Spatiotemporal and Socioeconomic Risk Factors for Dengue at the Province Level in Vietnam, 2013–2015: Clustering Analysis and Regression Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
- (1)
- Housing pressure: data on total population per housing area was not available, and so a ratio of ‘new’ people to ‘new’ housing was constructed using two datasets:
- inter-annual change in provincial population (numerator);
- square metres of new housing built (denominator).
- (2)
- Data on the percentage of households accessing electricity was only available in two-year intervals, so the mean of the year before and after was taken for inter-year values.
2.2. Study Design
2.2.1. Spatial Autocorrelation and Cluster Analysis
2.2.2. Regression Analysis
Model
Variables
- Factors likely to influence opportunities for human-vector contact: housing pressure (‘new’ people per new m² housing per annum), the percentage of provincial populations living in urban areas [19], population density (people per km²), and population mobility (million-person-km travelled each year) [20];
- Indicators of socioeconomic status: percentage of households accessing electricity and poverty rate (percentage of people with household income below the province-adjusted poverty line) [21];
- Indicators of healthcare access: clinicians per 1000 people and percentage of children under one year vaccinated. Note: vaccination rate is not an indicator for dengue vaccination as this was not available during the period of interest but is instead used a proxy indicator for healthcare access overall;
- A number of other socioeconomic variables, such as age profile and water access, were considered but data was unfortunately not available for this period. Definitions, units, rationale, and source of socioeconomic exposures are in supplementary materials.
- A province’s own case count the previous year;
- A province’s own case count two years previous;
- A province’s first and second order queen contiguous neighbours’ average case count the previous year;
- A province’s first and second order queen contiguous neighbours’ average case count two years previous.
Model Development and Testing Goodness of Fit
3. Results
3.1. Distribution of Dengue Case Counts
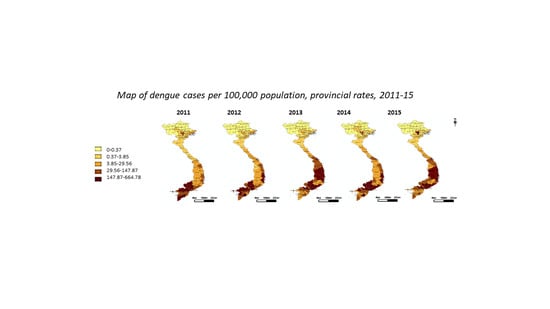
3.2. Spatiotemporal Trends, Clusters and Outliers
3.3. Regression Analysis
- Bivariate fixed-effects model with mobility and average dengue rates in first order contiguous neighbours two years previous, which showed that an increase of one million-person-km travelled indicates a decrease of one case in the year of interest (p = 0.022), and an increase of one case per 100,000 population averaged across first order contiguous neighbours two years previous indicates an increase of one case in the year of interest (p = 0.038). A two-year lag was selected on the basis of previous research indicating a two-year cycle of dengue prevalence may be present [3];
- A bivariate fixed-effects model with mobility and average dengue rates in second order contiguous neighbours two years previous, which showed that an increase of one million-person-km travelled indicates a decrease of one case in the year of interest (p = 0.033), and an increase of one case per 100,000 population averaged across second order contiguous neighbours two years previous indicates an increase of one case in the year of interest (p = 0.05);
- A univariate model with interacting terms taking a multiplication of mobility with dengue rates in the province of interest the previous year, which showed that an increase of one unit of (million-person-km travelled multiplied by dengue rates in the province of interest the previous year) indicates a decrease of one case in the year of interest (p = 0.017).
3.4. Testing Goodness of Fit and Link Function
4. Discussion
4.1. Discussion of Findings
4.2. Limitations
4.2.1. Modelling Limitations
4.2.2. Data Limitations
4.2.3. Spatiotemporal Scale Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization Dengue in Vietnam. Available online: https://www.who.int/vietnam/health-topics/dengue (accessed on 2 April 2020).
- Vietnam General Statistics Office Population and Area Data by Province. Available online: https://www.gso.gov.vn/default_en.aspx?tabid=774 (accessed on 5 April 2020).
- Lee, H.; Nguyen-Viet, H. Seasonal patterns of dengue fever and associated climate factors in 4 provinces in Vietnam from 1994 to 2013. BMC Infect. Dis. 2017, 17, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, H.; Doan, H. Ecological factors associated with dengue fever in a central Highlands province, Vietnam. BMC Infect. Dis. 2011, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Bett, B.; Grace, D. Spatiotemporal analysis of historical records (2001–2012) on dengue fever in Vietnam and development of a statistical model for forecasting risk. PLoS ONE 2019, 14, e0224353. [Google Scholar] [CrossRef] [PubMed]
- Phung, D.; Huang, C. Identification of the prediction model for dengue incidence in Can Tho city, a Mekong Delta area in Vietnam. Acta Trop. 2015, 141, 88–96. [Google Scholar] [PubMed]
- Xuan, L.T.T.; Hau, P.V.; Thu, D.T.; Toan, D.T.T. Estimates of meteorological variability in association with dengue cases in a coastal city in Northern Vietnam: An ecological study. Glob. Health Action 2013, 7, 1–7. [Google Scholar] [CrossRef]
- Phuong, L.T.D.; Hanh, T.T.T. Climate variability and dengue hemorrhagic fever in Ba Tri District, Ben Tre Province, Vietnam during 2004–2014. AIMS Public Health 2016, 3, 769–780. [Google Scholar] [CrossRef]
- Schmidt, W.P.; Suzuki, M. Population density, water supply, and the risk of dengue fever in Vietnam: Cohort study and spatial analysis. PLoS Med. 2011, 8, e1001082. [Google Scholar] [CrossRef]
- Chareonsook, O.; Foy, H.M. Changing epidemiology of dengue hemorrhagic fever in Thailand. Epidemiol. Infect. 1999, 122, 161–166. [Google Scholar] [CrossRef]
- Wijayanti, S.P.M.; Porphyre, T. The importance of socio-economic versus environmental risk factors for reported dengue cases in Java, Indonesia. PLoS Negl. Trop. Dis. 2016, 10, e0004964. [Google Scholar] [CrossRef]
- Qi, X.; Wang, Y. The effects of socioeconomic and environmental factors on the incidence of dengue fever in the Pearl river delta, China, 2013. PLoS Negl. Trop. Dis. 2015, 9, e0004159. [Google Scholar] [CrossRef]
- Cuong, H.Q.; Salje, H.; Rodriguez-Barraquer, I.; Yoon, I.-K.; Vinh, C.N.V.; Thanh, H.N.; Manh, T.H.; Trong, L.P.; Bridget, W.; Ananda, N.; et al. Synchrony of dengue incidence in Ho Chi Minh City and Bangkok. PLoS Negl. Trop. Dis. 2016, 11, e0005188. [Google Scholar]
- Cuong, H.; Vu, N. Spatiotemporal dynamics of dengue epidemics, southern Vietnam. Emerg. Infect. Dis. 2013, 19, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Lauer, S.A.; Sakrejda, K.; Ray, E.L.; Keegan, L.T.; Bi, Q.; Suangtho, P.; Hinjoy, S.; Iamsirithaworn, S.; Suthachana, S.; Laosiritaworn, Y.; et al. Prospective annual Thai dengue incidence forecasts. Proc. Natl. Acad. Sci. USA 2018, 115, E2175–E2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Department of Preventive Medicine. Communicable Diseases Yearbook from 1994 to 2015; Ministry of Health: Hanoi, Vietnam, 2016.
- Casenghi, M.; Kosack, C.; Li, R.; Bastard, M.; Ford, N. NS1 antigen detecting assays for diagnosing acute dengue infection in people living in or returning from endemic countries. Cochrane Database Syst. Rev. 2018, 2018. [Google Scholar] [CrossRef]
- Global Administrative Database. Available online: https://www.gadm.org (accessed on 10 April 2019).
- Vietnam National Statistics. Available online: https://www.gso.gov.vn/danhmuc/HTCT_tinh.aspx?ma_nhom=T0102a01 (accessed on 19 August 2019).
- Eritja, R.; Palmer, J.R.B. Direct evidence of adult Aedes albopictus dispersal by car. Sci. Rep. 2017, 7, 14399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulligan, K.; Dixon, J. Is dengue a disease of poverty? A systematic review. Pathog. Glob. Health 2015, 109, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Historical El Niño/La Niña Episodes (1950–Present). Available online: https://www.cpc.ncep.noaa.gov/products/analysis_monitoring/ensostuff/ensoyears.shtml (accessed on 8 August 2019).
- Thai, K.T.D.; Nagelkerke, N. Geographical heterogeneity of dengue transmission in two villages in southern Vietnam. Epidemiol. Infect. 2010, 138, 585–591. [Google Scholar] [CrossRef] [Green Version]
- Chikaki, E.; Ishikawa, H. A dengue transmission model in Thailand considering sequential infections with all four serotypes. J. Infect. Dev. Ctries. 2009, 3, 711–722. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.C.; Pavlicova, M. Zero-Inflated and hurdle models of count data with extra zeros: Examples from an HIV-risk reduction intervention trial. Am. J. Drug Alcohol Abus. 2011, 37, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Cuong, H.Q.; Hien, N.T.; Duong, T.N.; Phong, T.V.; Cam, N.N.; Farrar, J.; Nam, V.S.; Thai, K.T.; Horby, P. Quantifying the emergence of Dengue in Hanoi, Vietnam: 1998–2009. PLoS Negl. Trop. Dis. 2011, 5, e1322. [Google Scholar] [CrossRef]
- Lowe, R.; Coelho, C.A.; Barcellos, C.; Carvalho, M.S.; Catão Rde, C.; Coelho, G.E.; Ramalho, W.M.; Bailey, T.C.; Stephenson, D.B.; Rodó, X. Evaluating probabilistic dengue risk forecasts from a prototype early warning system for Brazil. eLife 2016, 5, e11285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummings, D.A.; Iamsirithaworn, S. The impact of the demographic transition on dengue in Thailand: Insights from a statistical analysis and mathematical modelling. PLoS Med. 2009, 6, e1000139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.T.; Rossi, S. Dengue epidemiology in selected endemic countries: Factors influencing expansion factors as estimates of underreporting. Trop. Med. Int. Health 2015, 20, 840–863. [Google Scholar]
- Delmelle, E.; Hagenlocherb, M. A spatial model of socioeconomic and environmental determinants of dengue fever in Cali, Colombia. Acta Trop. 2016, 164, 169–176. [Google Scholar] [CrossRef] [PubMed]




| Annual Dengue Rates, All Provinces, 2011–2015 | |
|---|---|
| Arithmetic mean | 66.41 |
| Maximum | 731.86 |
| Minimum | 0 |
| Standard deviation | 106.42 |
| Coefficient of variation | 1.60 |
| Regression Results | ||||||
|---|---|---|---|---|---|---|
| Model | Wald Chi p Value | Coefficient (Exponentiated) | z or t Test p Value | Constant/Intercept | BIC Number | |
| 1 | 0.02 | Mobility | −1.00 | 0.02 | −13.54 | 1419.83 |
| 2 | 0.0075 | Mobility | −1.00 | 0.022 | −13.71 | 1420.88 |
| First order neighbours two years previous | 1.00 | 0.038 | ||||
| 3 | 0.0093 | Mobility | −1.00 | 0.033 | −13.73 | 1421.30 |
| Second order neighbours two years previous | 1.00 | 0.05 | ||||
| 4 | 0.017 | Mobility | −1.00 | 0.017 | −13.63 | 1417.95 |
| Model | Predicted vs. Actual Summary Statistics (Provincial Dengue Case Counts, 2013–2015) | Pregibon Test p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | Standard Deviation | ||||||
| Predicted | Actual | Predicted | Actual | Predicted | Actual | Predicted | Actual | ||
| 1 | 0.2 | 0 | 9.39 | 5610 | 1.3 | 43.27 | 1.7 | 159.96 | 0.0006 |
| 2 | 0.25 | 9.49 | 1.27 | 1.72 | 0.0002 | ||||
| 3 | 0.22 | 8.5 | 1.27 | 1.72 | 0.0005 | ||||
| 4 | 0.37 | 9.03 | 1.3 | 1.68 | 0.0001 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashmore, P.; Lindahl, J.F.; Colón-González, F.J.; Sinh Nam, V.; Quang Tan, D.; Medley, G.F. Spatiotemporal and Socioeconomic Risk Factors for Dengue at the Province Level in Vietnam, 2013–2015: Clustering Analysis and Regression Model. Trop. Med. Infect. Dis. 2020, 5, 81. https://doi.org/10.3390/tropicalmed5020081
Ashmore P, Lindahl JF, Colón-González FJ, Sinh Nam V, Quang Tan D, Medley GF. Spatiotemporal and Socioeconomic Risk Factors for Dengue at the Province Level in Vietnam, 2013–2015: Clustering Analysis and Regression Model. Tropical Medicine and Infectious Disease. 2020; 5(2):81. https://doi.org/10.3390/tropicalmed5020081
Chicago/Turabian StyleAshmore, Polly, Johanna F. Lindahl, Felipe J. Colón-González, Vu Sinh Nam, Dang Quang Tan, and Graham F. Medley. 2020. "Spatiotemporal and Socioeconomic Risk Factors for Dengue at the Province Level in Vietnam, 2013–2015: Clustering Analysis and Regression Model" Tropical Medicine and Infectious Disease 5, no. 2: 81. https://doi.org/10.3390/tropicalmed5020081
APA StyleAshmore, P., Lindahl, J. F., Colón-González, F. J., Sinh Nam, V., Quang Tan, D., & Medley, G. F. (2020). Spatiotemporal and Socioeconomic Risk Factors for Dengue at the Province Level in Vietnam, 2013–2015: Clustering Analysis and Regression Model. Tropical Medicine and Infectious Disease, 5(2), 81. https://doi.org/10.3390/tropicalmed5020081