One Health—Its Importance in Helping to Better Control Antimicrobial Resistance
Abstract
:1. Introduction
2. Use of Antimicrobials in Humans, Animals and Plants
Growth Promotion Use
3. One Health Antimicrobial Resistance Case Studies
3.1. Third Generation Cephalosporins
3.2. Colistin
4. Risks to Public Health and Animal Health
5. One Health Considerations from the Environment
6. One Health Strategies to Address Antimicrobial Resistance
- Improve Awareness and Understanding of Antimicrobial Resistance through Effective Communication, Education and Training
- Strengthen the Knowledge and Evidence Base through Surveillance and Research
- Reduce the Incidence of Infection through Effective Sanitation, Hygiene and Infection Prevention Measures
- Optimize the Use of Antimicrobial Medicines in Human and Animal Health
- Develop the Economic Case tor Sustainable Investment that Takes Account of the Needs of All Countries, and Increase Investment in New Medicines, Diagnostic Tools, Vaccines and Other Interventions
7. Conclusions
Funding
Conflicts of Interest
References
- World Health Organization (WHO). Antimicrobial Resistance: Global Report on Surveillance; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Centers for Disease Control (CDC). Antibiotic Resistance Threats in the United States; CDC: Atlanta, GA, USA, 2013.
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations the Review On Antimicrobial Resistance. 2016. Available online: https://amr-review.org/sites/default/files/160525_Final paper_with cover.pdf (accessed on 15 January 2019).
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic Resistance—The Need for Global Solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Action Plan on Antimicrobial Resistance; WHO: Geneva, Switzerland, 2015; Available online: http://www.wpro.who.int/entity/drug_resistance/resources/global_action_plan_eng.pdf (accessed on 15 January 2019).
- Aarestrup, F.M.; Wegener, H.C.; Collignon, P. Resistance in Bacteria of The Food Chain: Epidemiology and Control Strategies. Expert Rev. Anti-Infect. Ther. 2008, 6, 733–750. [Google Scholar] [CrossRef]
- Collignon, P.; Beggs, J.J.; Walsh, T.R.; Gandra, S.; Laxminarayan, R. Anthropological and Socioeconomic Factors Contributing to Global Antimicrobial Resistance: A Univariate and Multivariable Analysis. Lancet Planet Heal. 2018, 2, e398–e405. [Google Scholar] [CrossRef]
- Holmes, A.H.; Moore, L.S.P.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J. Understanding the Mechanisms and Drivers of Antimicrobial Resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- Burow, E.; Käsbohrer, A. Risk Factors for Antimicrobial Resistance in Escherichia coli in Pigs Receiving Oral Antimicrobial Treatment: A Systematic Review. Microb. Drug Resist. 2017, 23, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Marti, E.; Variatza, E.; Balcazar, J.L. The Role of Aquatic Ecosystems as Reservoirs of Antibiotic Resistance. Trends Microbiol. 2014, 22, 36–41. [Google Scholar] [CrossRef]
- Bürgmann, H.; Frigon, D.; Gaze, W.H.; Manaia, C.M.; Pruden, A.; Singer, A.C.; Smets, B.F.; Zhang, T. Water and Sanitation: An Essential Battlefront in the War on Antimicrobial Resistance. FEMS Microbiol. Ecol. 2018, 94, fiy101. [Google Scholar] [CrossRef]
- Huijbers, P.M.C.; Blaak, H.; de Jong, M.C.M.; Graat, E.A.M.; Vandenbroucke-Grauls, C.M.J.E.; de Roda Husman, A.M. Role of the Environment in the Transmission of Antimicrobial Resistance to Humans: A Review. Environ. Sci. Technol. 2015, 49, 11993–12004. [Google Scholar] [CrossRef]
- Anonymous. Initiatives for Addressing Antimicrobial Resistance in the Environment: Current Situation and Challenges. 2018. Available online: https://wellcome.ac.uk/sites/default/files/antimicrobial-resistance-environment-report.pdf (accessed on 15 January 2019).
- Gaze, W.H.; Krone, S.M.; Larsson, D.G.J.; Li, X.-Z.; Robinson, J.A.; Simonet, P.; Smalla, K.; Timinouni, M.; Topp, E.; Wellington, E.M.; et al. Influence of Humans on Evolution and Mobilization of Environmental Antibiotic Resistome. Emerg. Infect. Dis. 2013, 19, e120871. [Google Scholar] [CrossRef]
- Perry, J.A.; Wright, G.D. Forces Shaping the Antibiotic Resistome. BioEssays 2014, 36, 1179–1184. [Google Scholar] [CrossRef]
- Woolhouse, M.E.J.; Ward, M.J. Sources of Antimicrobial Resistance. Science 2013, 341, 1460–1461. [Google Scholar] [CrossRef]
- Heuer, O.E.; Kruse, H.; Grave, K.; Collignon, P.; Karunasagar, I.; Angulo, F.J. Human Health Consequences of Use of Antimicrobial Agents in Aquaculture. Clin. Infect. Dis. 2009, 49, 1248–1253. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, J. Antimicrobials in Agriculture and the Environment: Reducing Unnecessary Use and Waste. The Review on Antimicrobial Resistance. 2015. Available online: https://amr-review.org/sites/default/files/Antimicrobials in agriculture and the environment—Reducing unnecessary use and waste.pdf (accessed on 15 January 2019).
- So, A.D.; Shah, T.A.; Roach, S.; Ling Chee, Y.; Nachman, K.E. An Integrated Systems Approach is Needed to Ensure the Sustainability of Antibiotic Effectiveness for Both Humans and Animals. J. Law Med. Ethics 2015, 43, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Collignon, P. The Importance of a One Health Approach to Preventing the Development and Spread of Antibiotic Resistance. In One Health: The Human-Animal-Environment Interfaces in Emerging Infectious Diseases: Food Safety and Security, and International and National Plans for Implementation of One Health Activities; Mackenzie, J.S., Jeggo, M., Daszak, P., Richt, J.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 19–36. [Google Scholar]
- Torren-Edo, J.; Grave, K.; Mackay, D. “One Health”: The Regulation and Consumption of Antimicrobials for Animal Use in the EU. IHAJ 2015, 2, 14–16. [Google Scholar]
- Robinson, T.P.; Bu, D.P.; Carrique-Mas, J.; Fèvre, E.M.; Gilbert, M.; Grace, D.; Hay, S.I.; Jiwakanon, J.; Kakkar, M.; Kariuki, S.; et al. Antibiotic Resistance is the Quintessential One Health Issue. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Zinsstag, J.; Meisser, A.; Schelling, E.; Bonfoh, B.; Tanner, M. From ‘Two Medicines’ to ‘One Health’ and Beyond. Onderstepoort J. Vet. Res. 2012, 79, a492. [Google Scholar] [CrossRef]
- World Health Organization. One Health. 2017. Available online: https://www.who.int/features/qa/one-health/en/ (accessed on 15 January 2019).
- One Health Commission. What is One Health? 2018. Available online: https://www.onehealthcommission.org/en/why_one_health/what_is_one_health/ (accessed on 15 January 2019).
- McEwen, S.A.; Fedorka-Cray, P.J. Antimicrobial Use and Resistance in Animals. Clin. Infect. Dis. 2002, 34, S93–S106. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global Trends in Antimicrobial Use in Food Animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Sales of Veterinary Antimicrobial Agents in 29 European Countries in 2014; EMA: London, UK, 2016. [Google Scholar]
- ECDC (European Centre for Disease Prevention and Control); EFSA (European Food Safety Authority); EMA (European Medicines Agency). ECDC/EFSA/EMA First Joint Report on The Integrated Analysis of The Consumption of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Humans and Food-Producing Animals. EFSA J. 2015, 13, 4006. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Drivers, Dynamics and Epidemiology of Antimicrobial Resistance in Animal Oroduction. 2016. Available online: http://www.fao.org/3/a-i6209e.pdf (accessed on 15 January 2019).
- Vidaver, A.K. Uses of Antimicrobials in Plant Agriculture. Clin. Infect. Dis. 2002, 34, S107–S110. [Google Scholar] [CrossRef]
- Sundin, G.W.; Wang, N. Antibiotic Resistance in Plant-Pathogenic Bacteria. Annu. Rev. Phytopathol. 2018, 56, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Collignon, P. Use of Critically Important Antimicrobials in Food Production. In Kucers’ The Use of Antibiotics: A Clinical Review of Antibacterial, Antifungal, Antiparasitic and Antivral Drugs, 7th ed.; Grayson, L., Ed.; American Society for Microbiology and CRC Press: Boca Raton, FL, USA, 2018; pp. 9–18. [Google Scholar]
- Sykes, J.E. Antimicrobial Drug Use in Dogs and Cats. In Antimicrobial Therapy in Veterinary Medicine, 5th ed.; Giguère, S., Prescott, J.F., Dowling, P.M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 473–494. [Google Scholar]
- Giguère, S.; Abrams-Ogg, A.C.G.; Kruth, S.A. Prophylactic Use of Antimicrobial Agents, and Antimicrobial Chemotherapy for the Neutropenic Patient. In Antimicrobial Therapy in Veterinary Medicine, 5th ed.; Giguère, S., Prescott, J.F., Dowling, P.M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 357–378. [Google Scholar]
- National Research Council. The Use of Drugs in Food Animals: Benefits and Risks; The National Academies Press: Washington, DC, USA, 1999. [Google Scholar]
- Murphy, D.; Ricci, A.; Auce, Z.; Beechinor, J.G.; Bergendahl, H.; Breathnach, R.; Bures, J.; Pedro, J.; da Silva, D.; Hederová, J.; et al. EMA and EFSA Joint Scientific Opinion on Measures to Reduce the Need to Use Antimicrobial Agents in Animal Husbandry in the European Union, and the Resulting Impacts on Food Safety (RONAFA). EFSA J. 2017, 15, 4666. [Google Scholar]
- Food and Agriculture Organization (FAO); World Organisation for Animal Health (OIE); World Health Organization (WHO) (FAO/OIE/WHO). Joint FAO/OIE/WHO Expert Workshop on Non-Human Antimicrobial Usage and Antimicrobial Resistance: Scientific Assessment; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- Food and Agriculture Organization (FAO); World Health Organization (WHO). FAO/WHO Expert Meeting on Foodborne Antimicrobial Resistance: Role of Environment, Crops and Biocides. Summary Report; FAO: Rome, Italy, 2018. [Google Scholar]
- Zuraw, L. Perdue Announces Dramatic Reduction in Antibiotic Use in its Chickens. Food Safety News. 2014. Available online: http://www.foodsafetynews.com/2014/09/perdue-dramatically-reduces-antibiotic-use-in-chickens/#.WjLZulQ-dTZ (accessed on 15 January 2019).
- World Health Organization (WHO). Impacts of Antimicrobial Growth Promoter Termination in Denmark. The WHO International Review Panel’s Evaluation of the Termination of the Use of Antimicrobial Growth Promoters in Denmark; WHO: Foulum, Denmark, 2003. [Google Scholar]
- World Health Organization (WHO). The Medical Impact of the Use of Antimicrobials in Food Animals; WHO: Berlin, Germany, 1997. [Google Scholar]
- European Union (EU). Guidelines for the Prudent Use of Antimicrobials in Veterinary Medicine (2015/C 299/04). Off. J. Eur. Union 2015, C299, C299:7–C299:26. [Google Scholar]
- Food and Drug Administration (FDA). Guidance for Industry #213. New Animal Drugs and New Animal Drug Combination Products Administered in or on Medicated Feed or Drinking Water of Food-Producing Animals: Recommendations for Drug Sponsors for Voluntarily Aligning Product Use Conditions with GFI #209; FDA: Rockville, MD, USA, 2013.
- Mehrotra, M.; Li, X.-Z.; Ireland, M.J. Enhancing Antimicrobial Stewardship by Strengthening the Veterinary Drug Regulatory Framework. Can. Commun. Dis. Rep. 2017, 43, 220–223. [Google Scholar] [CrossRef]
- World Organisation for Animal Health (OIE). OIE Annual Report on Antimicrobial Agents Intended for Use in Animals. Second Report; OIE: Paris, France, 2018; Available online: http://www.oie.int/fileadmin/Home/eng/Our_scientific_expertise/docs/pdf/AMR/Annual_Report_AMR_2.pdf (accessed on 15 January 2019).
- WHO Advisory Group on Integrated Surveillance of Antimicrobial Resistance (AGISAR). Critically Important Antimicrobials for Human Medicine; 4th revision 2013; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- European Medicines Agency (EMA). Revised Reflection Paper on the Use of 3rd and 4th Generation Cephalosporins in Food Producing Animals in the European Union: Development of Resistance and Impact on Human and Animal Health; EMA: London, UK, 2009. [Google Scholar]
- Prescott, J.F. Beta-lactam Antibiotics. In Antimicrobial Therapy in Veterinary Medicine, 5th ed.; Giguère, S., Prescott, J.F., Dowling, P.M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 153–173. [Google Scholar]
- Food and Drug Administration (FDA). 2017 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals; FDA: Washington, DC, USA, 2018.
- Food and Drug Administration (FDA). Drug Use Review. Food and Drug Administration, Department of Health and Human Services; FDA: Washington, DC, USA, 2012. Available online: http://www.fda.gov/downloads/Drugs/DrugSafety/InformationbyDrugClass/UCM319435.pdf (accessed on 15 January 2019).
- de Kraker, M.E.A.; Wolkewitz, M.; Davey, P.G.; Koller, W.; Berger, J.; Nagler, J.; Icket, C.; Kalenic, S.; Horvatic, J.; Seifert, H.; et al. Burden of Antimicrobial Resistance in European Hospitals: Excess Mortality and Length of Hospital Stay Associated with Bloodstream Infections due to Escherichia coli Resistant to Third-Generation Cephalosporins. J. Antimicrob. Chemother. 2011, 66, 398–407. [Google Scholar] [CrossRef]
- Park, S.H. Third-Generation Cephalosporin Resistance in Gram-Negative Bacteria in the Community: A Growing Public Health Concern. Korean J. Intern. Med. 2014, 29, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, N.; Scott, H.M.; Norby, B.; Loneragan, G.H.; Vinasco, J.; McGowan, M.; Cottell, J.L.; Chengappa, M.M.; Bai, J.; Boerlin, P. Effects of Ceftiofur and Chlortetracycline Treatment Strategies on Antimicrobial Susceptibility and on tet(A), tet(B), and blaCMY-2 Resistance Genes among E. coli Isolated from the Feces of Feedlot Cattle. PLoS ONE 2013, 8, e80575. [Google Scholar] [CrossRef]
- Canadian Integrated Program for Antimicrobial Resistance (CIPARS). Salmonella Heidelberg—Ceftiofur-Related Resistance in Human and Retail Chicken Isolates. 2009. Available online: http://www.phac-aspc.gc.ca/cipars-picra/heidelberg/pdf/heidelberg_e.pdf (accessed on 15 January 2019).
- Dutil, L.; Irwin, R.; Finley, R.; Ng, L.K.; Avery, B.; Boerlin, P.; Bourgault, A.M.; Cole, L.; Daignault, D.; Desruisseau, A.; et al. Ceftiofur Resistance in Salmonella enterica Serovar Heidelberg From Chicken Meat and Humans, Canada. Emerg. Infect. Dis. 2010, 16, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.E.; Medus, C.; Meyer, S.D.; Boxrud, D.J.; Leano, F.; Hedberg, C.W.; Elfering, K.; Braymen, C.; Bender, J.B.; Danila, R.N. Outbreaks of Salmonellosis in Minnesota (1998 through 2006) Associated with Frozen, Microwaveable, Breaded, Stuffed Chicken Products. Vol. 71. J. Food Prot. 2008, 71, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- Chicken Farmers of Canada. Antibiotics. 2018. Available online: https://www.chickenfarmers.ca/antibiotics/ (accessed on 15 January 2019).
- Hiki, M.; Kawanishi, M.; Abo, H.; Kojima, A.; Koike, R.; Hamamoto, S.; Asai, T. Decreased Resistance to Broad-Spectrum Cephalosporin in Escherichia coli from Healthy Broilers at Farms in Japan After Voluntary Withdrawal of Ceftiofur. Foodborne Pathog. Dis. 2015, 12, 639–643. [Google Scholar] [CrossRef]
- DANMAP 2014. Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark. Statens Serum Institut, National Veterinary Institute, Technical University of Denmark, National Food Institute, Technical University of Denmark, 2015. Available online: https://www.danmap.org/downloads/reports.aspx (accessed on 15 January 2019).
- Department of Health and Human Services, Food and Drug Administration. 2012, 21 CFR Part 530 [Docket No. FDA–2008–N–0326] New Animal Drugs; Cephalosporin Drugs; Extra Label Animal Drug Use; Order of Prohibition. Fed. Regist. 2012, 77, 735–745. [Google Scholar]
- European Medicines Agency (EMA). Answers to the Request for Scientific Advice on the Impact on Public Health and Animal Health of the Use of Antibiotics in Animals. 2014. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Other/2014/07/WC500170253.pdf (accessed on 15 January 2019).
- European Medicines Agency (EMA). Updated Advice on the Use of Colistin Products in Animals Within the European Union: Development of Resistance and Possible Impact on Human and Animal Health. In Committee for Medicinal Products for Veterinary use (CVMP), Committee for Medicinal Products; EMA: London, UK, 2016. [Google Scholar]
- Falagas, M.E.; Kasiakou, S.K. Toxicity of Polymyxins: A Systematic Review of the Evidence from Old and Recent Studies. Crit. Care 2006, 10, R27. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Kasiakou, S.K.; Saravolatz, L.D. Colistin: The Revival of Polymyxins for the Management of Multidrug-Resistant Gram-Negative Bacterial Infections. Clin. Infect. Dis. 2005, 40, 1333–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linden, P.K.; Kusne, S.; Coley, K.; Fontes, P.; Kramer, D.J.; Paterson, D. Use of Parenteral Colistin for the Treatment of Serious Infection Due to Antimicrobial-Resistant Pseudomonas aeruginosa. Clin. Infect. Dis. 2003, 37, e154–e160. [Google Scholar] [CrossRef]
- Fernandes, M.R.; Moura, Q.; Sartori, L.; Silva, K.C.; Cunha, M.P.V.; Esposito, F.; Lopes, R.; Otutumi, L.K.; Gonçalves, D.D.; Dropa, M.; et al. Silent Dissemination of Colistin-Resistant Escherichia coli in South America Could Contribute to the Global Spread of the mcr-1 Gene. Eurosurveillance 2016, 21. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of Plasmid-Mediated Colistin Rresistance Mechanism MCR-1 in Animals and Human Beings in China: A Microbiological and Molecular Biological Study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- European Centers for Disease Control and Prevention (ECDC). Summary of the Latest Data on Antibiotic Consumption in the European Union. Antibiotic Consumption in Europe; ECDC: Stockholm, Sweden, 2015.
- Landman, D.; Georgescu, C.; Martin, D.A.; Quale, J. Polymyxins Revisited. Clin. Microbiol. Rev. 2008, 21, 449–465. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority (EFSA), European Centre for Disease Prevention and Control (ECDC). The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2016. EFSA J. 2018, 16, e05182. [Google Scholar]
- Catry, B.; Cavaleri, M.; Baptiste, K.; Grave, K.; Grein, K.; Holm, A.; Jukes, H.; Liebana, E.; Navas, A.L.; Mackay, D.; et al. Use of Colistin-Containing Products Within the European Union and European Economic Area (EU/EEA): Development of Resistance in Animals and Possible Impact on Human and Animal Health. Int. J. Antimicrob Agents 2015, 46, 297–306. [Google Scholar] [CrossRef]
- Prim, N.; Rivera, A.; Rodríguez-Navarro, J.; Español, M.; Turbau, M.; Coll, P.; Mirelis, B. Detection of mcr-1 Colistin Resistance Gene in Polyclonal Escherichia coli Isolates in Barcelona, Spain, 2012 to 2015. Eurosurveillance 2016, 21. [Google Scholar] [CrossRef]
- Irrgang, A.; Roschanski, N.; Tenhagen, B.-A.; Grobbel, M.; Skladnikiewicz-Ziemer, T.; Thomas, K.; Roesler, U.; Käsbohrer, A. Prevalence of mcr-1 in E. coli from Livestock and Food in Germany, 2010–2015. PLoS ONE 2016, 11, e0159863. [Google Scholar] [CrossRef]
- Hasman, H.; Hammerum, A.M.; Hansen, F.; Hendriksen, R.S.; Olesen, B.; Agersø, Y.; Zankari, E.; Leekitcharoenphon, P.; Stegger, M.; Kaas, R.S.; et al. Detection of mcr-1 Encoding Plasmid-Mediated Colistin-Resistant Escherichia coli Isolates from Human Bloodstream Infection and Imported Chicken Meat, Denmark 2015. Eurosurveillance 2015, 20. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; van Dorp, L.; Shaw, L.P.; Bradley, P.; Wang, Q.; Wang, X.; Jin, L.; Zhang, Q.; Liu, Y.; Rieux, A.; et al. The Global Distribution and Spread of the Mobilized Colistin Resistance Gene mcr-1. Nat. Commun. 2018, 9, 1179. [Google Scholar] [CrossRef]
- von Wintersdorff, C.J.H.; Wolffs, P.F.G.; van Niekerk, J.M.; Beuken, E.; van Alphen, L.B.; Stobberingh, E.E.; Oude Lashof, A.M.L.; Hoebe, C.J.P.A.; Savelkoul, P.H.M.; Penders, J. Detection of the Plasmid-Mediated Colistin-Resistance Gene mcr-1 in Faecal Metagenomes of Dutch Travellers. J. Antimicrob Chemother. 2016, 71, 3416–3419. [Google Scholar] [CrossRef]
- Shen, Z.; Wang, Y.; Shen, Y.; Shen, J.; Wu, C. Early Emergence of mcr-1 in Escherichia coli from Food-Producing Animals. Lancet Infect. Dis. 2016, 16, 293. [Google Scholar] [CrossRef]
- Zurfuh, K.; Poirel, L.; Nordmann, P.; Nüesch-Inderbinen, M.; Hächler, H.; Stephan, R. Occurrence of the Plasmid-Borne mcr-1 Colistin Resistance Gene in Extended-Spectrum-β-Lactamase-Producing Enterobacteriaceae in River Water and Imported Vegetable Samples in Switzerland. Antimicrob. Agents Chemother. 2016, 60, 2594–2595. [Google Scholar] [CrossRef] [PubMed]
- Xavier, B.B.; Lammens, C.; Ruhal, R.; Kumar-Singh, S.; Butaye, P.; Goossens, H.; Malhotra-Kumar, S. Identification of a Novel Plasmid-Mediated Colistin-Resistance Gene, mcr-2, in Escherichia coli, Belgium, June 2016. Eurosurveillance 2016, 21. [Google Scholar] [CrossRef]
- Levine, D.P. Vancomycin: A History. Clin. Infect. Dis. 2006, 42, S5–S12. [Google Scholar] [CrossRef]
- Bager, F.; Madsen, M.; Christensen, J.; Aarestrup, F.M. Avoparcin Used as a Growth Promoter is Associated with the Occurrence of Vancomycin-Resistant Enterococcus faecium on Danish Poultry and Pig Farms. Prev. Vet. Med. 1997, 31, 95–112. [Google Scholar] [CrossRef]
- Barza, M. Potential Mechanisms of Increased Disease in Humans from Antimicrobial Resistance in Food Animals. Clin. Infect. Dis. 2002, 34, S123–S125. [Google Scholar] [CrossRef]
- Anderson, E.S. Drug Resistance in Salmonella typhimurium and its Implications. Br. Med. J. 1968, 3, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Swann, M.M. The Use of Antibiotics in Animal Husbandry and Veterinary Medicine; HMSO: London, UK, 1969.
- Institute of Medicine. Human Health Risks with the Subtherapeutic Use of Penicillin or Tetracyclines in Animal Feed; The National Academies Press: Washington, DC, USA, 1989. [Google Scholar]
- Hanning, I.B.; Nutt, J.D.; Ricke, S.C. Salmonellosis Outbreaks in the United States Due to Fresh Produce: Sources and Potential Intervention Measures. Foodborne Pathog. Dis. 2009, 6, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Endtz, H.; Ruijs, G.; van Klingeren, B.; Jansen, W.H.; Reijden, T.; Mouton, R.P. Quinolone Resistance in Campylobacter Isolated from Man and Poultry Following the Introduction of Fluoroquinolones in Veterinary Medicine. J. Antimicrob Chemother. 1991, 27, 199–208. [Google Scholar] [CrossRef] [PubMed]
- McDermott, P.F.; Bodeis, S.M.; English, L.L.; White, D.G.; WalkeR, R.D.; Zhao, S.; Simjee, S.; Wagne, D.D. Ciprofloxacin Resistance in Campylobacter jejuni Evolves Rapidly in Chickens Treated with Fluoroquinolones. J. Infect. Dis. 2002, 185, 837–840. [Google Scholar] [CrossRef]
- Nelson, J.M.; Chiller, T.M.; Powers, J.H.; Angulo, F.J. Fluoroquinolone-Resistant Campylobacter Species and the Withdrawal of Fluoroquinolones from Use in Poultry: A Public Health Success Story. Clin. Infect. Dis. 2007, 44, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.C.; Turnidge, J.; Collignon, P.; Looke, D.; Barton, M.; Gottlieb, T. Control of Fluoroquinolone Resistance through Successful Regulation, Australia. Emerg. Infect. Dis. 2012, 18, 1453–1460. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Use of Quinolones in Food Animals and Potential Impact on Human Health; WHO: Geneva, Switzerland, 1998. [Google Scholar]
- Chiu, C.H.; Wu, T.L.; Su, L.H.; Chu, C.; Chia, J.H.; Kuo, A.J.; Chien, M.S.; Lin, T.Y. The Emergence in Taiwan of Fluoroquinolone Resistance in Salmonella enterica Serotype Choleraesuis. N. Engl. J. Med. 2002, 346, 413–419. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Reflection Paper on the Use of Fluoroquinolones in Food-Producing Animals in the European Union: Development of Resistance and Impact on Human and Animal Health; EMEA/CVMP/SAGAM/184651/2005; EMA: London, UK, 2006. [Google Scholar]
- Helms, M.; Simonsen, J.; Mølbak, K. Quinolone Resistance Is Associated with Increased Risk of Invasive Illness or Death during Infection with Salmonella Serotype Typhimurium. J. Infect. Dis. 2004, 190, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Mollenkopf, D.F.; Stull, J.W.; Mathys, D.A.; Bowman, A.S.; Feicht, S.M.; Grooters, S.V.; Daniels, J.B.; Wittum, T.E. Carbapenemase-Producing Enterobacteriaceae Recovered from the Environment of a Swine Farrow-to-Finish Operation in the United States. Antimicrob. Agents Chemother. 2017, 61, e01298-16. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, K.; Collignon, P. Colonisation with Escherichia coli Resistant to “Critically Important” Antibiotics: A High Risk for International Travellers. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1501–1506. [Google Scholar] [CrossRef]
- Laupland, K.B.; Church, D.L. Population-Based Epidemiology and Microbiology of Community-Onset Bloodstream Infections. Clin. Microbiol. Rev. 2014, 27, 647–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazarus, B.; Paterson, D.L.; Mollinger, J.L.; Rogers, B.A. Do Human Extraintestinal Escherichia coli Infections Resistant to Expanded-Spectrum Cephalosporins Originate From Food-Producing Animals? A Systematic Review. Clin. Infect. Dis. 2015, 60, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Bou-Antoun, S.; Davies, J.; Guy, R.; Johnson, A.P.; Sheridan, E.A.; Hope, R.J. Descriptive Epidemiology of Escherichia coli Bacteraemia in England, April 2012 to March 2014. Eurosurveillance 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Hammerum, A.M.; Larsen, J.; Andersen, V.D.; Lester, C.H.; Skovgaard Skytte, T.S.; Hansen, F.; Olsen, S.S.; Mordhorst, H.; Skov, R.L.; Aarestrup, F.M.; et al. Characterization of Extended-Spectrum β-lactamase (ESBL)-Producing Escherichia coli Obtained from Danish Pigs, Pig farmers and Their Families from Farms with High or no Consumption of Third- or Fourth-Generation Cephalosporins. J. Antimicrob Chemother. 2014, 69, 2650–2657. [Google Scholar] [CrossRef] [PubMed]
- Collignon, P. Antibiotic Resistance: Are we all Doomed? Intern. Med. J. 2015, 45, 1109–1115. [Google Scholar] [CrossRef]
- Walsh, T.R.; Weeks, J.; Livermore, D.M.; Toleman, M.A. Dissemination of NDM-1 positive Bacteria in the New Delhi Environment and its Implications for Human Health: An Environmental Point Prevalence Study. Lancet Infect. Dis. 2011, 11, 355–362. [Google Scholar] [CrossRef]
- Graham, D.W.; Collignon, P.; Davies, J.; Larsson, D.G.J.; Snape, J. Underappreciated Role of Regionally Poor Water Quality on Globally Increasing Antibiotic Resistance. Environ. Sci. Technol. 2014, 48, 11746–11747. [Google Scholar] [CrossRef]
- Tängdén, T.; Cars, O.; Melhus, Å.; Löwdin, E. Foreign Travel Is a Major Risk Factor for Colonization with Escherichia coli Producing CTX-M-Type Extended-Spectrum β-Lactamases: A Prospective Study with Swedish Volunteers. Antimicrob. Agents Chemother. 2010, 54, 3564–3568. [Google Scholar] [CrossRef]
- Vieira, A.R.; Collignon, P.; Aarestrup, F.M.; McEwen, S.A.; Hendriksen, R.S.; Hald, T.; Wegener, H.C. Association Between Antimicrobial Resistance in Escherichia coli Isolates from Food Animals and Blood Stream Isolates From Humans in Europe: An Ecological Study. Foodborne Pathog. Dis. 2011, 8, 1295–1301. [Google Scholar] [CrossRef]
- De Been, M.; Lanza, V.F.; de Toro, M.; Scharringa, J.; Dohmen, W.; Du, Y.; Hu, J.; Lei, Y.; Li, N.; Tooming-Klunderud, A.; et al. Dissemination of Cephalosporin Resistance Genes between Escherichia coli Strains from Farm Animals and Humans by Specific Plasmid Lineages. PLOS Genet. 2014, 10, e1004776. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, L.; Kurbasic, A.; Skjøt-Rasmussen, L.; Ejrnæs, K.; Porsbo, L.J.; Pedersen, K.; Jensen, L.B.; Emborg, H.-D.; Agersø, Y.; Olsen, K.E.P.; et al. Escherichia coli Isolates from Broiler Chicken Meat, Broiler Chickens, Pork, and Pigs Share Phylogroups and Antimicrobial Resistance with Community-Dwelling Humans and Patients with Urinary Tract Infection. Foodborne Pathog. Dis. 2009, 7, 537–547. [Google Scholar] [CrossRef] [PubMed]
- ECDC (European Centre for Disease Prevention and Control); EFSA (European Food Safety Authority); EMA (European Medicines Agency). Joint Scientific Report of ECDC, EFSA and EMEA on Meticillin Resistant Staphylococcus aureus (MRSA) in Livestock, Companion Animals and Food. EFSA J. 2009, 7. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Antimicrobial Resistance Surveillance in Europe 2014. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net); ECDC: Stockholm, Sweden, 2015.
- Finley, R.L.; Collignon, P.; Larsson, D.G.J.; McEwen, S.A.; Li, X.-Z.; Gaze, W.H.; Reid-Smith, R.; Timinouni, M.; Graham, D.W.; Topp, E. The Scourge of Antibiotic Resistance: The Important Role of the Environment. Clin. Infect. Dis. 2013, 57, 704–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, L.B.; Stegger, M.; Hasman, H.; Aziz, M.; Larsen, J.; Andersen, P.S.; Pearson, T.; Waters, A.E.; Foster, J.T.; Schupp, J.; et al. Staphylococcus aureus CC398: Host Adaptation and Emergence of Methicillin Resistance in Livestock. mBio 2012, e00305-11. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S.; van Duijkeren, E. Methicillin-Resistant Staphylococcus aureus and Staphylococcus pseudintermedius in Veterinary Medicine. Vet. Microbiol. 2010, 140, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Boost, M.V.; O’Donoghue, M.M.; Siu, K.H.G. Characterisation of Methicillin-Resistant Staphylococcus aureus Isolates from Dogs and Their Owners. Clin. Microbiol. Infect. 2007, 13, 731–733. [Google Scholar] [CrossRef]
- Lewis, H.C.; Mølbak, K.; Reese, C.; Aarestrup, F.M.; Selchau, M.; Sørum, M.; Skov, R.L. Pigs as Source of Methicillin-Resistant Staphylococcus aureus CC398 Infections in Humans, Denmark. Emerg. Infect. Dis. 2008, 14, 1383–1389. [Google Scholar] [CrossRef]
- Voss, A.; Loeffen, F.; Bakker, J.; Klaassen, C.; Wulf, M. Methicillin-Resistant Staphylococcus aureus in Pig Farming. Emerg. Infect. Dis. J. 2005, 11, 1965. [Google Scholar] [CrossRef]
- Dorado-Garcia, A.; Dohmen, W.; Bos, M.E.H.; Verstappen, K.M.; Houben, M.; Wagenaar, J.A.; Heederik, D.J. Dose-Response Relationship Between Antimicrobial Drugs and Livestock-Associated MRSA in Pig Farming. Emerg. Infect. Dis. 2015, 21, 950–959. [Google Scholar] [CrossRef]
- Ruuskanen, M.; Muurinen, J.; Meierjohan, A.; Parnanen, K.; Tamminen, M.; Lyra, C.; Kronberg, L.; Virta, M. Fertilizing with Animal Manure Disseminates Antibiotic Resistance Genes to the Farm Environment. J. Environ. Qual. 2016, 45, 488–493. [Google Scholar] [CrossRef]
- Wellington, E.M.H.; Boxall, A.B.; Cross, P.; Feil, E.J.; Gaze, W.H.; Hawkey, P.M.; Johnson-Rollings, A.S.; Jones, D.L.; Lee, N.M.; Otten, W.; et al. The Role of the Natural Environment in the Emergence of Antibiotic Resistance in Gram-Negative Bacteria. Lancet Infect. Dis. 2013, 13, 155–165. [Google Scholar] [CrossRef]
- Aubertheau, E.; Stalder, T.; Mondamert, L.; Ploy, M.-C.; Dagot, C.; Labanowski, J. Impact of Wastewater Treatment Plant Discharge on the Contamination of River Biofilms by Pharmaceuticals and Antibiotic Resistance. Sci. Total Environ. 2017, 579, 1387–1398. [Google Scholar] [CrossRef]
- Singer, A.C.; Shaw, H.; Rhodes, V.; Hart, A. Review of Antimicrobial Resistance in the Environment and Its Relevance to Environmental Regulators. Front. Microbiol. 2016, 7, 1728. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban Wastewater Treatment Plants as Hotspots for Antibiotic Resistant Bacteria and Genes Spread into the Environment: A Review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef]
- Zhang, Q.-Q.; Ying, G.-G.; Pan, C.-G.; Liu, Y.-S.; Zhao, J.-L. Comprehensive Evaluation of Antibiotics Emission and Fate in the River Basins of China: Source Analysis, Multimedia Modeling, and Linkage to Bacterial Resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Cabello, F.C.; Godfrey, H.P.; Buschmann, A.H.; Dölz, H.J. Aquaculture as yet Another Environmental Gateway to the Development and Globalisation of Antimicrobial Resistance. Lancet Infect. Dis. 2016, 16, e127–e133. [Google Scholar] [CrossRef]
- Wang, H.; Yang, J.; Yu, X.; Zhao, G.; Zhao, Q.; Wang, N.; Jiang, Y.; Jiang, F.; He, G.; Chen, Y.; et al. Exposure of Adults to Antibiotics in a Shanghai Suburban Area and Health Risk Assessment: A Biomonitoring-Based Study. Environ. Sci. Technol. 2018, 52, 13942–13950. [Google Scholar] [CrossRef]
- Rahube, T.O.; Marti, R.; Scott, A.; Tien, Y.-C.; Murray, R.; Sabourin, L.; Duenk, P.; Lapen, D.R.; Topp, E. Persistence of antibiotic resistance and plasmid-associated genes in soil following application of sewage sludge and abundance on vegetables at harvest. Can. J. Microbiol. 2016, 62, 600–607. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Reflection Paper on Antimicrobial Resistance in the Environment: Considerations for Current and Future Risk Assessment of Veterinary Medicinal Products Draft. 2018. Available online: https://www.ema.europa.eu/documents/scientific-guideline/draft-reflection-paper-antimicrobial-resistance-environment-considerations-current-future-risk_en.pdf (accessed on 15 January 2019).
- Collignon, P.; Kennedy, K.J. Long-Term Persistence of Multidrug-Resistant Enterobacteriaceae after Travel. Clin. Infect. Dis. 2015, 61, 1766–1767. [Google Scholar] [CrossRef] [PubMed]
- Ashbolt, N.J.; Amézquita, A.; Backhaus, T.; Borriello, P.; Brandt, K.K.; Collignon, P.; Coors, A.; Finley, R.; Gaze, W.H.; Heberer, T.; et al. Human Health Risk Assessment (HHRA) for Environmental Development and Transfer of Antibiotic Resistance. Environ. Health Perspect. 2013, 121, 993–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Organisation for Animal Health (OIE). The OIE Strategy on Antimicrobial Resistance and the Prudent Use of Antimicrobials; OIE: Paris, France, 2016. [Google Scholar]
- Department of Health and Department for Environment Food & Rural Affairs. UK Five-Year Antimicrobial Resistance Strategy 2013 to 2018; Department of Health and Department for Environment Food & Rural Affairs: London, UK, 2013.
- Public Health Agency of Canada. Federal Action Plan on Antimicrobial Resistance and Use in Canada; Public Health Agency of Canada: Ottawa, ON, Cananda, 2015.
- Commonwealth of Australia. Responding to the Threat of Antimicrobial Resistance. Australia’s First National Antimicrobial Resistance Strategy 2015-2019; Commonwealth of Australia: Canberra, Australia, 2016.
- European Union (EU). Communication from the Commission to the European Parliament and the Council. Action Plan against the Rising Threats from Antimicrobial Resistance; EU: Brussels, Belgium, 2011. [Google Scholar]
- The White House. National Action Plan for Combating Antibiotic-Resistant Bacteria; The White House: Washington, DC, USA, 2015.
- Food and Agriculture Organization (FAO). The FAO Action Plan on Antimicrobial Resistance 2016-2020; FAO: Rome, Italy, 2016. [Google Scholar]
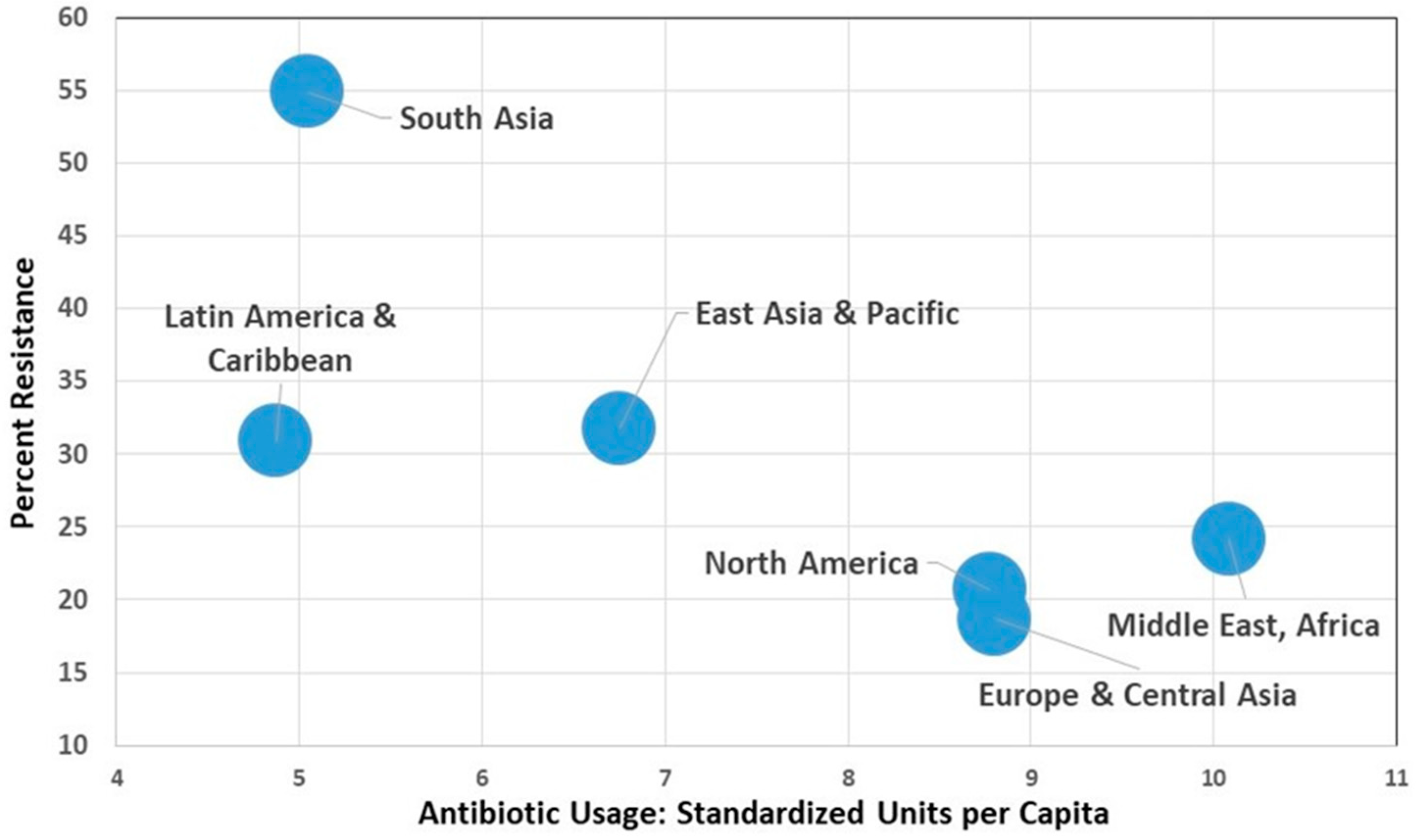

| Country | Antibiotic Usage (Standard units per 1000 pop - CCDEP) | E. coli % Resistance 3rd gen ceph (WHO) | E. coli % Resistance Fluoroquinolones (WHO) | Staphylococcus Aureus (MRSA Rates - WHO) | 2015 Corruption Index | GNP per capita 2015 (Purchasing Power Parity in 2011 Dollars) | % with Adequate Sanitation 2015 | Improved Water Source (% of Population with Access) |
|---|---|---|---|---|---|---|---|---|
| Algeria | 15.4 | 17 | 2 | 44.8 | 36 | $13,795 | 88 | 87.7 |
| Argentina | 6.2 | 5.1 | 7.8 | 54 | 32 | $19,102 | 96 | 98.9 |
| Australia | 11 | 7.7 | 10.6 | 30 | 79 | $43,631 | 100 | 100 |
| Austria | 7.2 | 9.1 | 22.3 | 7.4 | 76 | $44,048 | 100 | 100 |
| Bahrain | 55 | 62 | 10 | 51 | $43,754 | 99 | 100 | |
| Bangladesh | 4.3 | 57.4 | 89 | 46 | 25 | $3,137 | 61 | 86.2 |
| Belgium | 12.6 | 6 | 21.5 | 17.4 | 77 | $41,826 | 100 | 100 |
| Bhutan | 19.4 | 35.5 | 10 | 65 | $7,861 | 50 | 100 | |
| Bosnia and Herzegovina | 7.5 | 1.5 | 7.8 | 38 | $10,119 | 95 | 99.9 | |
| Brazil | 5.9 | 30 | 40 | 29.5 | 38 | $14,533 | 83 | 98.1 |
| Brunei Darussalam | 6.5 | 12 | 55 | $73,605 | 100 | 100 | ||
| Bulgaria | 9.4 | 22.9 | 30.2 | 22.4 | 41 | $17,000 | 86 | 99.6 |
| Burkina Faso | 36 | 52.8 | 38 | $1,593 | 20 | 82.1 | ||
| Burundi | 7.2 | 16 | 13 | 21 | $683 | 48 | 75.8 | |
| Cambodia | 45 | 71.8 | 21 | $3,278 | 42 | 73.4 | ||
| Canada | 7.2 | 8 | 26.9 | 21 | 83 | $42,983 | 100 | 99.8 |
| Central African Republic | 30 | 53 | 24 | $581 | 22 | 68.4 | ||
| Chile | 4.3 | 23.8 | 90 | 70 | $22,197 | 99 | 99 | |
| China | 3 | 51.9 | 55.1 | 38.3 | 70 | $13,572 | 77 | 94.8 |
| Colombia | 2.9 | 11.7 | 59 | 7.2 | 37 | $12,988 | 81 | 91.3 |
| Croatia | 10.6 | 6 | 14 | 13 | 51 | $20,664 | 97 | 99.6 |
| Cuba | 42.9 | 56 | 47 | $21,017 | 93 | 94.6 | ||
| Cyprus | 36.2 | 47.4 | 41.6 | 61 | $30,383 | 100 | 100 | |
| Czech Republic | 7.5 | 11.4 | 23.5 | 14.5 | 56 | $30,381 | 99 | 100 |
| Denmark | 6.7 | 8.5 | 14.1 | 1.2 | 91 | $45,484 | 100 | 100 |
| Dominican Republic | 2.4 | 33 | 49 | 30 | 33 | $13,372 | 84 | 86.5 |
| Ecuador | 6.7 | 15.1 | 43.8 | 29 | 32 | $10,777 | 85 | 86.9 |
| Egypt | 9.1 | 44.4 | 34.9 | 46 | 36 | $10,250 | 95 | 99.2 |
| Estonia | 4.4 | 9.9 | 1.7 | 70 | $27,345 | 97 | 99.6 | |
| Ethiopia | 62 | 71 | 31.6 | 33 | $1,530 | 28 | 55.4 | |
| Finland | 7.2 | 5.1 | 10.8 | 2.8 | 90 | $38,994 | 98 | 100 |
| France | 12.9 | 8.2 | 17.9 | 20.1 | 70 | $37,775 | 99 | 100 |
| Germany | 7.1 | 8 | 23.7 | 16.2 | 81 | $43,788 | 99 | 100 |
| Greece | 14.6 | 14.9 | 26.6 | 39.2 | 46 | $24,095 | 99 | 100 |
| Guatemala | 39.8 | 41.8 | 52 | 28 | $7,253 | 64 | 92.7 | |
| Honduras | 36.7 | 43.1 | 30 | 31 | $4,785 | 83 | 90.6 | |
| Hong Kong | 7.5 | 75 | $53,463 | 96 | 100 | |||
| Hungary | 7.3 | 15.1 | 31.2 | 26.2 | 51 | $24,831 | 98 | 100 |
| Iceland | 6.2 | 14 | 79 | $42,704 | 99 | 100 | ||
| India | 5 | 51.4 | 52.3 | 42.7 | 38 | $5,733 | 40 | 94.1 |
| Indonesia | 3.6 | 36 | $10,385 | 61 | 86.8 | |||
| Iran | 41 | 54 | 53 | 27 | $16,507 | 90 | 96.2 | |
| Ireland | 11.4 | 9 | 22.9 | 23.7 | 75 | $61,378 | 91 | 97.9 |
| Israel | 2.6 | 17.9 | 46.7 | 61 | $31,971 | 100 | 100 | |
| Italy | 11.5 | 19.8 | 40.5 | 38.2 | 44 | $34,220 | 100 | 100 |
| Japan | 5.3 | 16.6 | 34.3 | 53 | 75 | $37,872 | 100 | 100 |
| Jordan | 6.3 | 22.5 | 14.5 | 53 | $10,240 | 99 | 96.9 | |
| Kazakhstan | 7.5 | 28 | $23,522 | 98 | 93.5 | |||
| Kenya | 87.2 | 91.4 | 20 | 25 | $2,901 | 30 | 63.1 | |
| Kuwait | 6.3 | 20.1 | 32 | 49 | $70,107 | 100 | 99 | |
| Latvia | 5.2 | 15.9 | 16.8 | 9.9 | 55 | $23,080 | 88 | 99.3 |
| Lebanon | 9.3 | 27.7 | 47 | 20 | 28 | $13,089 | 81 | 99 |
| Lesotho | 2 | 14 | 44 | $2,770 | 30 | 81.6 | ||
| Lithuania | 7.6 | 7 | 12.9 | 5.8 | 61 | $26,971 | 92 | 96.6 |
| Luxembourg | 11 | 8.2 | 24.1 | 20.5 | 81 | $93,900 | 98 | 100 |
| Malaysia | 4.3 | 17.4 | 23 | 17.3 | 50 | $25,312 | 96 | 98.2 |
| Malta | 12.8 | 32 | 49.2 | 56 | $32,720 | 100 | 100 | |
| Mexico | 2.4 | 42.1 | 46.3 | 29.9 | 35 | $16,490 | 85 | 96.1 |
| Mongolia | 64.1 | 64.7 | 39 | $11,478 | 60 | 64.2 | ||
| Morocco | 6 | 4 | 23.3 | 19 | 36 | $7,365 | 77 | 85.3 |
| Myanmar | 68 | 55 | 26 | 22 | $4,931 | 80 | 80.5 | |
| Nepal | 37.9 | 64.3 | 44.9 | 27 | $2,312 | 46 | 90.7 | |
| Netherlands | 4.1 | 5.7 | 14.3 | 1.4 | 87 | $46,354 | 98 | 100 |
| New Zealand | 10.9 | 3 | 6.5 | 10.4 | 88 | $35,159 | 100 | 100 |
| Nicaragua | 48.1 | 42.9 | 27 | $4,884 | 68 | 86.9 | ||
| Nigeria | 6.7 | 36.5 | 47.1 | 26 | $5,639 | 29 | 67.6 | |
| Norway | 5.9 | 3.6 | 9 | 0.3 | 87 | $63,650 | 98 | 100 |
| Pakistan | 7.1 | 36.2 | 35.3 | 37.6 | 30 | $4,706 | 64 | 91.3 |
| Panama | 9.2 | 23.3 | 21.1 | 39 | $20,885 | 75 | 94.4 | |
| Papua New Guinea | 24.1 | 13.3 | 43.9 | 25 | $2,723 | 19 | 40 | |
| Paraguay | 1.4 | 22.1 | 27 | 27 | $8,639 | 89 | 96.6 | |
| Peru | 3.4 | 44.1 | 62.8 | 65.9 | 36 | $11,768 | 76 | 86.3 |
| Philippines | 2.2 | 26.7 | 40.9 | 54.9 | 35 | $6,938 | 74 | 91.5 |
| Poland | 9.3 | 11.7 | 27.3 | 24.3 | 62 | $25,323 | 97 | 98.3 |
| Portugal | 9.3 | 11.3 | 27.2 | 54.6 | 63 | $26,549 | 100 | 100 |
| Puerto Rico | 9.1 | 99 | ||||||
| Republic of Moldova | 28 | 15.3 | 50.3 | 33 | $4,742 | 76 | 88.4 | |
| Russian Federation | 6.2 | 18 | 25.7 | 29.3 | 29 | $24,124 | 72 | 96.9 |
| Rwanda | 21.4 | 54 | $1,655 | 62 | 75.5 | |||
| Serbia | 10.6 | 21.3 | 16 | 44.5 | 40 | $13,278 | 96 | 99.3 |
| Singapore | 5.7 | 20 | 37.8 | 85 | $80,192 | 100 | 100 | |
| Slovakia | 9.2 | 31 | 41.9 | 25.9 | 51 | $28,254 | 99 | 100 |
| Slovenia | 6.3 | 8.8 | 20.7 | 7.1 | 60 | $29,097 | 99 | 99.6 |
| South Africa | 8.7 | 8.2 | 16.1 | 52 | 44 | $12,393 | 66 | 92.8 |
| South Korea | 10.9 | 24.4 | 40.9 | 65.3 | 56 | $34,387 | 100 | 97.6 |
| Spain | 14.3 | 12 | 34.5 | 22.5 | 55 | $32,219 | 100 | 100 |
| Sri Lanka | 3.9 | 58.9 | 58.8 | 37 | $11,048 | 95 | 95.6 | |
| Sudan | 49.5 | 56.8 | 12 | $4,121 | 24 | 58.5 | ||
| Saudi Arabia | 11.1 | 15.9 | 40.9 | 41.9 | 52 | $50,284 | 100 | 97 |
| Sweden | 4.8 | 3 | 7.9 | 0.8 | 89 | $45,488 | 99 | 100 |
| Switzerland | 5.2 | 8.2 | 20.2 | 10.2 | 86 | $56,517 | 100 | 100 |
| Syrian Arab Republic | 49.8 | 18 | $- | 96 | 90.1 | |||
| Taiwan | 8.7 | 62 | ||||||
| Thailand | 7 | 37.9 | 52.5 | 22.4 | 38 | $15,347 | 93 | 97.8 |
| Tunisia | 18 | 20.6 | 9.4 | 55.8 | 38 | $10,770 | 92 | 97.7 |
| Turkey | 18.5 | 43.3 | 46.3 | 31.5 | 42 | $19,460 | 95 | 100 |
| United Arab Emirates | 10.5 | 23 | 32.5 | 33.4 | 70 | $65,717 | 98 | 99.7 |
| United Kingdom | 9 | 9.6 | 17.5 | 13.6 | 81 | $38,509 | 99 | 100 |
| United States of America | 10.3 | 14.6 | 33.3 | 51.3 | 76 | $52,704 | 100 | 99.2 |
| Uruguay | 6.6 | 0 | 15 | 40 | 74 | $19,952 | 96 | 99.6 |
| Venezuela | 8.1 | 12.5 | 37.2 | 31 | 17 | $16,769 | 94 | 93.1 |
| Vietnam | 9.4 | 0.2 | 31 | $5,667 | 78 | 96.4 | ||
| Zambia | 37.4 | 50.5 | 32 | 38 | $3,602 | 44 | 64.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collignon, P.J.; McEwen, S.A. One Health—Its Importance in Helping to Better Control Antimicrobial Resistance. Trop. Med. Infect. Dis. 2019, 4, 22. https://doi.org/10.3390/tropicalmed4010022
Collignon PJ, McEwen SA. One Health—Its Importance in Helping to Better Control Antimicrobial Resistance. Tropical Medicine and Infectious Disease. 2019; 4(1):22. https://doi.org/10.3390/tropicalmed4010022
Chicago/Turabian StyleCollignon, Peter J., and Scott A. McEwen. 2019. "One Health—Its Importance in Helping to Better Control Antimicrobial Resistance" Tropical Medicine and Infectious Disease 4, no. 1: 22. https://doi.org/10.3390/tropicalmed4010022
APA StyleCollignon, P. J., & McEwen, S. A. (2019). One Health—Its Importance in Helping to Better Control Antimicrobial Resistance. Tropical Medicine and Infectious Disease, 4(1), 22. https://doi.org/10.3390/tropicalmed4010022





