Early Growth Parameters as Predictors of Developmental Delay among Children Conceived During the 2015–2016 Zika Virus Outbreak in Northeastern Brazil
Abstract
:1. Introduction
2. Materials and Methods
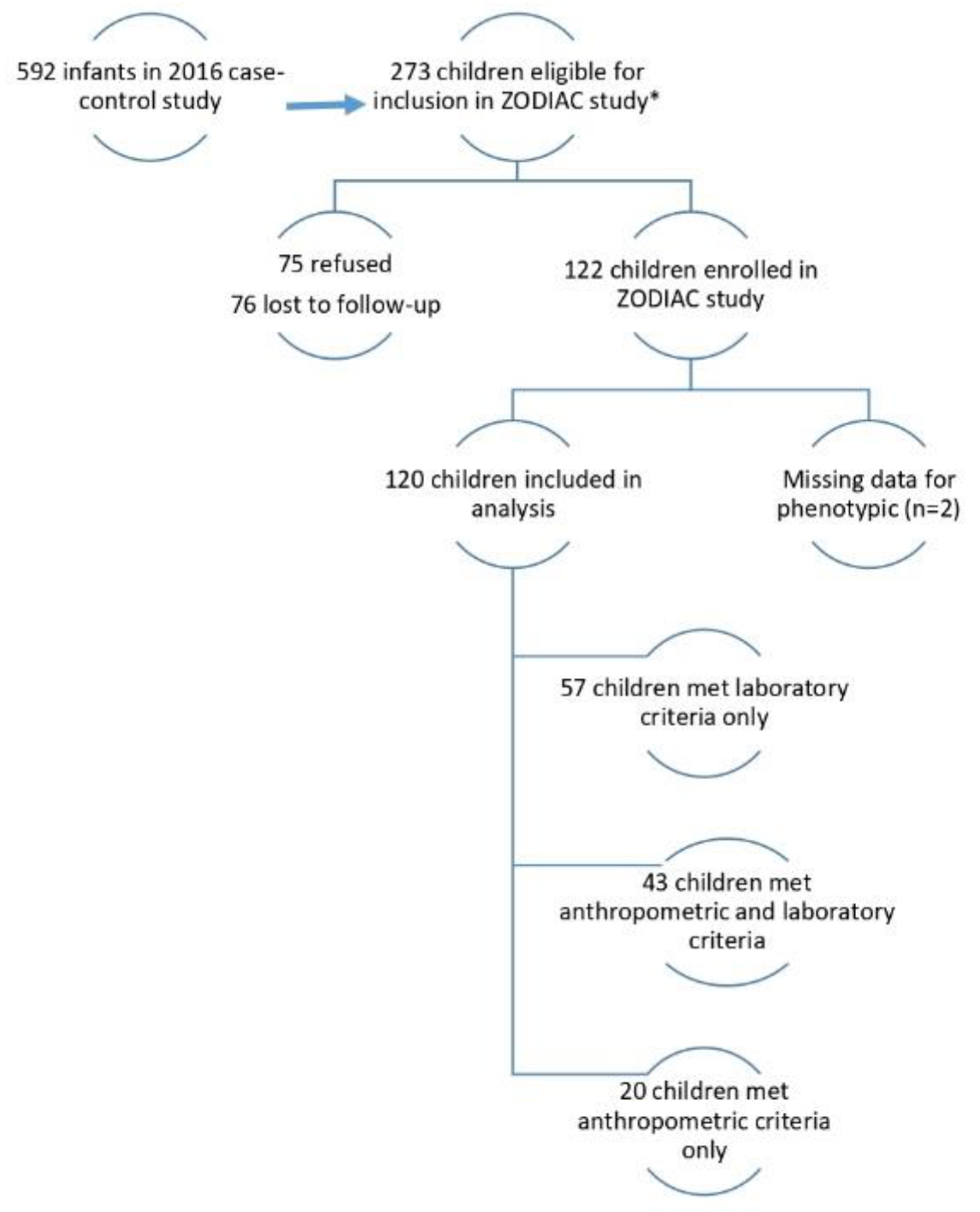
2.1. Study Population
- -
- Laboratory: non-negative test for Zika-specific neutralizing antibodies in an infant sample, and/or
- -
- Anthropometric: met case-control study criteria for assignment to the microcephaly, small, or disproportionate group [23], as defined above.
2.2. Phenotype Classifications
2.3. Ages and Stages Questionnaire
2.4. Head Circumference, Length, and Weight Assessment
2.5. Analysis Methods
2.6. Developmental Quotient z-score
2.7. Predictor Variables
2.8. Developmental Quotient z-score Modeling Using Head Circumference and Length
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Althouse, B.M.; Vasilakis, N.; Sall, A.A.; Diallo, M.; Weaver, S.C.; Hanley, K.A. Potential for Zika Virus to Establish a Sylvatic Transmission Cycle in the Americas. PLoS Negl. Trop. Dis. 2016, 10, e0005055. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P. Chikungunya and Zika: The Future. In Chikungunya and Zika Viruses, 1st ed.; Higgs, S., Vanlandingham, D., Powers, A., Eds.; Elsevier: London, UK, 2018; pp. 367–377. [Google Scholar]
- Munoz-Jordan, J.L. Diagnosis of Zika virus infections: Challenges and opportunities. J. Infect. Dis. 2017, 216, S951–S956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleber de Oliveira, W.; Cortez-Escalante, J.; Holanda de Oliveira, W.T.G.; Ikeda do Carmo, G.M.; Pessanha Henriques, C.M.; Coelho, G.E.; Araújo de França, G.V. Increase in reported prevalence of microcephaly in infants born to women living in areas with confirmed Zika virus transmission during the first trimester of pregnancy—Brazil, 2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 242–247. [Google Scholar] [CrossRef]
- Brasil, P.; Pereira, J.P., Jr.; Moreira, M.E.; Ribeiro Nogueira, R.M.; Damasceno, L.; Wakimoto, M.; Rabello, R.S.; Valderramos, S.G.; Halai, U.A.; Salles, T.S.; et al. Zika virus infection in pregnant women in Rio de Janeiro. N. Engl. J. Med. 2016, 375, 2321–2334. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.A.; Jamieson, D.J.; Honein, M.A.; Petersen, L.R. Zika virus and birth defects—Reviewing the evidence for causality. N. Engl. J. Med. 2016, 374, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.A.; Staples, J.E.; Dobyns, W.B.; Pessoa, A.; Ventura, C.V.; Fonseca, E.B.; Ribeiro, E.M.; Ventura, L.O.; Neto, N.N.; Arena, J.F.; et al. Characterizing the Pattern of Anomalies in Congenital Zika Syndrome for Pediatric Clinicians. JAMA Pediatr. 2017, 171, 288–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuler-Faccini, L.; Ribeiro, E.M.; Feitosa, I.M.; Horovitz, D.D.; Cavalcanti, D.P.; Pessoa, A.; Doriqui, M.J.; Neri, J.I.; Neto, J.M.; Wanderley, H.Y.; et al. Possible association between Zika virus infection and microcephaly—Brazil, 2015. MMWR Morb. Mortal. Wkl. Rep. 2016, 65, 59–62. [Google Scholar] [CrossRef]
- França, G.V.; Schuler-Faccini, L.; Oliveira, W.K.; Henriques, C.M.; Carmo, E.H.; Pedi, V.D.; Nunes, M.L.; Castro, M.C.; Serruya, S.; Silveira, M.F.; et al. Congenital Zika virus syndrome in Brazil: A case series of the first 1501 livebirths with complete investigation. Lancet 2016, 388, 891–897. [Google Scholar] [CrossRef] [Green Version]
- Van Der Linden, V.; Pessoa, A.; Dobyns, W.; Barkovich, A.J.; Júnior, H.V.; Filho, E.L.R.; Ribeiro, E.M.; Leal, M.C.; Coimbra, P.P.; Aragão, M.F.; et al. Description of 13 Infants Born During October 2015–January 2016 With Congenital Zika Virus Infection Without Microcephaly at Birth—Brazil. MMWR. Morb. Mortal. Wkly. Rep. 2016, 65, 1343–1348. [Google Scholar] [CrossRef]
- De França, T.L.B.; Medeiros, W.R.; De Souza, N.L.; Longo, E.; Pereira, S.A.; França, T.B.D.O.; Sousa, K.G. Growth and Development of Children with Microcephaly Associated with Congenital Zika Virus Syndrome in Brazil. Int. J. Environ. Res. Public Health 2018, 15, 1990. [Google Scholar] [CrossRef] [Green Version]
- Moura da Silva, A.A.; Ganz, J.S.S.; Sousa, P.D.S.; Doriqui, M.J.; Ribeiro, M.R.; Branco, M.D.; Queiroz, R.C.D.S.; Pacheco, M.D.J.T.; Da Costa, F.R.V.; Silva, F.D.S.; et al. Early Growth and Neurologic Outcomes of Infants with Probable Congenital Zika Virus Syndrome. Emerg. Infect. Dis. 2016, 22, 1953–1956. [Google Scholar] [CrossRef]
- Del Campo, M.; Feitosa, I.M.; Ribeiro, E.M.; Horovitz, D.D.; Pessoa, A.L.; França, G.V.; García-Alix, A.; Doriqui, M.J.; Wanderley, H.Y.; Sanseverino, M.V.; et al. The phenotypic spectrum of congenital Zika syndrome. Am. J. Med Genet. Part A 2017, 173, 841–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice, M.E.; Galang, R.R.; Roth, N.M.; Ellington, S.R.; Moore, C.A.; Valencia-Prado, M.; Ellis, E.M.; Tufa, A.J.; Taulung, L.A.; Alfred, J.M.; et al. Vital Signs: Zika-Associated Birth Defects and Neurodevelopmental Abnormalities Possibly Associated with Congenital Zika Virus Infection—U.S. Territories and Freely Associated States, 2018. MMWR. Morb. Mortal. Wkly. Rep. 2018, 67, 858–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Einspieler, C.; Utsch, F.; Brasil, P.; Panvequio Aizawa, C.Y.; Peyton, C.; Hydee Hasue, R.; Françoso Genovesi, F.; Damasceno, L.; Moreira, M.E.; Adachi, K.; et al. Association of Infants Exposed to Prenatal Zika Virus Infection With Their Clinical, Neurologic, and Developmental Status Evaluated via the General Movement Assessment Tool. Jama Netw. Open 2019, 2, e187235. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, M.L.; Nery Júnior, N.; Estofolete, C.F.; Bernardes Terzian, A.; Guimarães, G.; Zini, N.; Alves da Silva, R.; Dutra Silva, G.C.; Junqueira Franco, L.C.; Rahal, P.; et al. Adverse birth outcomes associated with Zika virus exposure during pregnancy in São José do Rio Preto, Brazil. Clin. Microbiol. Infect. 2018, 24, 646–652. [Google Scholar] [CrossRef] [Green Version]
- Adebanjo, T.; Godfred-Cato, S.; Viens, L.; Fischer, M.; Staples, J.E.; Kuhnert-Tallman, W.; Walke, H.; Oduyebo, T.; Polen, K.; Peacock, G.; et al. Update: Interim Guidance for the Diagnosis, Evaluation, and Management of Infants with Possible Congenital Zika Virus Infection—United States, October 2017. Morb. Mortal. Wkly. Rep. 2017, 66, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Lopes Moreira, M.E.; Nielsen-Saines, K.; Brasil, P.; Kerin, T.; Damasceno, L.; Pone, M.; Carvalho, L.M.A.; Pone, S.M.; Vasconcelos, Z.; Ribeiro, I.P.; et al. Neurodevelopment in Infants Exposed to Zika Virus In Utero. N. Engl. J. Med. 2018, 379, 2377–2379. [Google Scholar] [CrossRef]
- Nielsen-Saines, K.; Brasil, P.; Kerin, T.; Vasconcelos, Z.F.M.; Gabaglia, C.R.; Damasceno, L.; Pone, M.; De Carvalho, L.M.A.; Pone, S.M.; Zin, A.A.; et al. Delayed childhood neurodevelopment and neurosensory alterations in the second year of life in a prospective cohort of ZIKV-exposed children. Nat. Med. 2019, 25, 1213–1217. [Google Scholar] [CrossRef]
- Walker, C.L.; Merriam, A.A.; Ohuma, E.O.; Dighe, M.K.; Gale, M.; Rajagopal, L.; Papageorghiou, A.T.; Gyamfi-Bannerman, C.; Waldorf, K.M.A. Femur-sparing pattern of abnormal fetal growth in pregnant women from New York City after maternal Zika virus infection. Am. J. Obs. Gynecol. 2018, 219, 187.e1–187.e20. [Google Scholar] [CrossRef] [Green Version]
- O’Leary, D.R.; Kuhn, S.; Kniss, K.L.; Hinckley, A.F.; Rasmussen, S.A.; Pape, W.J.; Kightlinger, L.K.; Beecham, B.D.; Miller, T.K.; Neitzel, D.F.; et al. Birth Outcomes Following West Nile Virus Infection of Pregnant Women in the United States: 2003–2004. Pediatrics 2006, 117, 537–545. [Google Scholar] [CrossRef] [Green Version]
- Chiriboga-Klein, S.; Oberfield, S.E.; Casullo, A.M.; Holahan, N.; Fedun, B.; Cooper, L.Z.; Levine, L.S. Growth in congenital rubella syndrome and correlation with clinical manifestations. J. Pediatr. 1989, 115, 251–255. [Google Scholar] [CrossRef]
- Krow-Lucal, E.; De Andrade, M.R.; Cananéa, J.N.A.; Moore, C.A.; Leite, P.L.; Biggerstaff, B.J.; Cabral, C.M.; Itoh, M.; Percio, J.; Wada, M.Y.; et al. Association and birth prevalence of microcephaly attributable to Zika virus infection among infants in Paraíba, Brazil, in 2015–16: A case-control study. Lancet Child Adolesc. Health 2018, 2, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Bertolli, J.; Attell, J.E.; Rose, C.; Moore, C.A.; Melo, F.; Staples, J.E.; Kotzky, K.; Krishna, N.; Satterfield-Nash, A.; Pereira, I.O.; et al. Functional Outcomes among a Cohort of Children in Northeastern Brazil Meeting Criteria for Follow-Up of Congenital Zika Virus Infection. Am. J. Trop. Med. Hyg. 2020, 102, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Haby, M.M.; Pinart, M.; Elias, V.; Reveiz, L. Prevalence of asymptomatic Zika virus infection: A systematic review. Bull. World Heal. Organ. 2018, 96, 402D–413D. [Google Scholar] [CrossRef] [PubMed]
- Granger, D.; Theel, E.S. Evaluation of a Rapid Immunochromatographic Assay and Two Enzyme-Linked Immunosorbent Assays for Detection of IgM-Class Antibodies to Zika Virus. J. Clin. Microbiol. 2018, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Squires, J.; Twombly, E.; Bricker, D.; Potter, L. ASQ-3™ User’s Guide, 3rd ed.; Paul, H., Ed.; Brooks Publishing Company, Inc.: Baltimore, MD, USA, 2009; pp. 157–169. [Google Scholar]
- Filgueiras, A.; Pires, P.; Maissonette, S.; Landeira-Fernandez, J. Psychometric properties of the Brazilian-adapted version of the Ages and Stages Questionnaire in public child daycare centers. Early Hum. Dev. 2013, 89, 561–576. [Google Scholar] [CrossRef] [PubMed]
- Attell, J.E.; Rose, C.; Bertolli, J.; Kotzky, K.; Squires, J.; Krishna, N.K.; Satterfield-Nash, A.; Peacock, G.; Ornelas Pereira, I.; Faria E Silva Santelli, A.C.; et al. Adapting the Ages and Stages Questionnaire to Identify and Quantify Development Among Children With Evidence of Zika Infection. Infants & Young Children. 2020, 33, 95–107. [Google Scholar] [CrossRef]
- International Fetal and Newborn Growth Consortium for the 21st Century. Standards for Newborns and References for Very Preterm Infants. 2017. Available online: https://intergrowth21.tghn.org/ (accessed on 8 August 2020).
- World Health Organization. Child Growth Standards. Head Circumference for Age. 2017. Available online: http://www.who.int/childgrowth/standards/hc_for_age/en/ (accessed on 8 August 2020).
- Kotzky, K.; Allen, J.E.; Robinson, L.R.; Satterfield-Nash, A.; Bertolli, J.; Smith, C.; Ornelas Pereira, I.; Faria E Silva Santelli, A.C.; Peacock, G. Depressive Symptoms and Care Demands Among Primary Caregivers of Young Children with Evidence of Congenital Zika Virus Infection in Brazil. J. Dev. Behav. Pediatr. 2019, 40, 344–353. [Google Scholar] [CrossRef]
- Early Childhood Technical Assistance Center. States and Territories Definitions of/Criteria for IDEA Part C Eligibility. 2015. Available online: https://ectacenter.org/~pdfs/topics/earlyid/partc_elig_table.pdf (accessed on 8 August 2020).
- Karlberg, J. On the modelling of human growth. Stat. Med. 1987, 6, 185–192. [Google Scholar] [CrossRef]
- Berkey, C.S.; Reed, R.B. A model for describing normal and abnormal growth in early childhood. Hum. Biol. 1987, 59, 973–987. [Google Scholar]
- Surén, P.; Stoltenberg, C.; Bresnahan, M.; Hirtz, D.; Lie, K.K.; Lipkin, W.I.; Magnus, P.; Reichborn-Kjennerud, T.; Schjølberg, S.; Susser, E.; et al. Early Growth Patterns in Children with Autism. Epidemiology 2013, 24, 660–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aragão, M.F.V.V.; Holanda, A.C.; Brainer-Lima, A.M.; Petribu, N.C.L.; Castillo, M.; Van Der Linden, V.; Serpa, S.C.; Tenório, A.G.; Travassos, P.T.C.; Cordeiro, M.T.; et al. Nonmicrocephalic Infants with Congenital Zika Syndrome Suspected Only after Neuroimaging Evaluation Compared with Those with Microcephaly at Birth and Postnatally: How Large Is the Zika Virus “Iceberg”? Am. J. Neuroradiol. 2017, 38, 1427–1434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, M.R.; Jones, A.M.; Petersen, E.E.; Lee, E.H.; Rice, M.E.; Bingham, A.; Ellington, S.R.; Evert, N.; Reagan-Steiner, S.; Oduyebo, T.; et al. Vital Signs: Update on Zika Virus-Associated Birth Defects and Evaluation of All, U.S. Infants with Congenital Zika Virus Exposure—U.S. Zika Pregnancy Registry, 2016. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 366–373. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, T.V.B.; Ximenes, R.A.A.; Miranda-Filho, D.B.; De Souza, W.V.; Montarroyos, U.R.; de Melo, A.P.L.; Valongueiro, S.; de Albuquerque, M.D.F.P.M.; Braga, C.; Filho, S.P.B.; et al. Association between microcephaly, Zika virus infection, and other risk factors in Brazil: Final report of a case-control study. Lancet Infect. Dis. 2018, 18, 328–336. [Google Scholar] [CrossRef] [Green Version]
- Carvalho-Sauer, R.; Costa, M.D.C.N.; Barreto, F.R.; Teixeira, M.G. Congenital Zika Syndrome: Prevalence of low birth weight and associated factors. Bahia, 2015–2017. Int. J. Infect. Dis. 2019, 82, 44–50. [Google Scholar] [CrossRef] [Green Version]
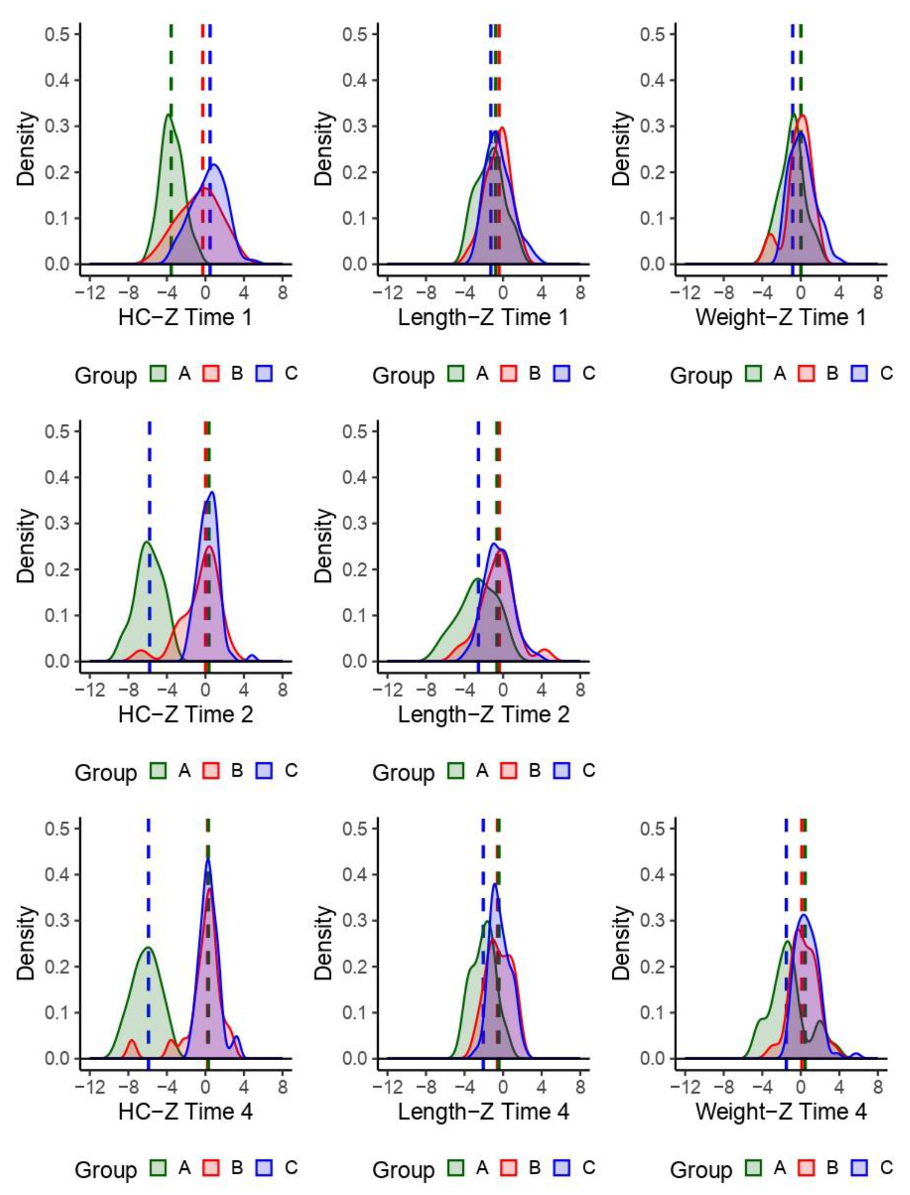
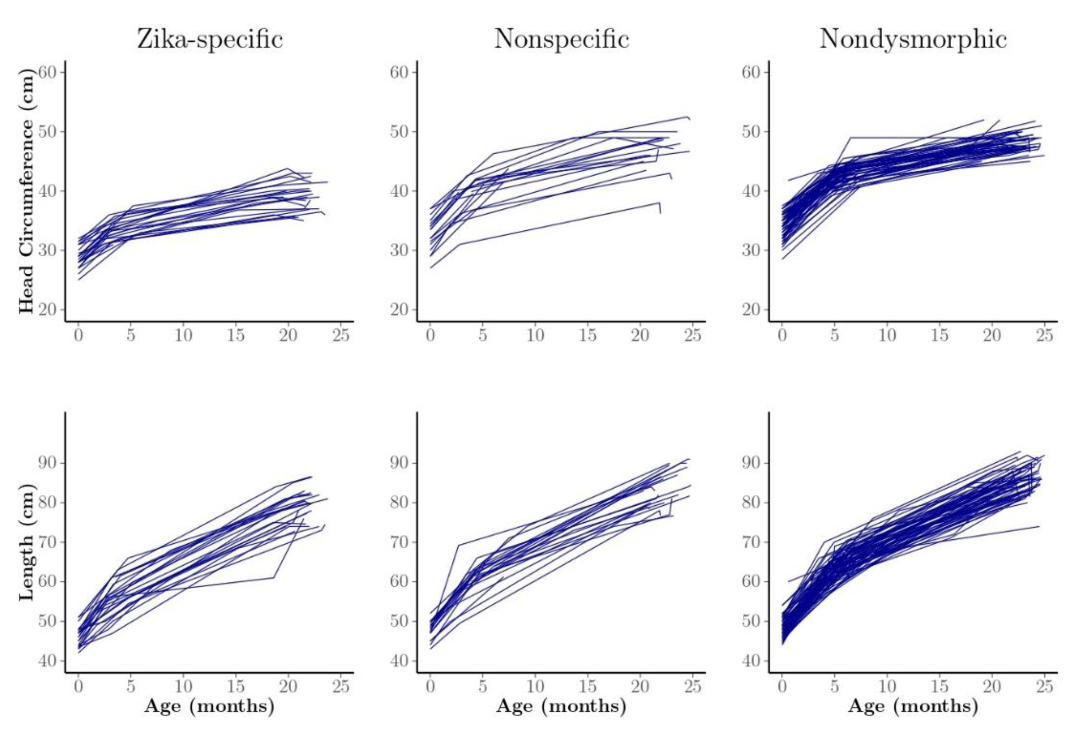

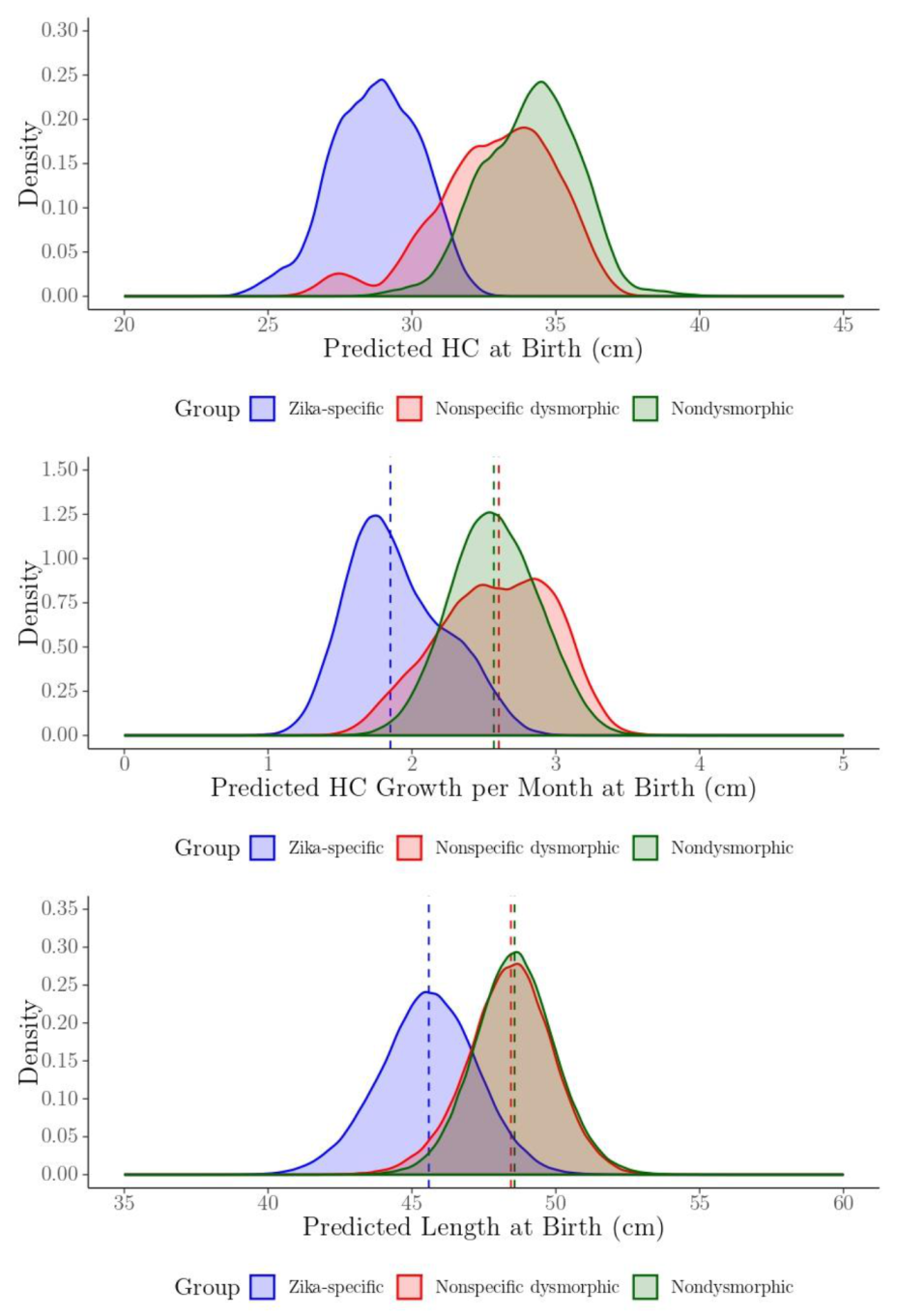
| Child Characteristics | Level | N | Percent |
|---|---|---|---|
| Sex | Male | 60 | 50.0 |
| Female | 60 | 50.0 | |
| Zika Phenotype | Zika-specific | 23 | 19.2 |
| Nonspecific dysmorphic | 22 | 18.3 | |
| Nondysmorphic | 75 | 62.5 | |
| Weight-to-length ratio (birth) | <12 | 17 | 14.2 |
| 12−16 | 84 | 70.0 | |
| >16 | 6 | 5.0 | |
| Missing | 13 | 10.8 | |
| Premature Child (<37 weeks) | Yes | 14 | 11.7 |
| No | 106 | 88.3 | |
| Caregiver Characteristics | |||
| Age (years) | ≤ 18 | 8 | 6.7 |
| 19−23 | 29 | 24.2 | |
| 24−28 | 23 | 19.2 | |
| 29−33 | 34 | 28.3 | |
| > 33 | 26 | 21.7 | |
| Education (years) | ≤ 6 | 24 | 20.0 |
| 7−8 | 24 | 20.0 | |
| 9−11 | 36 | 30.0 | |
| ≥ 12 | 36 | 30.0 | |
| Smoked during pregnancy | Yes | 7 | 5.8 |
| No | 111 | 92.5 | |
| Missing | 2 | 1.7 | |
| Previous Children | Yes | 72 | 60.0 |
| No | 44 | 36.7 | |
| Missing | 4 | 3.3 | |
| Breastfed Child (months) | 0−6 | 12 | 10.0 |
| 7−12 | 8 | 6.7 | |
| 13−18 | 36 | 30.0 | |
| 19−26 | 62 | 51.7 | |
| Missing | 2 | 1.7 |
| Developmental Classification | Phenotype Group | ||
|---|---|---|---|
| Zika-Specific N (%) | Nonspecific Dysmorphic N (%) | Nondysmorphic N (%) | |
| Communication | |||
| <1 SD below mean | 0 (0) | 14 (63.6) | 57 (76.0) |
| 1−1.9 SD below mean | 0 (0) | 5 (22.7) | 11 (14.7) |
| ≥2 SD below mean | 23 (100) | 3 (13.6) | 7 (9.3) |
| Gross Motor | |||
| <1 SD below mean | 0 (0) | 14 (63.6) | 59 (78.7) |
| 1−1.9 SD below mean | 0 (0) | 5 (22.7) | 11 (14.7) |
| ≥2 SD below mean | 23 (100) | 3 (13.6) | 5 (6.7) |
| Fine Motor | |||
| <1 SD below mean | 0 (0) | 17 (77.3) | 63 (84.0) |
| 1−1.9 SD below mean | 0 (0) | 3 (13.6) | 8 (10.7) |
| ≥2 SD below mean | 23 (100) | 2 (9.1) | 4 (5.3) |
| Problem-Solving | |||
| <1 SD below mean | 0 (0) | 14 (63.6) | 61 (81.3) |
| 1−1.9 SD below mean | 0 (0) | 6 (27.3) | 10 (13.3) |
| ≥2 SD below mean | 23 (100) | 2 (9.1) | 4 (5.3) |
| Personal-Social | |||
| <1 SD below mean | 0 (0) | 15 (68.2) | 69 (92.0) |
| 1−1.9 SD below mean | 0 (0) | 5 (22.7) | 2 (2.7) |
| ≥2 SD below mean | 23 (100) | 2 (9.1) | 4 (5.3) |
| Development Classification (overall) | |||
| All domains < 1 SD below mean | 0 (0) | 6 (27.3) | 40 (53.3) |
| ≥2 domains are ≥2 SD below mean | 23 (100) | 2 (9.1) | 7 (9.3) |
| Remaining | 0 (0) | 14 (63.6) | 28 (37.3) |
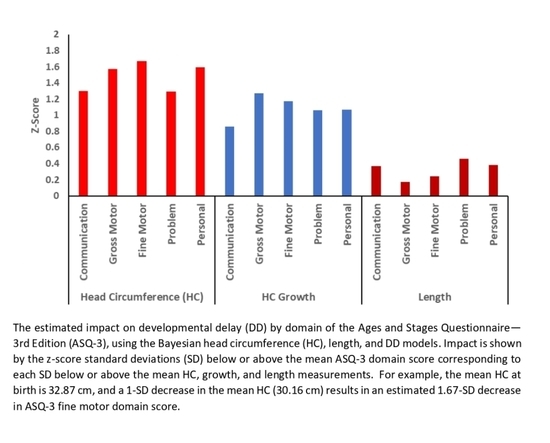
| Growth Parameter | Communication Mean (PI) | Gross Motor Mean (PI) | Fine Motor Mean (PI) | Problem-Solving Mean (PI) | Personal-Social Mean (PI) |
|---|---|---|---|---|---|
| Head Circumference | |||||
| Mean = 32.87 cm | −1.49 (−1.88 to −1.10) | −1.25 ( −1.67 to −0.83) | −1.10 (−1.51 to −0.70) | −1.52 (−1.90 to −1.13) | −1.07 (−1.50 to −0.65) |
| −1 SD = 30.16 cm | −2.79 (−3.56 to −2.00) | −2.82 (−3.66 to −1.97) | −2.77 (−3.56 to −1.97) | −2.81 (−3.57 to −2.02) | −2.66 (−3.54 to −1.77) |
| −2 SD = 27.45 cm | −4.10 (−5.48 to −2.67) | −4.40 (−5.91 to −2.87) | −4.44 (−5.86 to −3.01) | −4.09 (−5.49 to −2.65) | −4.25 (−5.84 to −2.63) |
| −3 SD = 24.74 cm | −5.40 (−7.42 to −3.30) | −5.97 ( −8.15 to −3.72) | −6.10 (−8.19 to −3.99) | −5.38 (−7.43 to −3.31) | −5.84 (−8.19 to −3.47) |
| Head Circumference Growth | |||||
| Mean = 2.45 cm/month | −1.49 (−1.88 to −1.10) | −1.25 ( −1.67 to −0.83) | −1.10 (−1.51 to −0.70) | −1.52 (−1.90 to −1.13) | −1.07 (−1.50 to −0.65) |
| −1 SD = 2.02 cm/month | −2.35 (−3.02 to −1.67) | −2.52 (−3.24 to −1.79) | −2.27 (−2.97 to −1.57) | −2.58 (−3.26 to −1.89) | −2.14 (−2.90 to −1.36) |
| −2 SD = 1.60 cm/month | −3.21 (−4.37 to −2.04) | −3.80 (−5.06 to −2.50) | −3.44 (−4.65 to −2.23) | −3.63 (−4.81 to −2.42) | −3.20 (−4.55 to −1.83) |
| −3 SD = 1.17 cm/month | −4.07 (−5.77 to −2.35) | −5.07 (−6.89 to −3.20) | −4.62 (−6.36 to −2.85) | −4.69 (−6.43 to −2.94) | −4.26 (−6.21 to −2.28) |
| Length | |||||
| Mean = 47.96 cm | −1.49 (−1.88 to −1.10) | −1.25 ( −1.67 to −0.83) | −1.10 (−1.51 to −0.70) | −1.52 (−1.90 to −1.13) | −1.07 (−1.50 to −0.65) |
| −1 SD = 46.09 cm | −1.86 (−2.80 to −0.95) | −1.42 (−2.45 to −0.43) | −1.34 (−2.28 to −0.43) | −1.98 (−2.92 to −1.06) | −1.45 (−2.53 to −0.40) |
| −2 SD = 44.22 cm | −2.24 (−4.00 to −0.53) | −1.60 (−3.55 to 0.25) | −1.58 (−3.34 to 0.11) | −2.44 (−4.23 to −0.71) | −1.83 (−3.88 to 0.14) |
| −3 SD = 42.35 cm | −2.62 (−5.19 to −0.11) | −1.77 (−4.64 to 0.96) | −1.82 (−4.40 to 0.68) | −2.89 (−5.51 to −0.38) | −2.20 (−5.21 to 0.69) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rose, C.E.; Bertolli, J.; Attell, J.E.; Moore, C.A.; Melo, F.; Kotzky, K.; Krishna, N.; Satterfield-Nash, A.; Pereira, I.O.; Pessoa, A.; et al. Early Growth Parameters as Predictors of Developmental Delay among Children Conceived During the 2015–2016 Zika Virus Outbreak in Northeastern Brazil. Trop. Med. Infect. Dis. 2020, 5, 155. https://doi.org/10.3390/tropicalmed5040155
Rose CE, Bertolli J, Attell JE, Moore CA, Melo F, Kotzky K, Krishna N, Satterfield-Nash A, Pereira IO, Pessoa A, et al. Early Growth Parameters as Predictors of Developmental Delay among Children Conceived During the 2015–2016 Zika Virus Outbreak in Northeastern Brazil. Tropical Medicine and Infectious Disease. 2020; 5(4):155. https://doi.org/10.3390/tropicalmed5040155
Chicago/Turabian StyleRose, Charles E., Jeanne Bertolli, Jacob Elijah Attell, Cynthia A. Moore, Flavio Melo, Kim Kotzky, Nevin Krishna, Ashley Satterfield-Nash, Isabela Ornelas Pereira, Andre Pessoa, and et al. 2020. "Early Growth Parameters as Predictors of Developmental Delay among Children Conceived During the 2015–2016 Zika Virus Outbreak in Northeastern Brazil" Tropical Medicine and Infectious Disease 5, no. 4: 155. https://doi.org/10.3390/tropicalmed5040155
APA StyleRose, C. E., Bertolli, J., Attell, J. E., Moore, C. A., Melo, F., Kotzky, K., Krishna, N., Satterfield-Nash, A., Pereira, I. O., Pessoa, A., Smith, D. C., Santelli, A. C. F. e. S., & Peacock, G. (2020). Early Growth Parameters as Predictors of Developmental Delay among Children Conceived During the 2015–2016 Zika Virus Outbreak in Northeastern Brazil. Tropical Medicine and Infectious Disease, 5(4), 155. https://doi.org/10.3390/tropicalmed5040155