Leptospirosis in Ruminants in Yogyakarta, Indonesia: A Serological Survey with Mixed Methods to Identify Risk Factors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Farm Survey
2.2. Serological Analyses
2.3. Risk Factor Analyses
2.4. Participatory Methods
3. Results
3.1. Farm Survey
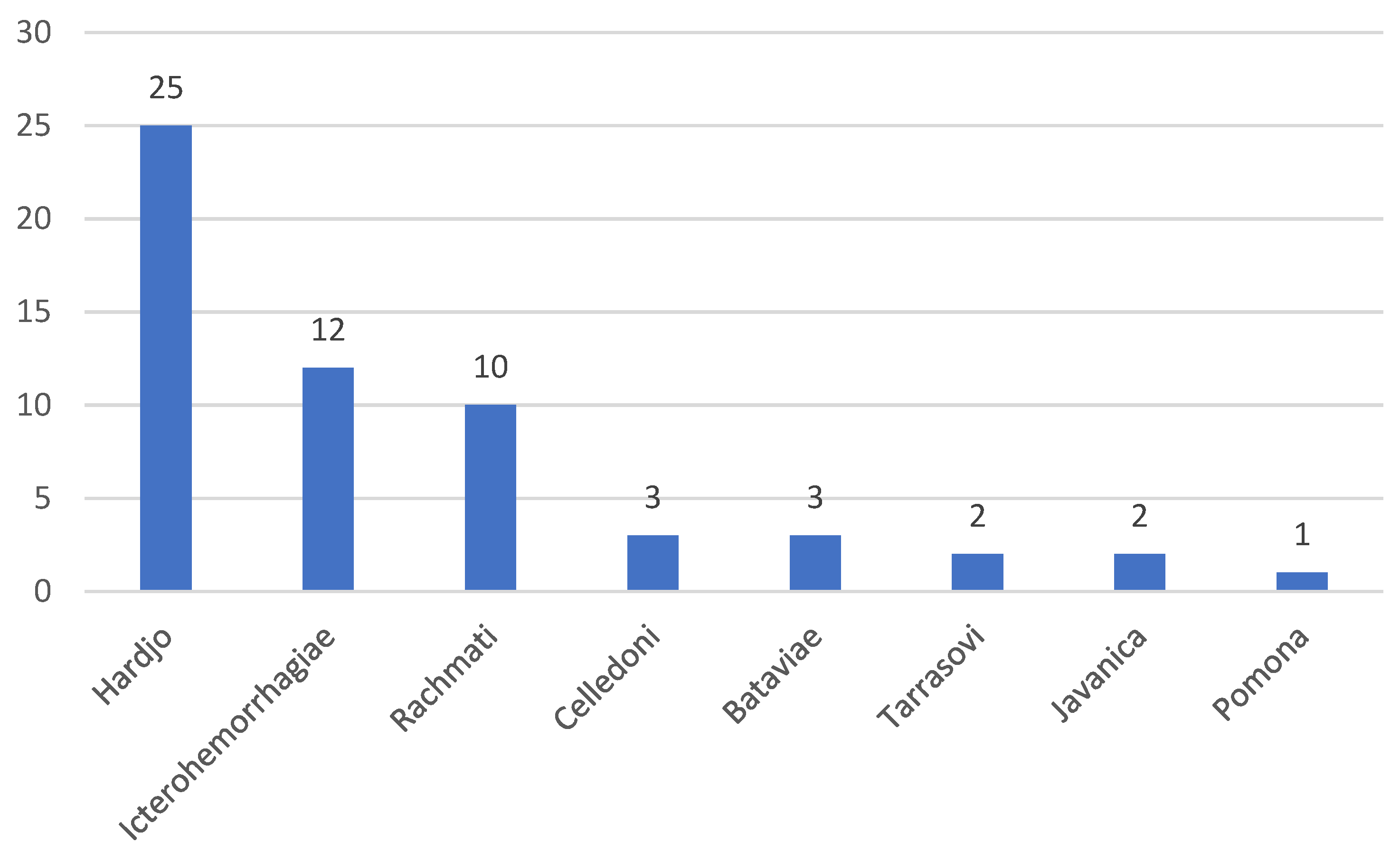
3.2. Serological Analyses
3.3. Risk Factor Analyses
3.3.1. Farm Level Analyses
3.3.2. Animal Level Analyses
3.4. Participatory Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pappas, G.; Papadimitriou, P.; Siozopoulou, V.; Christou, L.; Akritidis, N. The globalization of leptospirosis: Worldwide incidence trends. Int. J. Infect. Dis. 2008, 12, 351–357. [Google Scholar] [CrossRef] [Green Version]
- Hovingh, E. Abortions in Dairy Cattle I: Common Causes of Abortions; Virginia Cooperative Extension Report 404-288: Virginia-Maryland Regional College of Veterinary Medicine; Virginia Tech. Publishing: Blacksburg, VA, USA, 2009. [Google Scholar]
- Indrawati, F. Leptospirosis (Aspek Biomedis dan Epidemiologis). Bull. KEMAS 2009, 4, 2–7. [Google Scholar]
- Lau, C.; Smythe, L.; Weinstein, P. Leptospirosis: An emerging disease in travellers. Travel Med. Infect. Dis. 2010, 8, 33–39. [Google Scholar] [CrossRef]
- WHO. Report of the Second Meeting of the Leptospirosis Burden Epidemiology Reference Group; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Victoriano, A.F.B.; Smythe, L.D.; Gloriani-Barzaga, N.; Cavinta, L.L.; Kasai, L.; Limpakarnjanarat, K.; On, B.L.; Gongal, G.; Hall, J.; Coulombe, C.A.; et al. Leptospirosis in the Asia Pacific region. BMC Infect. Dis. 2009, 9, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadisaputro, A.; Sakundarno, S.; Suhartono, M. Lingkungan dan perilaku pada kejadian leptospirosis. Media Med. Indones. 2009, 43, 306–311. [Google Scholar]
- Gasem, M.H.; Hadi, U.; Alisjahbana, B.; Tjitra, E.; Hapsari, M.M.D.E.A.H.; Lestari, E.S.; Aman, A.T.; Lokida, D.; Salim, G.; Kosasih, H.; et al. Leptospirosis in Indonesia: Diagnostic challenges associated with atypical clinical manifestations and limited laboratory capacity. BMC Infect. Dis. 2020, 20, 179. [Google Scholar] [CrossRef]
- Rahayujati, T.; Municipal Health Services, Yogyakarta, Indonesia. Personal communication, 2013.
- Pirenaning, T.; Municipal Health Services, Yogyakarta, Indonesai. Personal communication, 2011.
- Nguyen-Viet, H.; Doria, S.; Tung, D.X.; Mallee, H.; Wilcox, B.A.; Grace, D. Ecohealth research in Southeast Asia: Past, present and the way forward. Infect. Dis. Poverty 2015, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Charron, D.F. Ecohealth Research in Practice: Innovative Applications of an Ecosystem Approach to Health; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Zinsstag, J. Convergence of ecohealth and one health. Ecohealth 2012, 9, 371–373. [Google Scholar] [CrossRef] [Green Version]
- WHO. Human Leptospirosis: Guidance for Diagnosis, Surveillance and Control; WHO: Geneva, Switzerland, 2003; Volume 45. [Google Scholar]
- Zaki, A.; Rahim, M.; Azme, M.; Mahmood, N. Animal reservoirs for leptospira spp. in south-east Asia: A meta-analysis. J. Adv. Res. Med. 2018, 5, 23–31. [Google Scholar]
- Zuerner, R.L.; Bolin, C.A. Repetitive sequence element cloned from Leptospira interrogans serovar hardjo type hardjo-bovis provides a sensitive diagnostic probe for bovine leptospirosis. J. Clin. Microbiol. 1988, 26, 2495–2500. [Google Scholar] [CrossRef] [Green Version]
- Ellis, W.A.; O’Brien, J.J.; Neill, S.D.; Bryson, D.G. Bovine leptospirosis: Experimental serovar hardjo infection. Vet. Microbiol. 1986, 11, 293–299. [Google Scholar] [CrossRef]
- Genc, O.; Otlu, S.; Sahin, M.; Aydin, F.; Gökce, H. Seroprevalence of brucellosis and leptospirosis in aborted dairy cows. TURKISH J. Vet. Anim. Sci. 2005, 29, 359–366. [Google Scholar]
- Kirkbride, C.A.; Johnson, M.W. Serologic examination of aborted ovine and bovine fetal fluids for the diagnosis of border disease, bluetongue, bovine viral diarrhea, and leptospiral infections. J. Vet. Diagn. Investig. 1989, 1, 132–138. [Google Scholar] [CrossRef]
- Langoni, H.; de Souza, L.C.; da Silva, A.V.; Luvizotto, M.C.R.; Paes, A.C.; Lucheis, S.B. Incidence of leptospiral abortion in Brazilian dairy cattle. Prev. Vet. Med. 1999, 40, 271–275. [Google Scholar] [CrossRef]
- Segura-Correa, V.M.; Solis-Calderon, J.J.; Segura-Correa, J.C. Seroprevalence of and risk factors for leptospiral antibodies among cattle in the state of Yucatan, Mexico. Trop. Anim. Health Prod. 2003, 35, 293–299. [Google Scholar] [CrossRef]
- Momtaz, H.; Moshkelani, S. Detection and characterization of Leptospira spp. isolated from aborted bovine clinical samples. Acta Vet. Brno 2012, 81, 21–25. [Google Scholar] [CrossRef]
- Scott-Orr, H.; Darodjat, M.; Achdiyati, J.; Soeroso, M. Kejadian leptospirosis dan brucellosis pada ternak di Indonesia. In Proceedings of the Risalah Seminar Penyakit Reproduksi dan Unggas, Lembaga Penelitian Penyakit (LPP), Hewan Tugu, Bogor, 13–15 March 1980; pp. 31–57. [Google Scholar]
- Budiharta, S. Leptospirosis in cows in special region of Yogyakarta. Bull. FVM UGM 1988, 8, 13–16. [Google Scholar]
- Diarmita, I.; Wasito, H.R. Indarjulianto prevalence of leptospirosis in seedling cattle on lombok island. Agrosains Bull. 2006, 19, 259–269. [Google Scholar]
- Noach, S.M.C.; Noach, Y.R. Prevalence rate and causes of leptospirosis serovar on cattle at Giwangan’s abattoir of Yogyakarta. J. Trop. Anim. Sci. Technol. 2020, 2, 37–42. [Google Scholar] [CrossRef]
- Lilenbaum, W.; Souza, G.N. Factors associated with bovine leptospirosis in Rio de Janeiro, Brazil. Res. Vet. Sci. 2003, 75, 249–251. [Google Scholar] [CrossRef]
- Murtiningsih, B. Setyawan Budiharta dan Suharyanto Supardi. Faktor Risiko Kejadian Leptospirosis di Provinsi DIY dan Sekitarnya. Berita Kedokt. Masy. 2005, XXI, 17–24. [Google Scholar] [CrossRef]
- Tirado, M.C.; Clarke, R.; Jaykus, L.A.; McQuatters-Gollop, A.; Frank, J.M. Climate change and food safety: A review. Food Res. Int. 2010, 43, 1745–1765. [Google Scholar] [CrossRef]
- Djati, R.R.A.P.; Kusnoputranto, H.; Utomo, S.W.; Sakundarno, M.; Dewabrata, P.W.; Fuad, H.A.H.; Wicaksono, M.A. Leptospirosis control based on eco-social factors: Modeling combination in Demak, Central Java, Indonesia. Biodiversitas 2020, 21, 5818–5828. [Google Scholar] [CrossRef]
- Sakundarno, M.; Bertolatti, D.; Maycock, B.; Spickett, J.; Dhaliwal, S. Risk factors for leptospirosis infection in humans and implications for public health intervention in indonesia and the Asia-Pacific region. Asia-Pac. J. Public Health 2014, 26, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Grace, D.; Lindahl, J.; Wanyoike, F.; Bett, B.; Randolph, T.; Rich, K.M. Poor livestock keepers: Ecosystem-poverty-health interactions. Philos. Trans. R. Soc. London B Biol. Sci. 2017, 372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Sub-District | Risk * | Village | Open Water Source | Cattle Sampled (Seropositives, %) | Small Ruminants Sampled (Seropositives, %) | ||
|---|---|---|---|---|---|---|---|
| Households | Animals | Households | Animals | ||||
| Girimulyo | High | 1 | 59.3% | 65 (9, 13.9%) | 132 (10, 7.6%) | 1 (0, 0%) | 4 (0, 0%) |
| Kalibawang | 2 | 55.6% | 18 (4, 22.2%) | 23 (5, 21.7%) | 2 (0, 0%) | 4 (0, 0%) | |
| Lendah | 3 | 0% | 0 (0, 0%) | 0 (0, 0%) | 2 (0, 0%) | 4 (0, 0%) | |
| Lendah | 4 | 0% | 129 (6, 4.7%) | 205 (6, 2.9%) | 1 (0, 0%) | 4 (0, 0%) | |
| Lendah | 5 | 0% | 51 (0, 0%) | 74 (0, 0%) | 2 (0, 0%) | 4 (0, 0%) | |
| Nanggulan | 6 | 0% | 25 (1, 4%) | 46 (1, 2.2%) | 4 (0, 0%) | 4 (0, 0%) | |
| Panjatan | Medium | 7 | 0% | 36 (1, 2.8%) | 79 (1, 1.3%) | 2 (0, 0%) | 4 (0, 0%) |
| Panjatan | Medium | 8 | 0% | 55 (2, 3.6%) | 118 (2, 1.7%) | 3 (1, 33%) | 4 (1, 25%) |
| Pengasih | Low | 9 | 97.5% | 25 (0, 0%) | 37 (0, 0%) | 2 (0, 0%) | 4 (0, 0%) |
| Pengasih | Low | 10 | 0% | 109 (3, 2.7%) | 179 (4, 2.2%) | 2 (0, 0%) | 4 (0, 0%) |
| Samigaluh | 11 | 74.2% | 16 (4, 25%) | 27 (4, 14.8%) | 2 (1, 50%) | 4 (1, 25%) | |
| Wates | 12 | 0% | 76 (1, 1.3%) | 137(1, 0.7%) | 2 (0, 0%) | 4 (0, 0%) | |
| Wates | 13 | 2.4% | 52 (0, 0%) | 123 (0, 0%) | 2 (0, 0%) | 4 (0, 0%) | |
| Wates | 14 | 0% | 20 (7, 35%) | 61 (11, 18%) | 2 (0, 0%) | 4 (0, 0%) | |
| Wates | 15 | 0% | 76 (3, 4.0%) | 163 (7, 4.3%) | 2 (0, 0%) | 4 (0, 0%) | |
| Total | 9.2% | 757 (41, 5.4%) | 1404 (52, 3.7%) | 31 (2, 6.4%) | 60 (2, 3.3%) | ||
| Sub-District | Low Pengasih | Medium Panjatan | High Girimulyo | |||
|---|---|---|---|---|---|---|
| FGD 1 | FGD 2 | FGD 3 | FGD 4 | FGD 5 | FGD 6 | |
| Farmer | 5 | 4 | 3 | 5 | 4 | 4 |
| Head of village | 2 | 1 | 3 | 1 | 2 | 1 |
| Cattle farmer leader | 3 | 3 | 2 | 2 | 2 | 3 |
| Municipal health officer | 1 | 1 | 1 | 1 | 2 | 1 |
| Livestock services | 1 | 1 | 4 | 1 | 5 | 1 |
| Total | 12 | 10 | 13 | 10 | 15 | 10 |
| Odds Ratio | z | p-Value | 95% Confidence Interval | |
|---|---|---|---|---|
| Having another primary occupation | 3.0 | 2.7 | 0.007 | 1.4–6.8 |
| Using open water source compared to well or tap water | 4.0 | 3.4 | 0.001 | 1.8–9.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Widiasih, D.A.; Lindahl, J.F.; Artama, W.T.; Sutomo, A.H.; Kutanegara, P.M.; Mulyani, G.T.; Widodo, E.; Djohan, T.S.; Unger, F. Leptospirosis in Ruminants in Yogyakarta, Indonesia: A Serological Survey with Mixed Methods to Identify Risk Factors. Trop. Med. Infect. Dis. 2021, 6, 84. https://doi.org/10.3390/tropicalmed6020084
Widiasih DA, Lindahl JF, Artama WT, Sutomo AH, Kutanegara PM, Mulyani GT, Widodo E, Djohan TS, Unger F. Leptospirosis in Ruminants in Yogyakarta, Indonesia: A Serological Survey with Mixed Methods to Identify Risk Factors. Tropical Medicine and Infectious Disease. 2021; 6(2):84. https://doi.org/10.3390/tropicalmed6020084
Chicago/Turabian StyleWidiasih, Dyah Ayu, Johanna Frida Lindahl, Wayan T. Artama, Adi Heru Sutomo, Pande Made Kutanegara, Guntari Titik Mulyani, Estu Widodo, Tjut Sugandawaty Djohan, and Fred Unger. 2021. "Leptospirosis in Ruminants in Yogyakarta, Indonesia: A Serological Survey with Mixed Methods to Identify Risk Factors" Tropical Medicine and Infectious Disease 6, no. 2: 84. https://doi.org/10.3390/tropicalmed6020084
APA StyleWidiasih, D. A., Lindahl, J. F., Artama, W. T., Sutomo, A. H., Kutanegara, P. M., Mulyani, G. T., Widodo, E., Djohan, T. S., & Unger, F. (2021). Leptospirosis in Ruminants in Yogyakarta, Indonesia: A Serological Survey with Mixed Methods to Identify Risk Factors. Tropical Medicine and Infectious Disease, 6(2), 84. https://doi.org/10.3390/tropicalmed6020084







