1. Introduction
Lymphatic filariasis (LF) is a common parasitic disease of major public health importance in tropical and subtropical countries, which are economically less endowed. About 90% of all lymphatic filariasis is caused by
Wuchereria bancrofti, one of the three parasitic filarial nematodes [
1]. These parasites are transmitted through the bite of infective mosquitoes of various genera. In highly endemic areas the disease may result in the disfiguring of the limbs and enlargement of the scrotum (hydrocele).
Owing to the availability of tools, the Global Programme to Eliminate Lymphatic filariasis (GPELF) was launched in 2000, with the aim of eliminating the disease as a public health problem by the year 2020 [
2]. Albendazole (ALB) in combination with ivermectin (IVM) or diethylcarbamazine (DEC) were the main drugs available for the large-scale control of LF [
3]. However, a combination of all three drugs—ivermectin + diethylcarbamazine + albendazole (IDA)—was recently approved for use in settings where LF is not co-endemic with onchocerciasis or loiasis [
4]. The treatment strategy relies on the assumption that if the microfilaria (mf) reservoir in the human host is reduced below a certain threshold, transmission of
W. bancrofti by anopheline vectors could be interrupted [
5]. By this strategy it was estimated that five to six rounds of mass drug administration (MDA) will be required to eliminate the disease. Ghana is one of the first countries to have started the implementation of the GPELF in 2000 [
6]. Being co-endemic for onchocerciasis, the recommended treatment regimen is IVM + ALB given once a year. These drugs mostly target mf in the blood, with limited macrofilaricidal and sterilization effect on the adult worms [
7]. Recent studies, however, suggest that administering ALB twice a year has macrofilaricidal activity [
8]. In Ghana, despite several years of treatment activities, districts with persistent transmission have been identified [
9,
10], necessitating the search for alternative and improved strategies for the control of LF. Alternative and effective treatment regimens and strategies have been recommended in order to achieve the LF elimination goals, such as, treatment to be given at shorter intervals or at increased dosage [
3,
7,
11]. Thus, a cluster-randomized study was initiated in 2017, with the aim of assessing the impact of twice-yearly treatment with IVM + ALB in communities with persistent transmission in Ghana [
12]. Following the baseline parasitological surveys, the data was analyzed to better understand the epidemiology of LF in the study areas. The information from this baseline analysis will help to implement targeted interventions, including adequate community sensitization required to achieve maximum impact, as well as monitoring the effects of the interventions.
2. Materials and Methods
2.1. Ethical Approval and Consent
Approval for the study was received from the Ghana Health Service Ethics Review Committee (GHS-ERC: 04112/2016) and the NMIMR IRB (CPN 062/16-17) with Federal Wide Assurance Registration (FWA 00001824). Community consent was sought for the study at community durbars, during which the aims of the study, procedures, risks, and benefits were explained to community leaders and members. Written informed consent was received from all study participants. For participants below the age of 18 years parental consent was sought, with written assent from children 12–17 years.
2.2. Study Design
The study was undertaken as part of a cluster randomized survey previously described [
12]. The baseline parasitological study was conducted in selected LF endemic communities with a history of at least 16 years of MDA. Following community entry, study participants were recruited and enrolled into the study. Each participant was interviewed through a questionnaire, administered to obtain data on age, sex, occupation, place of residence, use of treated bednet, and participation in MDA. Each participant also provided daytime finger-prick blood, which was tested for
W. bancrofti antigen by the rapid method. Antigen-positive individuals were followed up for night-blood samples, for the identification of mf by microscopy and detection of
W. bancrofti L3 larval stage antigen-specific IgG4 antibodies by ELISA. Filter paper blood spots were obtained from all child participants aged 5–10 years old and tested for ELISA. Mosquitoes were also collected from some of the communities and dissected to ascertain infection/infectivity with
W. bancrofti.
2.3. Study Sites and Participants
The study was conducted in 18 LF endemic villages in the Ahanta West, Nzema East and Ellembele Districts in the Western Region of Ghana. The Ahanta West and Ellembele districts share borders with the Nzema East district, and have the same ecology. The main occupations in the districts are farming and fishing. The Nzema East and Ellembele districts started MDA in 2002 while the Ahanta West district started in 2001. Details of the study districts, communities and sample size calculation were previously described [
12].
Study sites were selected following a review of the Neglected Tropical Disease Programme (NTDP) sentinel and spot check site monitoring data, as well as recommendation from the District Health Management Team (DHMT). The sample size determination took into consideration the null hypothesis that an additional MDA is not more effective than the standard single dose per annum treatment, and the alternate hypothesis that an additional MDA is more effective than the standard single dose. With prevalence between sites ranging from 1% to 18%, the sample size was determined assuming an effect size of 0.4, power of 0.80, 37% non-response rate (determined from a previous study). Thus, 80 participants were targeted from each community, with a total of 1440 participants for the entire study.
2.4. Demographic Data Collection
A computer-assisted personal interviewing (CAPI) using Census and Survey Processing System (CSPro) was employed to obtain data on age, sex, occupation, place of residence, use of treated bednet, and participation in MDA.
2.5. Blood Sample Collection and Analyses
Daytime finger-prick blood was collected from all study participants and tested for
W. bancrofti antigen using the Filaria Test Strip (FTS) [
13]. Filter paper blood spots were prepared for all participating children 5–10 years old.
Two milliliters of night-blood was collected from each
W. bancrofti antigen-positive individuals into EDTA tubes for the identification of mf using the nucleopore filtration method [
14]. Briefly, 1 mL of blood was transferred into a test tube containing 9 mL of 3% acetic acid, and the mixture filtered through a 25 mm diameter Nucleopore Track-Etch filter membrane with pore size 5 µm. The membrane was placed with the top down on a clean glass slide, and examined under a light microscope for the identification of mf. The intensity of infection was estimated as mf per mL (mf/mL) of blood.
All collected filter paper blood spots from children and night-blood samples were tested for the detection of IgG4 antibodies specific for
W. bancrofti L3 larval stage antigen [
15], using the Wb123 ELISA and following the manufacturer’s protocol [
16].
2.6. Mosquito Collection and Dissection for W. bancrofti
Mosquitoes were collected in six of the study communities, where antigen-positive individuals were identified. Two communities were selected in each study district. In each community, ten houses were randomly selected and mosquitoes were collected from houses, using the pyrethrum spray method [
17]. In each community, the collection was done in the morning, from 5 a.m. to 8 a.m. The collected mosquitoes were identified using morphological identification keys [
18], and subsequently dissected for identification of
W. bancrofti larval stages. Briefly, mosquitoes were individually placed on a labeled glass slide, separated into head, thorax, and abdomen, and gently teased apart in a drop of saline solution. The dissected parts were observed under the microscope for any
W. bancrofti larval stages (L
1–L
3).
2.7. Data Analysis
The data was combined for all districts since they shared borders and were similar in terms of ecology, occupation, and language. The data collected was analyzed using STATA (Version 15) and MedCalc Software (Version 18.6). Prevalences were estimated as percentages, with 95% confidence intervals. The chi-square test was used in the comparison of proportions. Statistical significance was estimated at p value ≤ 0.05.
4. Discussion
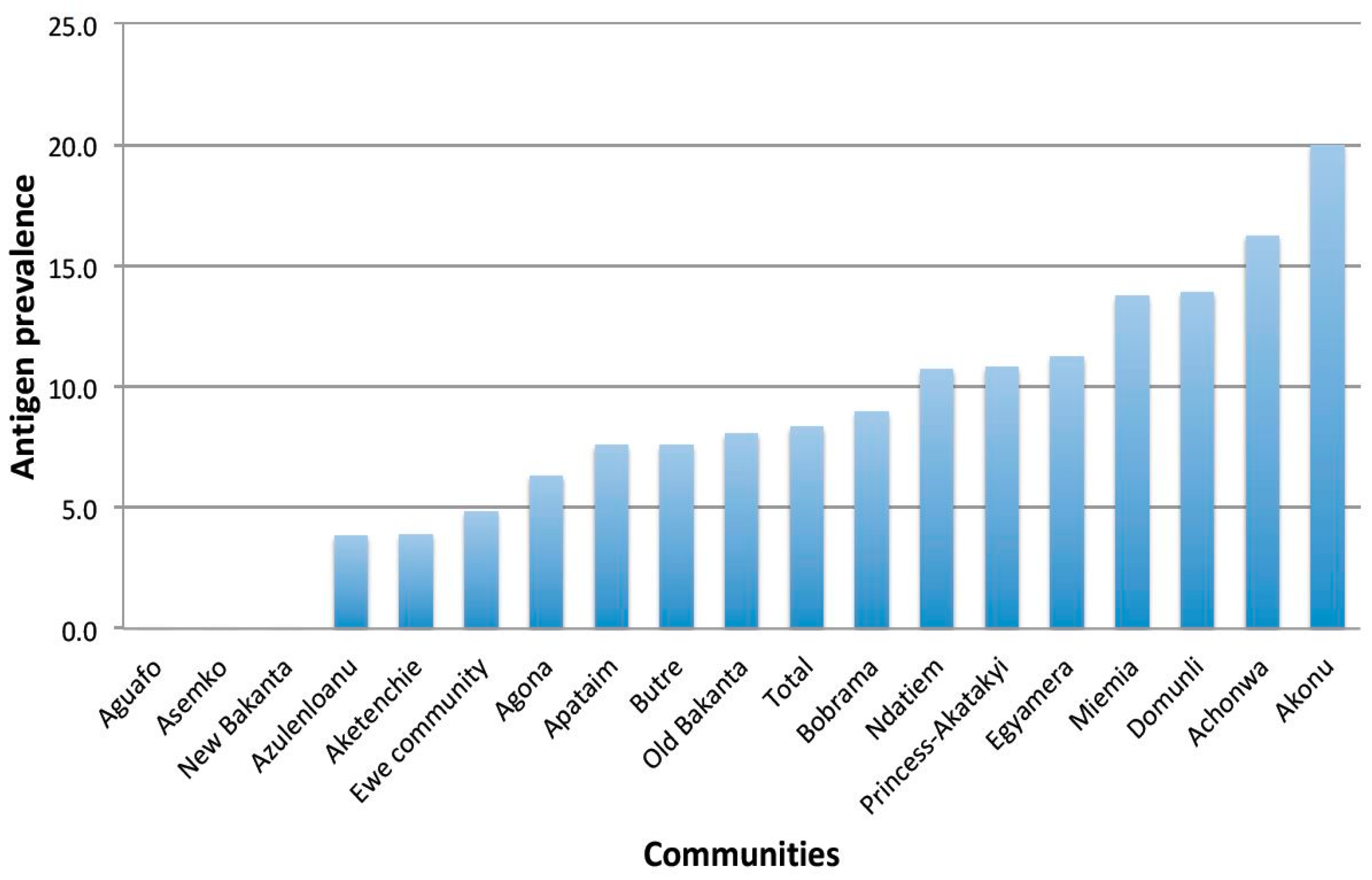
This paper presents the baseline epidemiological situation of LF in 18 communities in three endemic districts in Ghana that have received 16 to 18 years of MDA without interrupting transmission, prior to randomization and assignment to treatment groups for the evaluation of a twice-yearly treatment regimen with IVM + ALB. In this study, 1462 participants consented and provided demographic information, of which 1418 provided day blood samples for antigen testing using the FTS. The overall antigen prevalence in all the study districts was 8.3%, which is far above the minimum 2% level, below which transmission could be said to have been interrupted [
20,
21]. Similarly, the district level antigen prevalences were also higher than the recommended level.
The mf levels, on the other hand, were lower than expected. The overall mf prevalence was 1.2%, which was just slightly higher than the 1% level recommended for the interruption of transmission [
20,
21]. However, except for the mf prevalence in Ahanta West that was above the threshold (1.6%), both Nzema East and Ellembele had lower mf prevalence of 0.5% and 0.8%, respectively. While the mf prevalence might qualify Nzema East and Ellembele to proceed to carry out transmission assessment surveys (TAS) in children [
20], the levels of antigen prevalence do not. The high antigen prevalence could be due to residual adult antigen from resolved infections, with studies reporting the circulation of antigen in the blood for up to three years after treatment [
22].
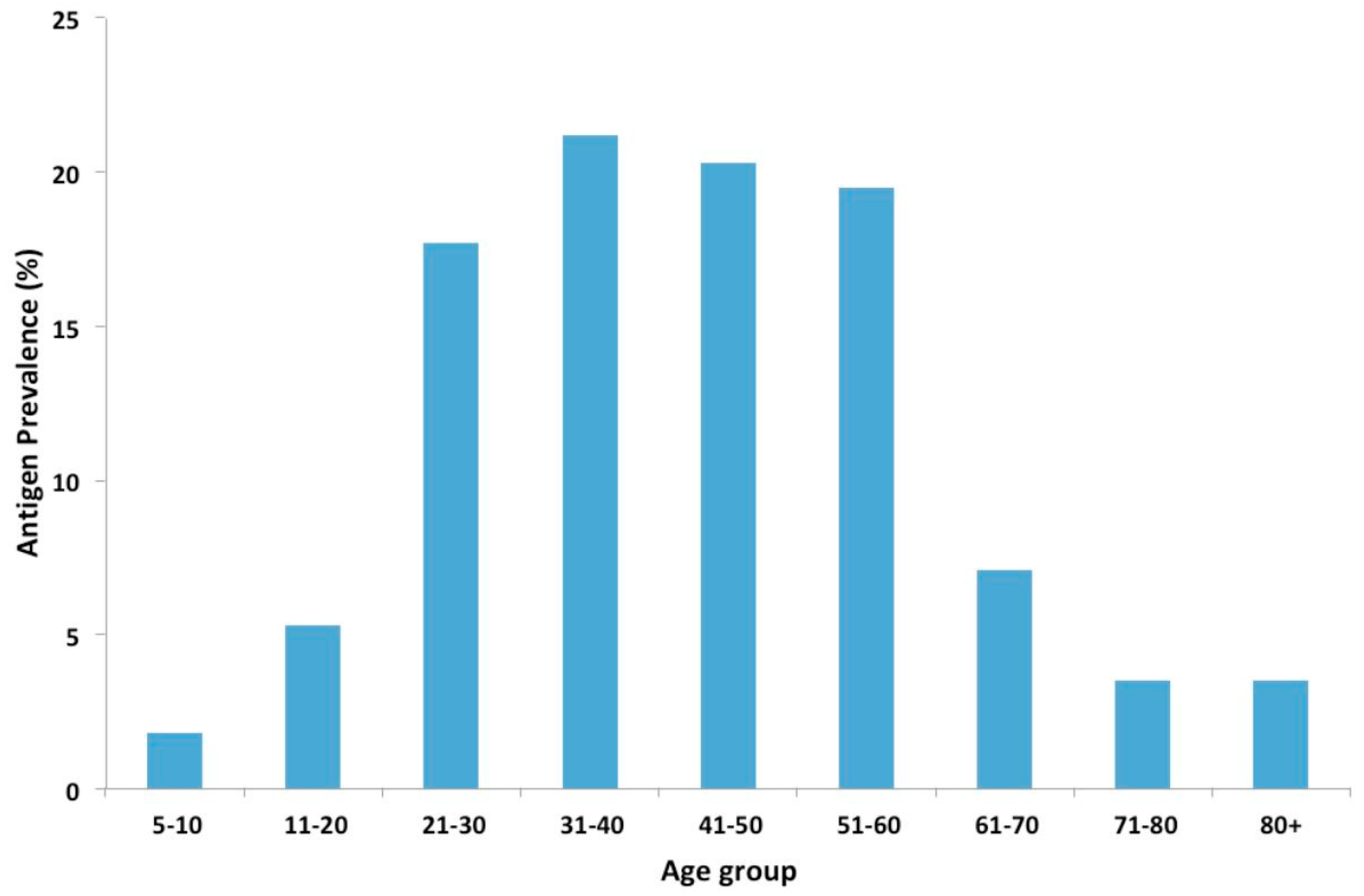
In this study, antigen prevalence increased with age, with similar trends reported in other studies [
23,
24]. This increase in antigen prevalence with age can be attributed to the increasing exposure of the population to
W. bancrofti infection. Higher antigen prevalence was also observed in males compared to females. This is consistent with studies, which showed a lower mean prevalence of infection in females than in males [
25,
26]. Higher prevalence with increased risks of infection was also observed in people involved in outdoor commercial activities such as farmers, fishermen, and drivers. Earlier studies in Ghana attributed infections to the occupational activities [
27,
28], with activities such as farming and fishing exposing individuals to mosquito bites [
29,
30]. Individuals involved in outdoor activities, and especially males, may therefore represent high-risk groups that could be targeted during MDAs, in order to reduce the prevalence of infection in LF endemic areas.
The presence of antigen-positive but mf-negative individuals could be due to very low levels of mf circulating in the blood or infections where adults worms do not produce mf [
31]. This could be a result of the sterilization effect of albendazole on adult worms [
8,
32], especially with repeated treatment.
The assessment of Wb123 antibodies for the detection of early exposure to infection [
15] indicated that 14.4% of antigen-positive adults were infected. Incidentally, antibodies were only detected in individuals found with mf. Given that antibodies may persist in the blood stream for years, it is impossible to determine if the presence of antibodies in individuals with mf is due to recent infections. The mf prevalence observed could be due to low compliance to MDA as only 8.6% of the population reported receiving at least five rounds of MDA. Since IVM and ALB, administered once a year, have mainly microfilaricidal effects with limited macrofilaricidal effect, any lapse in treatment will result in a large number of mf circulating in the blood, thus perpetuating the transmission cycle. As a result of the low compliance, adult worms in infected individuals may continue to produce mf, leading to the high prevalence observed among antigen-positive individuals.
The use of the Wb123 as a new tool for assessment in post-MDA monitoring is promising [
15,
33]. While none of the children below the age of 10 years were found infected, it is important to note that the study was not designed to assess infection in children, as undertaken during transmission assessment surveys. Further studies and guidelines to implementation for the Wb123 will be required [
34].
The generally low mf levels observed in the study population raise questions about the importance of low-level transmission, especially in communities that have received several years of treatment. The importance of vector-parasite interaction therefore comes to play. In our study communities, the main LF vectors belong to the
Anopheles gambiae complex, with
An. melas being a major vector [
35,
36].
Mansonia species have also been reported in the districts, with both
An. melas and
Mansonia spp. reported to exhibit the vector-parasite process of limitation [
37,
38], thus facilitating disease transmission at low mf levels in the community. The dissection of
An. gambiae collected from some of the study communities revealed the presence of L
3 infective stage larvae, an indication of active transmission. The exophilic nature of the vector species in the study communities also implies that transmission may occur outdoors, and may explain why individuals involved in outdoor commercial activities are at higher risks of infection. Despite these findings, there are limitations to the mosquito infection data as these did not take into consideration a sample size and the power required to make meaningful deductions. From the results, it can be observed that
W. bancrofti larvae were not identified in communities where low mosquito numbers were collected, implying that larger sample sizes will be required in such communities. However, collecting a large enough mosquito sample size would have required significant financial inputs, which unfortunately was not possible due to the funding available for the study. Nonetheless, the collection of mosquitoes in all communities was based on the same methods in the same month. As such, the numbers of mosquitoes collected could reflect the transmission potential in the communities.
This study provides information on the persistent LF transmission in three districts of Ghana that have received at least 16 years of MDA. Earlier studies in selected communities in the study areas indicated that mf load and antigen prevalence in infected individuals decreases after treatment and gradually increases within six months [
10]. Thus, administering ivermectin and albendazole to infected individuals twice in the year may help accelerate LF elimination in these settings [
10]. However, compliance to MDA was found to be low. Qualitative assessments indicated that while community members were aware of the purpose of the MDA, factors such as personal attitudes, the health system, absence of overt disease, social structure, and fear of adverse effects of drugs affected the compliance to the MDA [
39]. Considering the importance of low-level transmission in these settings, it may be useful to target interventions at high-risk individuals such as males, farmers, and fishermen in order to increase compliance in individuals most likely to serve as reservoirs of infection.
This study also emphasizes the need for comprehensive assessments in communities with persistent transmission, using a combination of assessment tools in human and vector populations. The parasitological, immunological and xenomonitoring assessments all revealed the presence of infection and transmission of the disease. Taken together, the results indicate the need for a thorough epidemiological understanding in communities with persistent LF transmission, in order to plan appropriate/tailored control interventions and achieve the LF elimination goals.