Combined Tuberculosis and Diabetes Mellitus Screening and Assessment of Glycaemic Control among Household Contacts of Tuberculosis Patients in Yangon, Myanmar
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Sample
2.3. Sample Size Calculation
2.4. Data Collection Tools
2.5. Diagnosis of DM in Index TB Patients and Household Contacts
2.6. Diagnosis of TB among Household Contacts
2.7. Data Collection Procedure
2.8. Data Analysis
2.9. Ethical Approval
3. Results
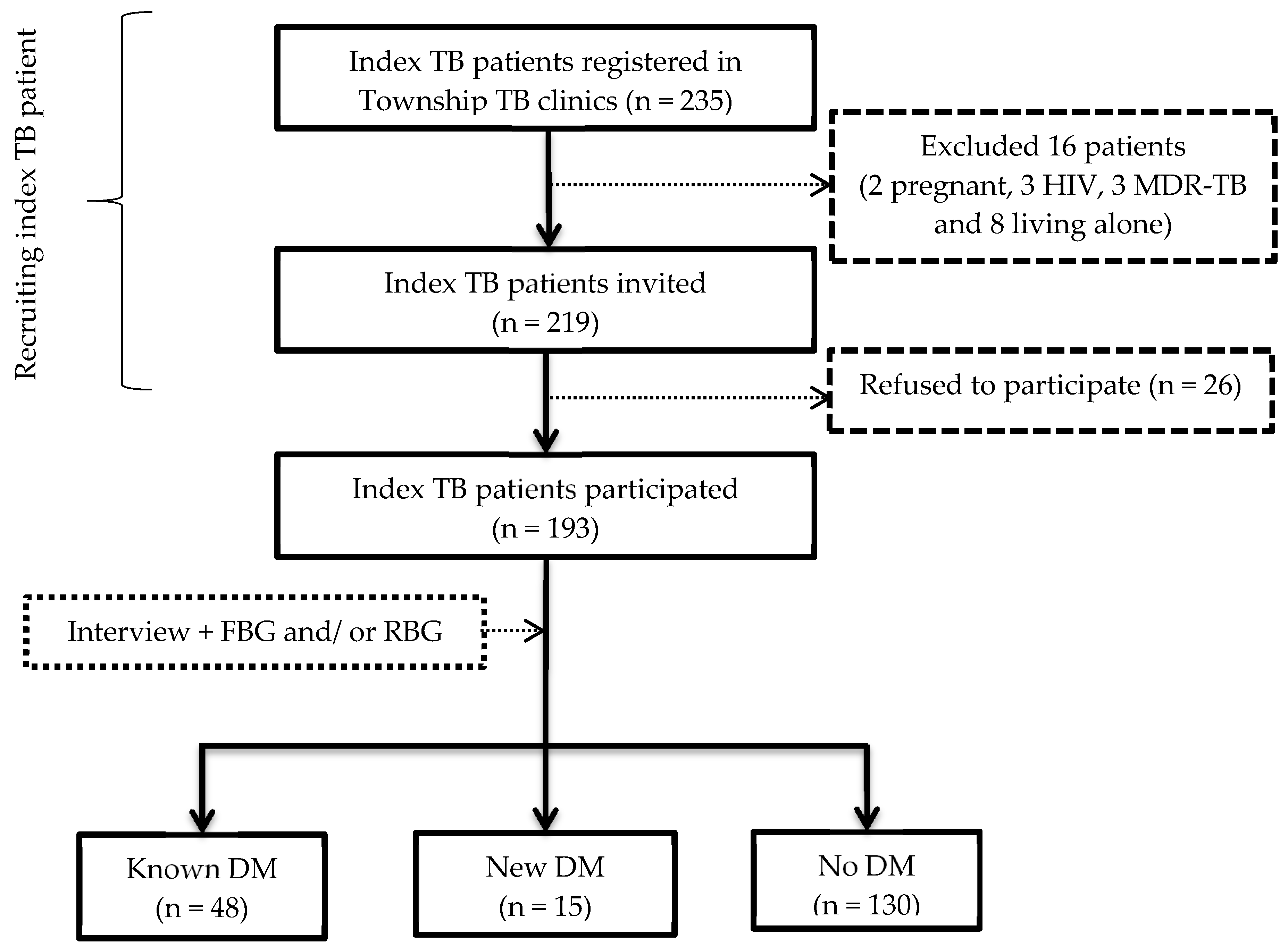
3.1. Investigation for DM among Index TB Patients
Prevalence of DM among Index TB Patients
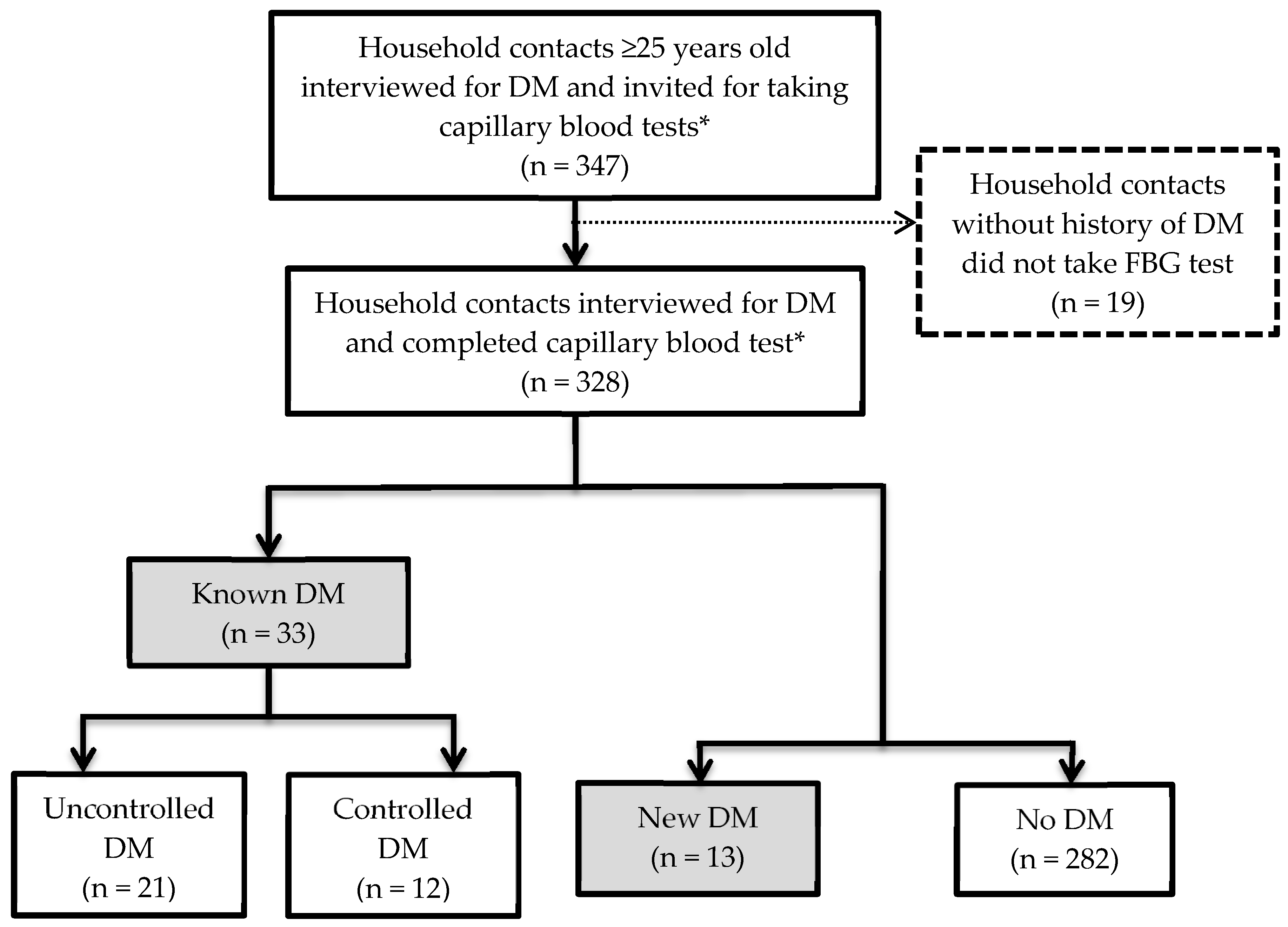
3.2. Prevalence and Glycaemic Control of DM among Household Contacts
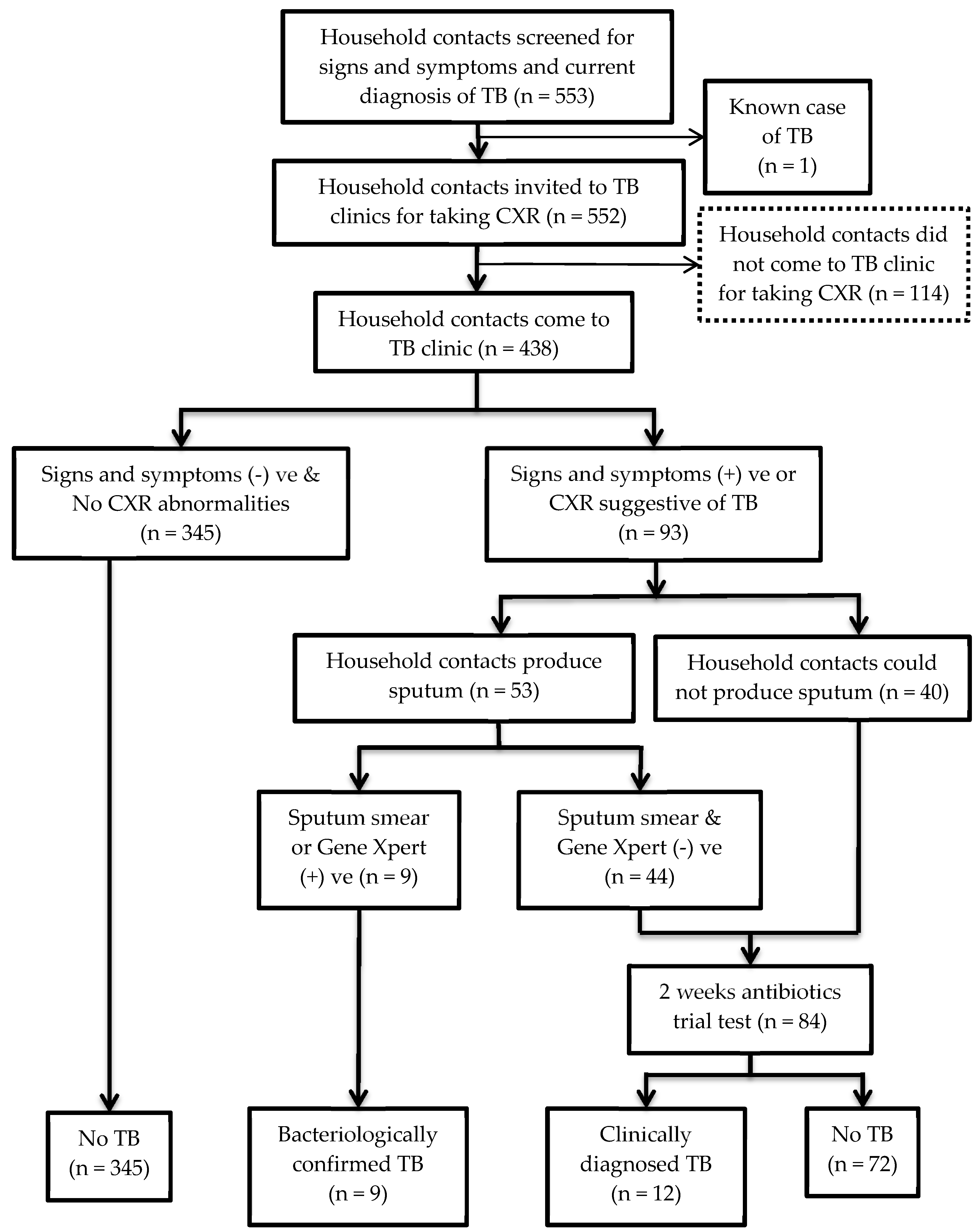
3.3. Prevalence of TB among Household Contacts
3.4. Comparing Prevalence of DM and TB among Household Contacts of Index TB Patients with and without DM
3.5. Number of Patients with DM among Household Contacts ≥ 25 Years Old
3.6. Odds Ratio of Getting DM and TB among Household Contacts Based on DM Status of Index TB Patients
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Girardi, E.; Sañé Schepisi, M.; Goletti, D.; Bates, M.; Mwaba, P.; Yeboah-Manu, D.; Ntoumi, F.; Palmieri, F.; Maeurer, M.; Zumla, A.; et al. The global dynamics of diabetes and tuberculosis: The impact of migration and policy implications. Int. J. Infect. Dis. 2017, 56, 45–53. [Google Scholar] [CrossRef]
- Jeon, C.Y.; Murray, M.B. Diabetes Mellitus Increases the Risk of Active Tuberculosis: A Systematic Review of 13 Observational Studies. PLoS Med. 2008, 5, e152. [Google Scholar] [CrossRef]
- Pan, S.-C.; Ku, C.-C.; Kao, D.; Ezzati, M.; Fang, C.-T.; Lin, H.-H. Effect of diabetes on tuberculosis control in 13 countries with high tuberculosis: A modelling study. Lancet Diabetes Endocrinol. 2015, 3, 323–330. [Google Scholar] [CrossRef]
- World Health Organization. World Health Organization Global Tuberculosis Report 2019; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-156571-4. [Google Scholar]
- Lönnroth, K.; Roglic, G.; Harries, A.D. Improving tuberculosis prevention and care through addressing the global diabetes epidemic: From evidence to policy and practice. Lancet Diabetes Endocrinol. 2014, 2, 730–739. [Google Scholar] [CrossRef]
- Yangon Region Plans Two-Year Campaign against Tuberculosis. Available online: https://www.mmtimes.com/news/yangon-region-plans-two-year-campaign-against-tuberculosis.html (accessed on 26 May 2020).
- Incidence of Tuberculosis (per 100,000 People) Data. Available online: https://data.worldbank.org/indicator/SH.TBS.INCD (accessed on 26 May 2020).
- Aung, W.P.; Htet, A.S.; Bjertness, E.; Stigum, H.; Chongsuvivatwong, V.; Kjøllesdal, M.K.R. Urban–rural differences in the prevalence of diabetes mellitus among 25–74 year-old adults of the Yangon Region, Myanmar: Two cross-sectional studies. BMJ Open 2018, 8, e020406. [Google Scholar] [CrossRef]
- International Diabetes Federation. Diabetes Atlas—2017 Atlas. Available online: https://diabetesatlas.org/resources/2017-atlas.html (accessed on 30 October 2019).
- Baker, M.A.; Lin, H.-H.; Chang, H.-Y.; Murray, M.B. The Risk of Tuberculosis Disease among Persons with Diabetes Mellitus: A Prospective Cohort Study. Clin. Infect. Dis. 2012, 54, 818–825. [Google Scholar] [CrossRef]
- Morrison, J.; Pai, M.; Hopewell, P.C. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: A systematic review and meta-analysis. Lancet Infect. Dis. 2008, 8, 359–368. [Google Scholar] [CrossRef]
- Hemminki, K.; Li, X.; Sundquist, K.; Sundquist, J. Familial Risks for Type 2 Diabetes in Sweden. Diabetes Care 2010, 33, 293–297. [Google Scholar] [CrossRef]
- Khan, A.; Lasker, S.S.; Chowdhury, T.A. Are Spouses of Patients with Type 2 Diabetes at Increased Risk of Developing Diabetes? Diabetes Care 2003, 26, 710–712. [Google Scholar] [CrossRef]
- Nielsen, J.; Bahendeka, S.K.; Whyte, S.R.; Meyrowitsch, D.W.; Bygbjerg, I.C.; Witte, D.R. Household and familial resemblance in risk factors for type 2 diabetes and related cardiometabolic diseases in rural Uganda: A cross-sectional community sample. BMJ Open 2017, 7. [Google Scholar] [CrossRef]
- Viswanathan, V.; Kumpatla, S.; Aravindalochanan, V.; Rajan, R.; Chinnasamy, C.; Srinivasan, R.; Selvam, J.M.; Kapur, A. Prevalence of Diabetes and Pre-Diabetes and Associated Risk Factors among Tuberculosis Patients in India. PLoS ONE 2012, 7, e41367. [Google Scholar] [CrossRef]
- Aye, S.; Majumdar, S.S.; Oo, M.M.; Tripathy, J.P.; Satyanarayana, S.; Kyaw, N.T.T.; Kyaw, K.W.Y.; Oo, N.L.; Thein, S.; Thu, M.K. Evaluation of a tuberculosis active case finding project in peri-urban areas, Myanmar: 2014–2016. Int. J. Infect. Dis. 2018, 70, 93–100. [Google Scholar] [CrossRef]
- Kyaw Soe, T.; Soe, K.T.; Satyanarayana, S.; Saw, S.; San, C.C.; Aung, S.T. Gaps in Implementing Bidirectional Screening for Tuberculosis and Diabetes Mellitus in Myanmar: An Operational Research Study. Trop. Med. Infect. Dis. 2020, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-H.; Fu, H.; Lai, T.-C.; Chiang, C.-Y.; Chan, C.-C.; Lin, H.-H. Glycemic Control and the Risk of Tuberculosis: A Cohort Study. PLoS Med. 2016, 13, e1002072. [Google Scholar] [CrossRef] [PubMed]
- Annual Report 2016—National Tuberculosis Programme; Ministry of Health and Sports: Nay Pyi Taw, Myanmar, 2016.
- Takasu, N.; Yamada, T.; Miura, H.; Sakamoto, S.; Korenaga, M.; Nakajima, K.; Kanayama, M. Rifampicin-induced Early Phase Hyperglycemia in Humans. Am. Rev. Respir. Dis. 1982, 125, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Ben-Haroush, A.; Yogev, Y.; Hod, M. Epidemiology of gestational diabetes mellitus and its association with Type 2 diabetes. Diabet. Med. 2004, 21, 103–113. [Google Scholar] [CrossRef]
- Lin, S.P.; Wu, C.-Y.; Wang, C.-B.; Li, T.-C.; Ko, N.-Y.; Shi, Z.-Y. Risk of diabetes mellitus in HIV-infected patients receiving highly active antiretroviral therapy: A nationwide population-based study. Medicine 2018, 97, e12268. [Google Scholar] [CrossRef]
- Liu, Q.; Li, W.; Xue, M.; Chen, Y.; Du, X.; Wang, C.; Han, L.; Tang, Y.; Feng, Y.; Tao, C.; et al. Diabetes mellitus and the risk of multidrug resistant tuberculosis: A meta-analysis. Sci. Rep. 2017, 7, 1090. [Google Scholar] [CrossRef]
- Feltbower, R.G.; McKinney, P.A.; Campbell, F.M.; Stephenson, C.R.; Bodansky, H.J. Type 2 and other forms of diabetes in 0–30 year olds: A hospital based study in Leeds, UK. Arch. Dis. Child. 2003, 88, 676–679. [Google Scholar] [CrossRef]
- Htet, K.K.K.; Liabsuetrakul, T.; Thein, S.; McNeil, E.B.; Chongsuvivatwong, V. Improving detection of tuberculosis among household contacts of index tuberculosis patients by an integrated approach in Myanmar: A cross-sectional study. BMC Infect. Dis. 2018, 18. [Google Scholar] [CrossRef]
- World Health Organization NCDs STEPS Manual. Available online: http://www.who.int/ncds/surveillance/steps/manual/en/ (accessed on 25 June 2019).
- Ferreira, F.H.G.; Chen, S.; Dabalen, A.L.; Dikhanov, Y.M.; Hamadeh, N.; Jolliffe, D.M.; Narayan, A.; Prydz, E.B.; Revenga, A.L.; Sangraula, P. A Global Count of the Extreme Poor in 2012: Data Issues, Methodology and Initial Results; The World Bank: Washington, DC, USA, 2015; pp. 1–66. [Google Scholar]
- World Health Organization. NCDs STEP Wise Approach to Chronic Disease Risk Factor Surveillance. Available online: http://www.who.int/ncds/surveillance/steps/myanmar/en/ (accessed on 21 May 2019).
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Available online: http://www.who.int/entity/nutrition/publications/obesity/WHO_TRS_894/en/index.html (accessed on 23 June 2019).
- World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation, GENEVA, 8–11 December 2008; World Health Organization: Geneva, Switzerland, 2011; ISBN 978-92-4-150149-1. [Google Scholar]
- Accu-Chek Performa. Available online: https://www.accu-chek.com.au/meter-systems/performa (accessed on 21 May 2019).
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019, 42, S61–S70. [Google Scholar] [CrossRef]
- World Health Organization. Chest Radiography in Tuberculosis Detection: Summary of Current WHO Recommendations and Guidance on Programmatic Approaches; World Health Organization: Geneva, Switzerland, 2016; ISBN 978-92-4-151150-6. [Google Scholar]
- Soh, A.Z.; Chee, C.B.E.; Wang, Y.-T.; Yuan, J.-M.; Koh, W.-P. Alcohol drinking and cigarette smoking in relation to risk of active tuberculosis: Prospective cohort study. BMJ Open Respir. Res. 2017, 4, e000247. [Google Scholar] [CrossRef]
- Acuña-Villaorduñ, C.; Jones-López, E.C.; Fregona, G.; Marques-Rodrigues, P.; Gaeddert, M.; Geadas, C.; Hadad, D.J.; White, L.F.; Molina, L.P.D.; Vinhas, S.; et al. Intensity of exposure to pulmonary tuberculosis determines risk of tuberculosis infection and disease. Eur. Respir. J. 2018, 51. [Google Scholar] [CrossRef]
- Triasih, R.; Rutherford, M.; Lestari, T.; Utarini, A.; Robertson, C.F.; Graham, S.M. Contact Investigation of Children Exposed to Tuberculosis in South East Asia: A Systematic Review. J. Trop. Med. 2012. [Google Scholar] [CrossRef]
- Kigozi, N.G.; Heunis, J.C.; Engelbrecht, M.C. Yield of systematic household contact investigation for tuberculosis in a high-burden metropolitan district of South Africa. BMC Public Health 2019, 19. [Google Scholar] [CrossRef]
- Shivakumar, S.V.B.Y.; Chandrasekaran, P.; Kumar, A.M.V.; Paradkar, M.; Dhanasekaran, K.; Suryavarshini, N.; Thomas, B.; Kohli, R.; Thiruvengadam, K.; Kulkarni, V.; et al. Diabetes and pre-diabetes among household contacts of tuberculosis patients in India: Is it time to screen them all? Int. J. Tuberc. Lung Dis. 2018, 22, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, S.; Obuchowski, N.A.; Lieber, M.L. Clinical Evaluation of Diagnostic Tests. Am. J. Roentgenol. 2005, 184, 14–19. [Google Scholar] [CrossRef]
- Xu, C.; Hu, B. Prevalence of active pulmonary tuberculosis among household contacts of recently diagnosed pulmonary tuberculosis patients with positive sputum-smear. Zhonghua Liu Xing Bing Xue Za Zhi 2008, 29, 693–695. [Google Scholar]
- Nishikiori, N.; Weezenbeek, C.V. Target prioritization and strategy selection for active case-finding of pulmonary tuberculosis: A tool to support country-level project planning. BMC Public Health 2013, 13, 1–10. [Google Scholar] [CrossRef]
- Martinez, L.; Zhu, L.; Castellanos, M.E.; Liu, Q.; Chen, C.; Hallowell, B.D.; Whalen, C.C. Glycemic Control and the Prevalence of Tuberculosis Infection: A Population-based Observational Study. Clin. Infect. Dis. 2017, 65, 2060–2068. [Google Scholar] [CrossRef] [PubMed]
- Freckmann, G.; Schmid, C.; Baumstark, A.; Pleus, S.; Link, M.; Haug, C. System Accuracy Evaluation of 43 Blood Glucose Monitoring Systems for Self-Monitoring of Blood Glucose according to DIN EN ISO 15197. J. Diabetes Sci. Technol. 2012, 6, 1060. [Google Scholar] [CrossRef] [PubMed]
| DM Screening in Household Contacts | Total | Household Contacts of Index TB with DM | Household Contacts of Index TB without DM | p Value |
| Number of household and household contact | ||||
| Total number of households visited (a) | 193 | 63 | 130 | N/A |
| Total number of household contacts screened for DM * (b) | 328 | 104 | 224 | N/A |
| Number of DM patients | ||||
| Known case of DM (c) | 33 | 15 | 18 | N/A |
| Newly diagnosed DM (d) | 13 | 6 | 7 | N/A |
| Prevalence of DM among household contacts, % (95%CI) | ||||
| Overall DM ((c + d)/b) | 14.0 (10.6–18.4) | 20.2 (13.2–29.4) | 11.2 (7.5–16.2) | 0.03 |
| Known case of DM (c/b) | 10.1 (7.1,13.9) | 14.4 (8.6–22.9) | 8.0 (4.9–12.6) | 0.07 |
| Newly diagnosed DM (d/b) | 4.0 (2.2–6.9) | 5.8 (2.4–12.6) | 3.1 (1.4–6.6) | 0.36 † |
| TB Screening in Household Contacts | Total | Household Contacts of Index TB with DM | Household Contacts of Index TB without DM | p Value |
| Number of household and household contact | ||||
| Total number of households visited (a) | 193 | 63 | 130 | N/A |
| Total number of household contacts screened for TB (b) | 439 | 134 | 305 | N/A |
| Number of TB patient | ||||
| Known case of TB (c) | 1 | 0 | 1 | N/A |
| Newly diagnosed TB (d) | 21 | 6 | 15 | N/A |
| Prevalence of TB among household contacts, % (95%CI) | ||||
| Overall TB prevalence ((c + d)/b) | 5.0 (3.2–7.6) | 4.5 (1.8–9.9) | 5.3 (3.1–8.6) | 0.73 |
| Newly diagnosed TB (d/b) | 4.8 (3.1–7.3) | 4.5 (1.9–10.0) | 4.9 (2.9–8.2) | 0.84 |
| Outcome—DM Status of Household Contacts | |||||||||
| Model | Main Hypothesis Exposure | Covariate Included in the Model | OR (95% CI) | AIC | |||||
| DM in Index TB Patient | Age | Gender | SES | Low Physical Activity | BMI | Central Obesity | |||
| D1 | + | - | - | - | - | - | - | 2.01 (1.07–3.79) * | 265.4 |
| D2 | + | + | - | - | - | - | - | 2.13 (1.10–4.12) * | 248.1 |
| D3 | + | + | + | - | - | - | - | 2.24 (1.15–4.35) * | 248.3 |
| D4 | + | + | + | + | - | - | - | 2.39 (1.22–4.72) * | 254.0 |
| D5 | + | + | + | + | + | - | - | 2.37 (1.19–4.69) * | 253.5 |
| D6 | + | + | + | + | + | + | - | 2.27 (1.13–4.56) * | 253.5 |
| D7 | + | + | + | + | + | + | + | 2.28 (1.13–4.57) * | 254.6 |
| Outcome—Active TB Status of Household Contacts | |||||||||
| Model | Main Hypothesis Exposure | Covariate Included in the Model | OR (95% CI) | AIC | |||||
| DM in Index TB Patient | Age | Gender | SES | Closeness to Index TB Patient | |||||
| T1 | + | - | - | - | - | 0.85 (0.32–2.21) | 178.5 | ||
| T2 | + | + | - | - | - | 0.85 (0.33–2.23) | 178.1 | ||
| T3 | + | + | + | - | - | 0.82 (0.31–2.17) | 175.3 | ||
| T4 | + | + | + | + | - | 0.81 (0.30–2.21) | 174.4 | ||
| T5 | + | + | + | + | + | 0.87 (0.31–2.39) | 175.7 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zayar, N.-N.; Sangthong, R.; Saw, S.; Aung, S.T.; Chongsuvivatwong, V. Combined Tuberculosis and Diabetes Mellitus Screening and Assessment of Glycaemic Control among Household Contacts of Tuberculosis Patients in Yangon, Myanmar. Trop. Med. Infect. Dis. 2020, 5, 107. https://doi.org/10.3390/tropicalmed5030107
Zayar N-N, Sangthong R, Saw S, Aung ST, Chongsuvivatwong V. Combined Tuberculosis and Diabetes Mellitus Screening and Assessment of Glycaemic Control among Household Contacts of Tuberculosis Patients in Yangon, Myanmar. Tropical Medicine and Infectious Disease. 2020; 5(3):107. https://doi.org/10.3390/tropicalmed5030107
Chicago/Turabian StyleZayar, Nyi-Nyi, Rassamee Sangthong, Saw Saw, Si Thu Aung, and Virasakdi Chongsuvivatwong. 2020. "Combined Tuberculosis and Diabetes Mellitus Screening and Assessment of Glycaemic Control among Household Contacts of Tuberculosis Patients in Yangon, Myanmar" Tropical Medicine and Infectious Disease 5, no. 3: 107. https://doi.org/10.3390/tropicalmed5030107
APA StyleZayar, N.-N., Sangthong, R., Saw, S., Aung, S. T., & Chongsuvivatwong, V. (2020). Combined Tuberculosis and Diabetes Mellitus Screening and Assessment of Glycaemic Control among Household Contacts of Tuberculosis Patients in Yangon, Myanmar. Tropical Medicine and Infectious Disease, 5(3), 107. https://doi.org/10.3390/tropicalmed5030107





