aegypti and Ae. albopictus persistence and competence for dengue virus transmission. Parasites Vectors 2014, 7, 338. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Gething, P.W.; Patil, A.P.; Smith, D.L.; Guerra, C.A.; Elyazar, I.R.F.; Johnston, G.L.; Tatem, A.J.; Hay, S.I. A new world malaria map: Plasmodium falciparum endemicity in 2010. Malar. J. 2011, 10, 378. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Li, Z.; Yang, F.; Zheng, J.; Feng, Y.; Guo, H.; Li, Y.; Wang, Y.; Su, N.; Zhang, F.; et al. Virome profiling of bats from Myanmar by Metagenomic Analysis of Tissue Samples Reveals More Novel Mammalian Viruses. PLoS ONE 2013, 8, e61950. [Google Scholar] [CrossRef]
- Peterson, A.T.; Samy, A.M. Geographic potential of disease caused by Ebola and Marburg viruses in Africa. Acta Trop. 2016, 162, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.T. Ecological niche conservatism: A time-structured review of evidence. J. Biogeogr. 2011, 38, 817–827. [Google Scholar] [CrossRef]
- Peterson, A.T. Biogeography of diseases: A framework for analysis. Naturwissenschaften 2008, 95, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Institute of Epidemiology, Disease Control, and Research. Available online: http://www.iedcr.gov.bd/ (accessed on 4 February 2018).
- Yob, J.M.; Field, H.; Rashdi, A.M.; Morrissy, C.; van der Heide, B.; Rota, P.; bin Adzhar, A.; White, J.; Daniels, P.; Jamaluddin, A.; et al. Nipah virus infection in bats (order Chiroptera) in Peninsular Malaysia. Emerg. Infect. Dis. 2001, 7, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.B.; Chua, B.H.; Wang, C.W. Anthropogenic deforestation, El Niño and the emergence of Nipah virus in Malaysia. Malays. J. Pathol. 2002, 24, 15–21. [Google Scholar] [PubMed]
- Reynes, J.M.; Counor, D.; Ong, S.; Faure, C.; Seng, V.; Molia, S.; Walston, J.; Georges-Courbot, M.C.; Deubel, V.; Sarthou, J.L. Nipah virus in Lyle’s flying foxes, Cambodia. Emerg. Infect. Dis. 2005, 11, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Hickey, A.C.; Zhang, Y.; Li, Y.; Wu, Y.; Zhang, H.; Yuan, J.; Han, Z.; McEachern, J.; et al. Antibodies to Nipah or Nipah-like viruses in bats, China. Emerg. Infect. Dis. 2008, 14, 1974–1976. [Google Scholar] [CrossRef] [PubMed]
- Hasebe, F.; Thuy, N.T.T.; Inoue, S.; Yu, F.; Kaku, Y.; Watanabe, S.; Akashi, H.; Dat, D.T.; Mai, L.T.Q.; Morita, K. Serologic evidence of Nipah virus infection in bats, Vietnam. Emerg. Infect. Dis. 2012, 18, 536–537. [Google Scholar] [CrossRef] [PubMed]
- Sendow, I.; Ratnawati, A.; Taylor, T.; Adjid, R.M.A.; Saepulloh, M.; Barr, J.; Wong, F.; Daniels, P.; Field, H. Nipah virus in the fruit bat Pteropus vampyrus in Sumatera, Indonesia. PLoS ONE 2013, 8, e69544. [Google Scholar] [CrossRef] [PubMed]
- Reisen, W.K. Landscape epidemiology of vector-borne diseases. Annu. Rev. Entomol. 2010, 55, 461–483. [Google Scholar] [CrossRef] [PubMed]
- Longbottom, J.; Browne, A.J.; Pigott, D.M.; Sinka, M.E.; Golding, N.; Hay, S.I.; Moyes, C.L.; Shearer, F.M. Mapping the spatial distribution of the Japanese encephalitis vector, Culex tritaeniorhynchus Giles, 1901 (Diptera: Culicidae) within areas of Japanese encephalitis risk. Parasites Vectors 2017, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- ESRI. ArcGIS Desktop: Release 10.5.1; Environmental Systems Research Institute: Redlands, CA, USA, 2017. [Google Scholar]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Deblauwe, V.; Droissart, V.; Bose, R.; Sonke, B.; Blach-Overgaard, A.; Svenning, J.-C.; Wieringa, J.J.; Ramesh, B.R.; Stevart, T.; Couvreur, T.L.P. Remotely sensed temperature and precipitation data improve species distribution modelling in the tropics. Glob. Ecol. Biogeogr. 2016, 25, 443–454. [Google Scholar] [CrossRef]
- Vega, G.C.; Pertierra, L.R.; Olalla-Tárraga, M.Á. MERRAclim, a high-resolution global dataset of remotely sensed bioclimatic variables for ecological modelling. Sci. Data 2017, 4, 170078. [Google Scholar] [CrossRef] [PubMed]
- Rienecker, M.M.; Suarez, M.J.; Gelaro, R.; Todling, R.; Bacmeister, J.; Liu, E.; Bosilovich, M.G.; Schubert, S.D.; Takacs, L.; Kim, G.K.; et al. MERRA: NASA’s modern-era retrospective analysis for research and applications. J. Clim. 2011, 24, 3624–3648. [Google Scholar] [CrossRef]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-resolution global maps of 21st-century forest cover change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Khan, S.U.; Crameri, G.; Epstein, J.H.; Broder, C.C.; Islam, A.; Peel, A.J.; Barr, J.; Daszak, P.; Wang, L.F.; et al. Serological evidence of henipavirus exposure in cattle, goats and pigs in Bangladesh. PLoS Negl. Trop. Dis. 2014, 8, e3302. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.; Monette, G. Generalized collinearity diagnostics. J. Am. Stat. Assoc. 1992, 87, 178–183. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Thuiller, A.W.; Georges, D.; Engler, R.; Georges, M.D.; Thuiller, C.W. The Biomod2 Package: The Updated Object-Oriented Version of BIOMOD Package. 2016. Available online: https://cran.r-project.org/web/packages/biomod2/biomod2.pdf (accessed on 15 May 2018).
- Phillips, S.B.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Int. J. Glob. Environ. Issues 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Drew, C.A.; Wiersma, Y.F.; Huettmann, F. Predictive Species and Habitat Modeling in Landscape Ecology: Concepts and Applications; Springer: New York, NY, USA; Dordrecht, The Netherlands; Heidelberg, Germany; London, UK, 2011. [Google Scholar]
- Jaynes, E.T. Information theory and statistical mechanics. Phys. Rev. 1957, 106, 620–630. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distribution with MaxEnt: New extensions and a comprehensive evaluation. Ecograpy 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species distributions: What it does, and why inputs and setting matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Gama, M.; Crespo, D.; Dolbeth, M.; Anastácio, P.M. Ensemble forecasting of Corbicula fluminea Worldwide distribution: Projections of the impact of climate change. Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 27, 675–684. [Google Scholar] [CrossRef]
- Guisan, A.; Edwards, T.C., Jr.; Hastie, T. Generalized linear and generalized additive models in studies of species distributions: setting the scene. Ecol. Model. 2002, 157, 89–100. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R.; Hastie, T. A working guide to boosted regression trees. J. Anim. Ecol. 2008, 77, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Vayssières, M.P.; Plant, R.E.; Allen-Diaz, B.H. Classification trees: An alternative non-parametric approach for predicting species distributions. J. Veg. Sci. 2000, 11, 679–694. [Google Scholar] [CrossRef]
- Lek, S.; Guégan, J.F. Artificial neural networks as a tool in ecological modelling, an introduction. Ecol. Model. 1999, 120, 65–73. [Google Scholar] [CrossRef]
- Busby, J.R. BIOCLIM—A bioclimatic analysis and prediction system. In Nature Conservation: Cost Effective Biological Surveys and Data Analysis; Csiro Publishing: Melbourne, Australia, 1991; pp. 64–68. [Google Scholar]
- Hastie, T.; Tibshirani, R.; Buja, A. Flexible discriminant analysis by optimal scoring. J. Am. Stat. Assoc. 1994, 89, 1255–1270. [Google Scholar] [CrossRef]
- Friedman, J.H. Multivariate adaptive regression splines. Ann. Stat. 1991, 19, 1–67. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Jurka, T.P. Maxent: An R package for low-memory multinomial logistic regression with support for semi-automated text classification. R J. 2012, 4, 56–59. [Google Scholar]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Sim, J.; Wright, C.C. The Kappa statistic in reliability studies: use, interpretation, and sample size requirements. Phys. Ther. 2005, 85, 257–268. [Google Scholar] [PubMed]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 993–1009. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, Kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Getis, A.; Ord, J.K. The analysis of spatial association by use of distance statistics. Geogr. Anal. 1992, 24, 189–206. [Google Scholar] [CrossRef]
- Ord, J.K.; Getis, A. Local spatial autocorrelation statistics: Distributional issues and an application. Geogr. Anal. 1995, 27, 286–306. [Google Scholar] [CrossRef]
- Wang, J.F.; Zhang, T.L.; Fu, B.J. A measure of spatial stratified heterogeneity. Ecol. Indic. 2016, 67, 250–256. [Google Scholar] [CrossRef]
- Schoener, T.W. The Anolis lizards of Bimini: Resource partitioning in a complex fauna. Ecology 1968, 49, 704–726. [Google Scholar] [CrossRef]
- Racine, J.-B.; Bailly, A.S. Geography and geographical space: Towards an epistemology of geography. Espac. Géogr. 1993, 1, 125–134. [Google Scholar] [CrossRef]
- Aryal, A.; Shrestha, U.B.; Ji, W.; Ale, S.B.; Shrestha, S.; Ingty, T.; Maraseni, T.; Cockfield, G.; Raubenheimer, D. Predicting the distributions of predator (snow leopard) and prey (blue sheep) under climate change in the Himalaya. Ecol. Evol. 2016, 6, 4065–4075. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Ostfeld, R.S.; Peterson, A.T.; Poulin, R.; de la Fuente, J. Effects of environmental change on zoonotic disease risk: An ecological primer. Trends Parasitol. 2014, 30, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Wiens, J.J.; Ackerly, D.D.; Allen, A.P.; Anacker, B.L.; Buckley, L.B.; Cornell, H.V.; Damschen, E.I.; Jonathan Davies, T.; Grytnes, J.A.; Harrison, S.P.; et al. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol. Lett. 2010, 13, 1310–1324. [Google Scholar] [CrossRef] [PubMed]
- Voigt, C.C.; Phelps, K.L.; Aguirre, L.F.; Corrie Schoeman, M.; Vanitharani, J.; Zubaid, A. Bats and buildings: The conservation of synanthropic bats. In Bats in the Anthropocene: Conservation of Bats in a Changing World; Voigt, C.C., Kingston, T., Eds.; Springer Publishing: New York City, NY, USA, 2015; pp. 427–462. [Google Scholar]
- Hahn, M.B.; Patz, J.A.; Gurley, E.S.; Epstein, J.H.; Daszak, P.; Islam, M.S.; Luby, S.P. The role of landscape composition and configuration on Pteropus giganteus roosting ecology and Nipah virus spillover risk in Bangladesh. Am. J. Trop. Med. Hyg. 2014, 90, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Roy, U.S.; Chattopadhyay, S. Distribution and abundance of three populations of Indian flying fox (Pteropus giganteus) from Purulia district of West Bengal, India. TAPROBANICA J. Asian Biodivers. 2013, 5, 60–66. [Google Scholar] [CrossRef]
- Costenbader, J.; Broadhead, J.; Yasmi, Y.; Durst, P.B. Drivers Affecting Forest Change in the Greater Mekong Subregion (GMS): An Overview; FAO: Rome, Italy, 2015. [Google Scholar]
- Sodhi, N.S.; Koh, L.P.; Brook, B.W.; Ng, P.K.L. Southeast Asian biodiversity: An impending disaster. Trends Ecol. Evol. 2004, 19, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Wagenaar, J.A.; Yue, H.; Pritchard, J.; Broekhuizen-Stins, M.; Huijsdens, X.; Mevius, D.J.; Bosch, T.; Van Duijkeren, E. Unexpected sequence types in livestock-associated methicillin-resistant Staphylococcus aureus (MRSA): MRSA ST9 and a single locus variant of ST9 in pig farming in China. Vet. Microbiol. 2009, 139, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, A.; Knowles, N.J.; Paton, D.J. Combining livestock trade patterns with phylogenetics to help understand the spread of foot and mouth disease in sub-Saharan Africa, the Middle East, and Southeast Asia. Rev. Sci. Tech. 2011, 30, 63–85. [Google Scholar] [CrossRef] [PubMed]
- Sinka, M.E.; Rubio-Palis, Y.; Manguin, S.; Patil, A.P.; Temperley, W.H.; Gething, P.W.; Van Boeckel, T.; Kabaria, C.W.; Harbach, R.E.; Hay, S.I. The dominant Anopheles vectors of human malaria in the Americas: Occurrence data, distribution maps and bionomic précis. Parasites Vectors 2010, 3, 72. [Google Scholar] [CrossRef] [PubMed]
- Hay, S.I. An overview of remote sensing and geodesy for epidemiology and public health application. Adv. Parasitol. 2000, 47, 1–35. [Google Scholar] [PubMed]
- Vancutsem, C.; Ceccato, P.; Dinku, T.; Connor, S.J. Evaluation of MODIS land surface temperature data to estimate air temperature in different ecosystems over Africa. Remote Sens. Environ. 2010, 114, 449–465. [Google Scholar] [CrossRef]
- Pigott, D.M.; Golding, N.; Mylne, A.; Huang, Z.; Henry, A.J.; Weiss, D.J.; Brady, O.J.; Kraemer, M.U.G.; Smith, D.L.; Moyes, C.L.; et al. Mapping the zoonotic niche of Ebola virus disease in Africa. Elife 2014, 3, e04395. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. Global Animal Disease Intelligence Report No. 1; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015. [Google Scholar]
- Hutson, A.M.; Racey, P. Pteropus Niger. The IUCN Red List of Threatened Species 2013. e.T18743A22084054. Available online: http://dx.doi.org/10.2305/IUCN.UK.2013-2.RLTS.T18743A22084054.en (accessed on 23 May 2018).
- Lange, M. Alternative control strategies against ASF in wild boar populations. EFSA Support. Publ. 2015, 12. [Google Scholar] [CrossRef]
- Cortes, M.C.; Cauchemez, S.; Lefrancq, N.; Luby, S.P.; Hossain, M.J.; Sazzad, H.M.S.; Rahman, M.; Daszak, P.; Salje, H.; Gurley, E.S. Characterization of the spatial and temporal distribution of Nipah virus spillover events in Bangladesh 2007–2013. J. Infect. Dis. 2018, 217, 1390–1394. [Google Scholar] [CrossRef] [PubMed]
- Nahar, N.; Sultana, R.; Gurley, E.S.; Hossain, M.J.; Luby, S.P. Date palm sap collection: Exploring opportunities to prevent Nipah transmission. Ecohealth 2010, 7, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Hewlett, B.S.; Amolat, R.P. Cultural contexts of Ebola in northern Uganda. Emerg. Infect. Dis. 2003, 9, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Gurley, E.S.; Montgomery, J.M.; Hossain, M.J.; Bell, M.; Azad, A.K.; Islam, M.R.; Molla, M.A.R.; Carroll, D.S.; Ksiazek, T.G.; Rota, P.A.; et al. Person-to-person transmission of Nipah virus in a Bangladeshi community. Emerg. Infect. Dis. 2007, 13, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.U.; Alam, M.M.; Sultana, F.; Sayeed, S.N.; Pressman, A.M.; Powers, M.B. Reaching the unreachable: Barriers of the poorest to accessing NGO healthcare services in Bangladesh. J. Health Popul. Nutr. 2006, 24, 456–466. [Google Scholar] [PubMed]
- Luby, S.P.; Gurley, E.S.; Hossain, M.J. Transmission of human infection with Nipah virus. Clin. Infect. Dis. 2009, 49, 1743–1748. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L. Ecology and Field Biology; Benjamin-Cummings Publishing Company: San Francisco, CA, USA, 2001; Volume 740. [Google Scholar]
- Olival, K.J.; Daszak, P. The ecology of emerging neurotropic viruses. J. Neurovirol. 2005, 11, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Despommier, D.; Ellis, B.R.; Wilcox, B.A. The role of ecotones in emerging infectious diseases. EcoHealth 2006, 3, 281–289. [Google Scholar] [CrossRef]
- Walsh, M.G.; Wiethoelter, A.; Haseeb, M.A. The impact of human population pressure on flying fox niches and the potential consequences for Hendra virus spillover. Sci. Rep. 2017, 7, 8226. [Google Scholar] [CrossRef] [PubMed]
- Hayman, D.T.S.; Bowen, R.A.; Cryan, P.M.; Mccracken, G.F.; O’Shea, T.J.; Peel, A.J.; Gilbert, A.; Webb, C.T.; Wood, J.L.N. Ecology of zoonotic infectious diseases in bats: Current knowledge and future directions. Zoonoses Public Health 2013, 60, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, B.A.; Gubler, D.J. Disease ecology and the global emergence of zoonotic pathogens. Environ. Health Prev. Med. 2005, 10, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.K.; Sato, N. Deforestation, land conversion and illegal logging in Bangladesh: The case of the Sal (Shorea robusta) forests. IForest 2012, 5, 171–178. [Google Scholar] [CrossRef]
- Morshed, N.; Yorke, C.; Zhang, Q. Urban expansion pattern and land use dynamics in Dhaka, 1989–2014. Prof. Geogr. 2017, 69, 396–411. [Google Scholar] [CrossRef]
- Smolinski, M.S.; Hamburg, M.A.; Lederberg, J. Microbial Threats to Health: Emergence, Detection, and Response; National Academies Press: Washington, DC, USA, 2003. [Google Scholar]
- Hughes, J.M.; Wilson, M.E.; Halpin, K.; Hyatt, A.D.; Plowright, R.K.; Epstein, J.H.; Daszak, P.; Field, H.E.; Wang, L.; Daniels, P.W. Emerging viruses: Coming in on a wrinkled wing and a prayer. Clin. Infect. Dis. 2007, 44, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Morse, S.S. Factors in the emergence of infectious diseases. Emerg. Infect. Dis. 1995, 1, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Lobo, J.M.; Jiménez-Valverde, A.; Real, R. AUC: A misleading measure of the performance of predictive distribution models. Glob. Ecol. Biogeogr. 2008, 1, 145–151. [Google Scholar] [CrossRef]
- Bever, L. Rare, Brain-damaging virus spreads panic in India as death toll rises. The Washington Post. 22 May 2018. Available online: www.washingtonpost.com/news/to-your-health/wp/2018/05/22/rare-brain-damaging-virus-spreads-panic-in-india-as-death-toll-rises/?noredirect=on&utm_term=.83bb250dcfdc (accessed on 23 May 2018).
- Vinobha, K. Nipah Virus: Two Suspected Cases Reported in Karnataka. The Times of India. Available online: http://timesofindia.indiatimes.com/city/mangaluru/nipah-virus-2-suspected-cases-reported-in-karnataka/articleshow/64274959.cms (accessed on 23 May 2018).





| Variable | Data Type | Resolution |
|---|---|---|
| Annual Mean Temperature | Environmental/continuous | 2.5 arc-minutes |
| Mean Diurnal Range | Environmental/continuous | 2.5 arc-minutes |
| Isothermality | Environmental/continuous | 2.5 arc-minutes |
| Temperature Seasonality | Environmental/continuous | 2.5 arc-minutes |
| Max. Temperature of Warmest Month | Environmental/continuous | 2.5 arc-minutes |
| Min. Temperature of Coldest Month | Environmental/continuous | 2.5 arc-minutes |
| Temperature Annual Range | Environmental/continuous | 2.5 arc-minutes |
| Mean Temperature of Wettest Quarter | Environmental/continuous | 2.5 arc-minutes |
| Mean Temperature of Driest Quarter | Environmental/continuous | 2.5 arc-minutes |
| Mean Temperature of Warmest Quarter | Environmental/continuous | 2.5 arc-minutes |
| Mean Temperature of Coldest Quarter | Environmental/continuous | 2.5 arc-minutes |
| Annual Precipitation | Environmental/continuous | 2.5 arc-minutes |
| Precipitation of Wettest Month | Environmental/continuous | 2.5 arc-minutes |
| Precipitation of Driest Month | Environmental/continuous | 2.5 arc-minutes |
| Precipitation Seasonality | Environmental/continuous | 2.5 arc-minutes |
| Precipitation of Wettest Quarter | Environmental/continuous | 2.5 arc-minutes |
| Precipitation of Driest Quarter | Environmental/continuous | 2.5 arc-minutes |
| Precipitation of Warmest Quarter | Environmental/continuous | 2.5 arc-minutes |
| Precipitation of Coldest Quarter | Environmental/continuous | 2.5 arc-minutes |
| Evergreen Broadleaf | Landscape/categorical | 1-km |
| Croplands | Landscape/categorical | 1-km |
| Gross Canopy Loss (2000–2016) | Human environment/categorical | 30-m |
| Elevation (SRTM) | Landscape/continuous | 1-km |
| Mean MODIS EVI (2001–2012) | Landscape/continuous | 1-km |
| Mean Value 8-day MODIS day-time Land Surface Temperature (LST) (2011–2012) | Landscape/continuous | 1-km |
| Tree Plantations (Southeast Asia) | Landscape/categorical | 1-km |
| Mixed Forests | Landscape/categorical | 1-km |
| Mosaic Cropland/Vegetation | Landscape/categorical | 1-km |
| Woody Savannas | Landscape/categorical | 1-km |
| Human Population Density (2015) | Human environment/continuous | 1-km |
| Cattle Density | Landscape/categorical | 1-km |
| Goat Density | Landscape/categorical | 1-km |
| Sheep Density | Landscape/categorical | 1-km |
| Pig Density | Landscape/categorical | 1-km |
| Model | ROC | KAPPA | TSS |
|---|---|---|---|
| GLM | 0.873 | 0.606 | 0.627 |
| GBM | 0.886 | 0.654 | 0.663 |
| FDA | 0.867 | 0.616 | 0.616 |
| MARS | 0.868 | 0.607 | 0.621 |
| RF | 0.897 | 0.661 | 0.681 |
| Variable | Contribution | Sample Averages |
|---|---|---|
| Population Density | 0.286 | 1357 per cell |
| Mean Temperature of the Driest Quarter | 0.252 | 23 °C |
| Precipitation of the Warmest Quarter | 0.143 | 1124 mm |
| Land Surface Temperature (LST) | 0.124 | - |
| Temperature Seasonality | 0.117 | 30 °C |
| Elevation (SRTM) | 0.074 | 709 m |
| EVI MODIS (2001–2012) | 0.060 | - |
| Mean Temperature of Warmest Quarter | 0.039 | 28°C |
| Mosaic Vegetation | 0.036 | - |
| Cattle Density | 0.028 | 24.85 |
| Pig Density | 0.025 | 18.84 |
| Model | ROC | KAPPA | TSS |
|---|---|---|---|
| GLM | 0.862 | 0.615 | 0.629 |
| GBM | 0.917 | 0.752 | 0.772 |
| FDA | 0.857 | 0.611 | 0.623 |
| MARS | 0.902 | 0.729 | 0.734 |
| RF | 0.770 | 0.747 | 0.789 |
| Maxent. Phillips | 0.770 | 0.526 | 0.517 |
| Maxent. Tsuruoka | 0.878 | 0.627 | 0.675 |
| Variable | Contribution | Sample Averages |
|---|---|---|
| Cattle Density | 0.509 | 45.5 |
| Temperature Seasonality | 0.201 | 40.2 °C |
| Elevation (SRTM) | 0.115 | 857.45 m |
| Land Surface Temperature (LST) | 0.105 | - |
| Population Density | 0.103 | 348.71 per cell |
| Mean Temperature of the Driest Quarter | 0.097 | 21 °C |
| Mean Temperature of the Warmest Quarter | 0.088 | 28 °C |
| Sheep Density | 0.06 | 16.7 |
| Pig Density | 0.06 | 16 |
| Mosaic Vegetation | 0.051 | - |
| Tree Plantations | 0.032 | - |
| Precipitation of the Warmest Quarter | 0.029 | 980 mm |
| Country | Area km2 |
|---|---|
| Afghanistan | 15.29 |
| Australia | 1258 |
| Bangladesh | 122,951 |
| Brunei | 2929 |
| Cambodia | 96,421 |
| China | 68,223 |
| Hong Kong | 263 |
| India | 549,588 |
| Indonesia | 1,107,777 |
| Laos | 19,640 |
| Macau | 4.88 |
| Malaysia | 206,031 |
| Myanmar | 90,354 |
| Nepal | 8092 |
| Pakistan | 18,626 |
| Papua New Guinea | 17,540 |
| Philippines | 233,641 |
| Singapore | 0.97 |
| Sri Lanka | 51,430 |
| Taiwan | 8187 |
| Thailand | 192,403 |
| Timor-Leste | 23,554 |
| Vietnam | 148,584 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deka, M.A.; Morshed, N. Mapping Disease Transmission Risk of Nipah Virus in South and Southeast Asia. Trop. Med. Infect. Dis. 2018, 3, 57. https://doi.org/10.3390/tropicalmed3020057
Deka MA, Morshed N. Mapping Disease Transmission Risk of Nipah Virus in South and Southeast Asia. Tropical Medicine and Infectious Disease. 2018; 3(2):57. https://doi.org/10.3390/tropicalmed3020057
Chicago/Turabian StyleDeka, Mark A., and Niaz Morshed. 2018. "Mapping Disease Transmission Risk of Nipah Virus in South and Southeast Asia" Tropical Medicine and Infectious Disease 3, no. 2: 57. https://doi.org/10.3390/tropicalmed3020057
APA StyleDeka, M. A., & Morshed, N. (2018). Mapping Disease Transmission Risk of Nipah Virus in South and Southeast Asia. Tropical Medicine and Infectious Disease, 3(2), 57. https://doi.org/10.3390/tropicalmed3020057





