In health facilities where scabies has already been diagnosed and treated, the main objective is often to understand if persistent itching is caused by the side effects of acaricides (e.g., benzyl benzoate), pre-existing co-morbidities, failure of therapy, or re-infestation. Since direct examination is not always sufficient to resolve these questions and optical microscopy is time consuming, any other instrumental help, including dermatoscopy, is worthy of attention and investigation. The methodical use of dermatoscopes has led to the introduction of a list of eight points, some already known and others previously undescribed, as an innovative morphological/functional key.
3.1. Definition of the Mite-Gallery Unit (MGU)
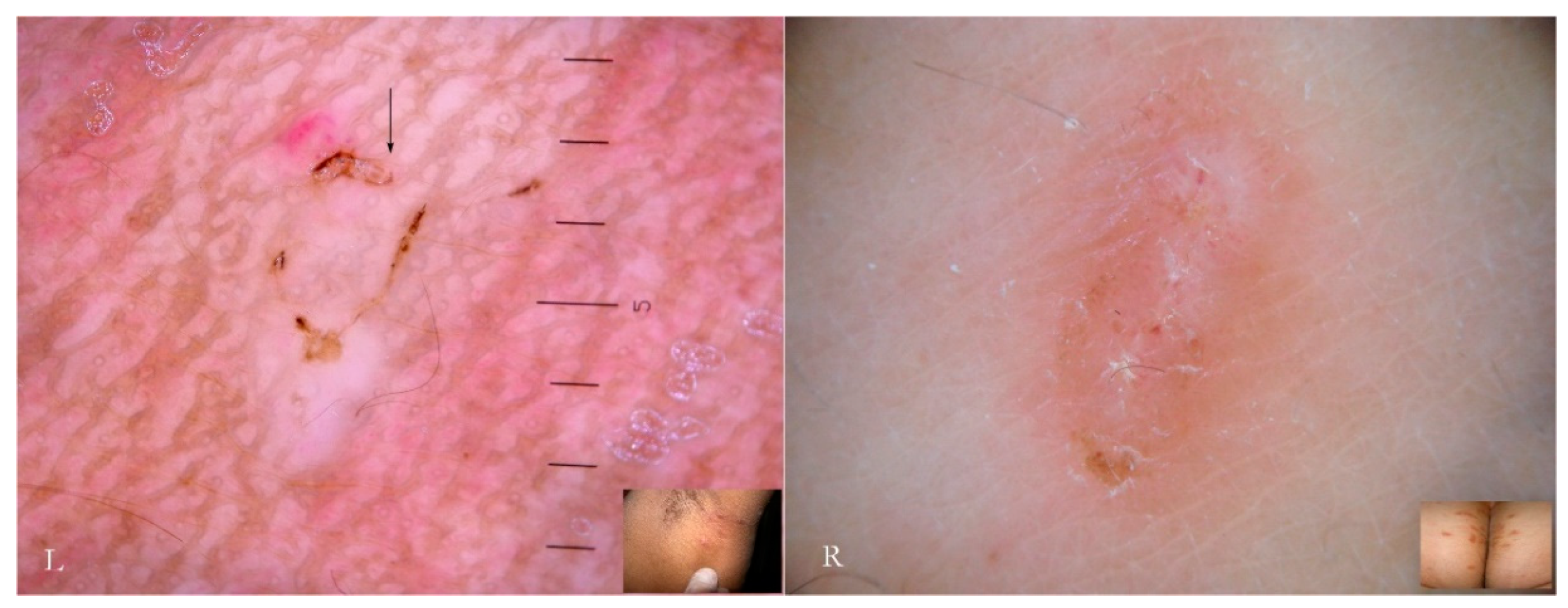
Generally, the burrow of scabies is described as a linear formation a few millimeters in length that has always been considered a unitary structure (
Figure 1).
An intact burrow is a whitish, slightly raised linear structure with a tortuous path a few millimeters long set against a variably erythematous background (circles, L-R). The appearance is pathognomonic. However, scratching that partially or completely destroys it can change its macroscopic and dermatoscopic appearance (arrow, R).
Dermatoscopy reveals different
anatomical and functional sections determined by both the mite life cycle and host epidermal turnover usually not taken into consideration but, none the less, able to affect the morphology of the tunnel itself. The gallery can be divided into three contiguous segments—Head, Body, and Tail—that together provide a new set of diagnostic markers called the Mite-Gallery Unit (MGU) (
Figure 2).
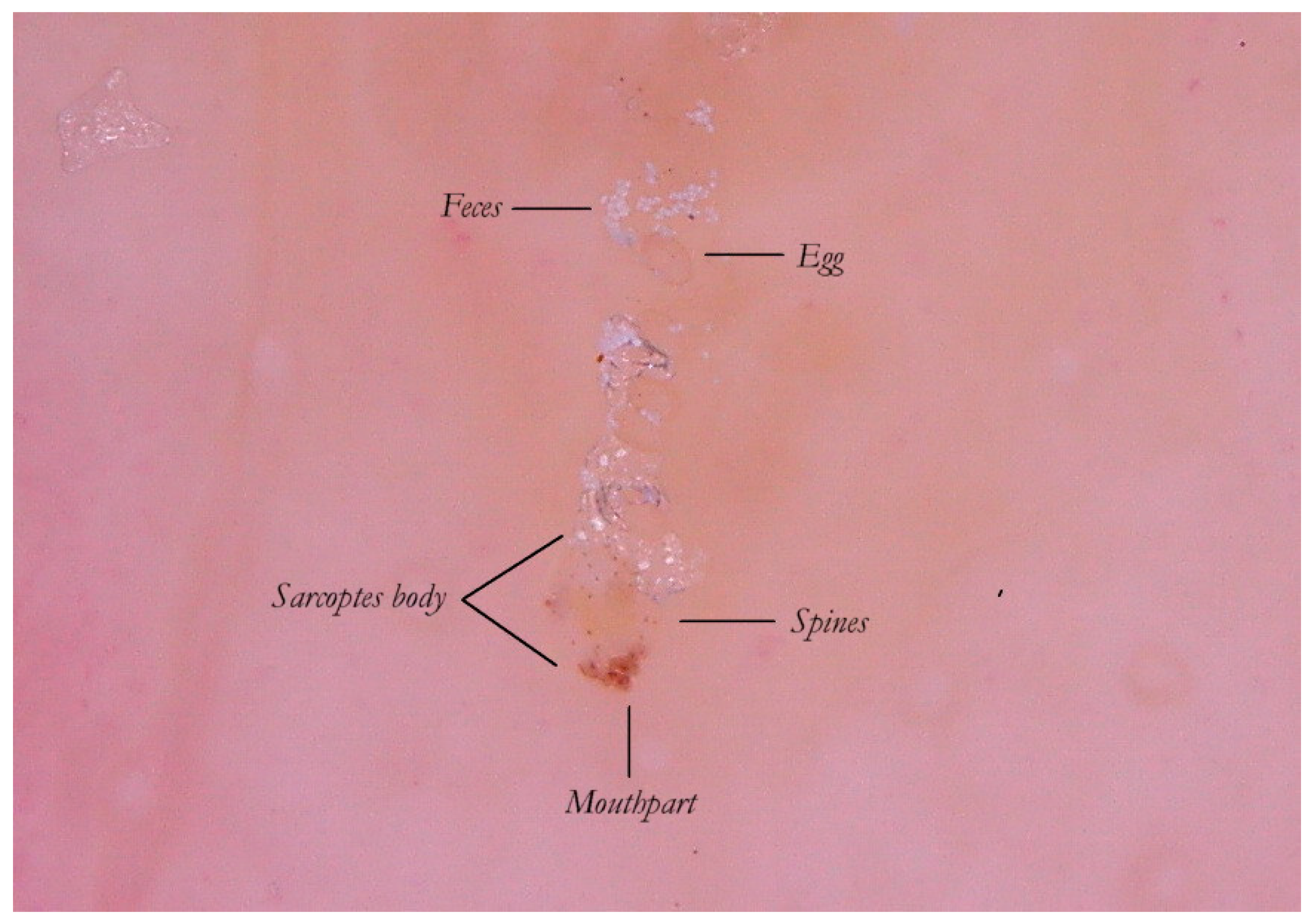
The Mite-Gallery Unit (MGU) structure can be divided into three parts: the Head hosting the mite; the Body, which represents what is clinically defined as the burrow containing the eggs and feces of the parasite; and the Tail at the end of the tunnel, which provides an incomplete structure as it is without a roof but is made of keratin collarettes, visible only in d-DS. Erythema may be present in the background around and immediately behind the mite.
The dermatoscopic observation should preferably be conducted at magnifications between 10 and 30× with different results depending on whether a liquid interface (wet dermatoscopy) or dry skin surface free from the compression of the plate (dry dermatoscopy) are used.
The MGU Head houses the mite which is visible due to its refractile area located anteriorly between the buccal apparatus (gnatosoma) and the second pair of legs. It appears as a dark “V” formation with the vertex that indicates the point of progression of the tunnel towards the healthy skin. In this segment, the roof of the tunnel is intact.
The MGU Body represents the longest part of the entire tunnel and appears white if observed with wet dermatoscopy. In this segment, the roof of the tunnel is no longer intact because it is interrupted by holes at roughly regular intervals.
The MGU Tail is the terminal segment of the tunnel even if the roof is completely absent. This part can only be identified in dry dermatoscopy as this is able to define the edges of the tunnel in the form of small desquamatory collarettes. In w-DS the liquid medium prevents these from being visible, which explains why in previous studies this morphological variation was not reported.
The first description of the tunnel compared its appearance to that of a jet plane with delta wings followed by a white trail as a result of observation under wet dermatoscopy [
5]. The observation of this phenomenon, however, was not accompanied by any functional interpretation. Entodermoscopy by studying the microstructure of the three components of the tunnel explains the “jet trail” effect as the interaction between the liquid interface and air contained in the tunnel that, due to the pressure of the dermatoscope, emerges from the small holes of the roof, forming a strip of small reflective bubbles under the optical plate.
In fact, if dry dermatoscopy is used, the white trail is no longer visible and only a tunnel can be observed whose roof is interrupted by several openings from which air escapes if compressed (
Figure 3).
When viewed using d-DS, the roof of the tunnel appears to be interrupted by several holes that allow air to exit (L). The first description of an MGU resembling a white jet trail was due to trapped air between the tunnel and the glass plate used in the w-DS (C). Under continued pressure, this picture disappears as all of air bubbles are pushed away and the liquid medium passes inside (R). Beneath there is a collection of serum that reflects the LEDs of the dermatoscope (L).
Another consequence of these holes, not previously described in the literature, is that the microbubble trail is by no means a persistent sign as it disappears when MGU observation is repeated several times or for excessive time in the wet modality. After the first contact with the dermatoscope, all the air inside the tunnel will be largely dispersed on the surface and replaced by the interface fluid; this makes the roof indistinguishable from the surrounding skin (
Figure 3 R). In this situation, the tunnel body can only be recognized because of the head that houses the
Sarcoptes, reducing the local diagnostic sensitivity which relies on the tunnel’s refractability. Therefore, in order to obtain good photographic documentation in w-DS of an intact MGU, it is necessary to keep the microbubbles under the plate without breaking off skin contact and to avoid repeating the viewing session. However, if air leakage decreases the dermatoscopic sensitivity as regards the external structure of the MGU, it provides ideal conditions to better observe the internal components of the tunnel (local diagnostic specificity).
3.2. Content of the Gallery and Other Markers of the Mite
Thanks to the higher magnifications (e-DS) and to the fact that the roof of the tunnel in w-DS becomes transparent when liquid medium is inside, it is possible to distinguish, with sufficient clarity, the eggs of the
Sarcoptes located behind the mite. The oval eggs are grouped at a short distance from each other with a major axis that is generally at right angles to that of the MGU, which also allows the edges of the embryo inside to be glimpsed, if enlarged further. In this part of the gallery there are also the parasite’s feces derived by the digestion of keratin and cellular fluids; under the optical microscope, feces usually appear as dark refractile dots (stercoraceous bullets), whereas under dermatoscopy, they appear as small white-gray spheres (
Figure 4).
The absence of bubbles and the penetration of the liquid film into the tunnel makes the roof transparent and allows one to glimpse the row of eggs, which is otherwise not easily visible. The white dots are the feces of the mite. A seropurulent exudate is located to the left.
If the examiner is interested in studying the inside of the tunnel, he/she must use wet dermatoscopy and make sure that all the air in the tunnel is extruded, pushing the dermatoscope plate several times onto the skin to ensure entry of the liquid medium.
It is commonly thought that the only visible part of the mite in w-DS is the front end, in the shape of a “V” which corresponds to the
gnatosoma and the first pair of legs. But enhanced dermatoscopy allows the identification of novel structures on the body of the
Sarcoptes scabiei that, under conditions of optimal magnification, show some visible refraction. These are mechano-sensory structures [
6] located on the back of the mite called “bristles” (
spine-like type) whose thicker bases (
sockets) appear under a dermatoscope as fine brown dots (
ladybird sign). This feature, not yet reported in the literature, is a good example of convergence between dermatoscopy and entomology. Even the body of the mite, which is usually transparent, can be distinguished by the slight opalescence of its cuticle under enhanced wet dermatoscopy (
Figure 5).
3.3. Spatio-Temporal Evolution of the Mite-Gallery Unit
The MGU is not a static structure. This concept is not usually explored further because it is irrelevant to clinical diagnosis. On the contrary, patterns change if we access the microscopic details that are appreciable only in situ and in vivo in the context of the entodermoscopy.
The MGU is a dynamic structure because its morphology derives from a reciprocal interference of two different movements: the horizontal one of the mite and the vertical one of the epidermis. The epidermis has an estimated turnover time of around 45 days [
7] during which the cellular layers are pushed upwards, ending with desquamation. In the presence of local inflammation (e.g., psoriasis) the upwards growth rate is greatly accelerated.
Histologically, the scabies burrow was always thought to be allocated in the
stratum corneum even though confocal microscopy has recently revealed that the
Sarcoptes mite is located closer to the
stratum spinosum [
8].
The integrity of the tunnel is therefore subordinate to the mite’s ability to maintain the same height in the epidermis to resist the tangential (rubbing) and elastic (contraction and distension) forces that act on the skin during the natural movements of the human body. The mite must out of necessity compensate for the direction of growth of the epidermis [
9] in order to protected itself, but especially to ensure that the eggs can hatch just inside the tunnel. It follows that the tunnel represents two different time sequences depending on the point being observed, with the head being the newest part and the tail the initial and oldest part.
Therefore, as a dynamic structure the gallery moves closer to the skin surface with the passing of time and is at its most superficial at the tail where the roof becomes thin enough to collapse under the effect of the forces acting on it [
10]. For this reason, near the tail there is no longer an intact tunnel but, rather, the borders of its base as collarettes visible only with dry dermatoscopy. Entodermoscopy, through linking the local factors of the host to the biology of the parasite, highlights an anatomic and functional difference of the MGU not previously appreciated under conditions of ordinary optical microscopy which cannot provide a detailed view of the mite’s whole microenvironment. Dermatoscopy instead reveals that the MGU is a dynamic entity in terms of both space (elongation phase) and time (maturation phase).
The collapsed roof is the result of an autonomous phenomenon and cannot be attributed to the scratching by the host as that would also destroy the other parts of the gallery rather than a very small part of it (the tail). This is further confirmed by the fact that the skin immediately around and adjacent to the tail remains intact (
Figure 2).
3.5. Moulting and Coupling Pockets
The greater part of the MGU consists of the tunnel body where the most important events of the mite life cycle take place. In this segment, eggs are collected and hatch. After about four days, these eggs produce six-legged larvae morphologically similar to a miniature adult [
12]. The larvae must undergo two more moults to the eight legged stage (proto/trito nymph) before becoming an adult; this process appears to take place mainly outside the mother tunnel in small niches, called “moulting pockets”, dug in order to provide limited shelter. Only when fully developed and after fertilization does the female mite dig a new definitive tunnel as it is known in temperate climates [
13].
The moulting pockets, well known to entomologists, present as small dimples in the most superficial layer of the
stratum corneum; in these, the larvae remain attached, maturing into nymphs and then into the adult [
12].
Mating is also performed in a pocket in which the adult female is found by a male whose track is lost afterwards. Normally, these niches are not described in dermatological textbooks because they have no diagnostic relevance and are of dimensions invisible to the naked eye. However, their significance increases if we return to dermatoscopic examination in which they appear as an incomplete MGU composed only of a head without a body and a tail (
Figure 7), regardless of the dermatoscope used. The preferred mode of observation is still the d-DS with normal or polarized light.
According to another report, the immature forms, besides digging a niche or pocket from scratch, can also take refuge in the openings of hair follicles [
14]. A rash of pseudo-folliculitis is traditionally considered to be a secondary, non-specific symptom in the course of scabies (
Figure 8). Assuming that the juvenile mite may shelter in human follicles, too, then the erythematous change associated with them may be regarded as a new “specific” sign. At the moment, personal observations and those of other authors have not been able to identify any traces of immature forms as features of in vivo refraction in these forms are unknown. It is possible that under higher magnification (>200×) these forms may become appreciable if present and visible.
3.6. Inflammatory Response Induced By the MGU
In a primary infestation, the host’s immunity to scabies develops after about four weeks. If we consider only the skin around the MGU, using dermatoscopy we can distinguish different types of response over time and depending on the anatomical sites involved. Generally, there is erythema around the head of the gallery where the mite is located or along the entire length of the MGU.
A vesicular or free exudate appears when the spongiotic inflammatory process [
15] reaches the skin surface. If an infection coexists, this phenomenon is more pronounced. Where the skin is thicker, as on the palm of the hand and on the wrist, the exudate remains trapped in the superficial epidermis, forming semitransparent (
dyshidrotic-like) circular areas [
16] that, over time, become superficial as yellowish desquamations (
Figure 9). The mite, the feces, and the eggs activate a different humoral and cellular immune response, probably depending on the time of exposure to the antigens that gradually accumulate over the course of the infestation. This reflects the life cycle of the mite.
In other cases, the inflammatory response may be nodular as in the buttocks, genital, and flexural regions. This phenomenon, known for its resistant pruritic symptoms, is interpreted as the effect of cell-mediated hyperreactivity to mite antigens [
17] even if scrapings for mites are generally negative. Dermatoscopy, however, can identify the parasite, especially in the initial phases of the formation of nodules compared to the older lesions (
Figure 10). Serial dermatoscopic observations could clarify the exact progression of the host’s local immune response, probably stimulated by deeper penetration of antigens (intradermal) in areas undergoing physical pressure.