Lymphocytic Choriomeningitis—Emerging Trends of a Neglected Virus: A Narrative Review
Abstract
:1. Introduction
2. Structure and Genome Organization of LCMV
3. Epidemiology of LCMV
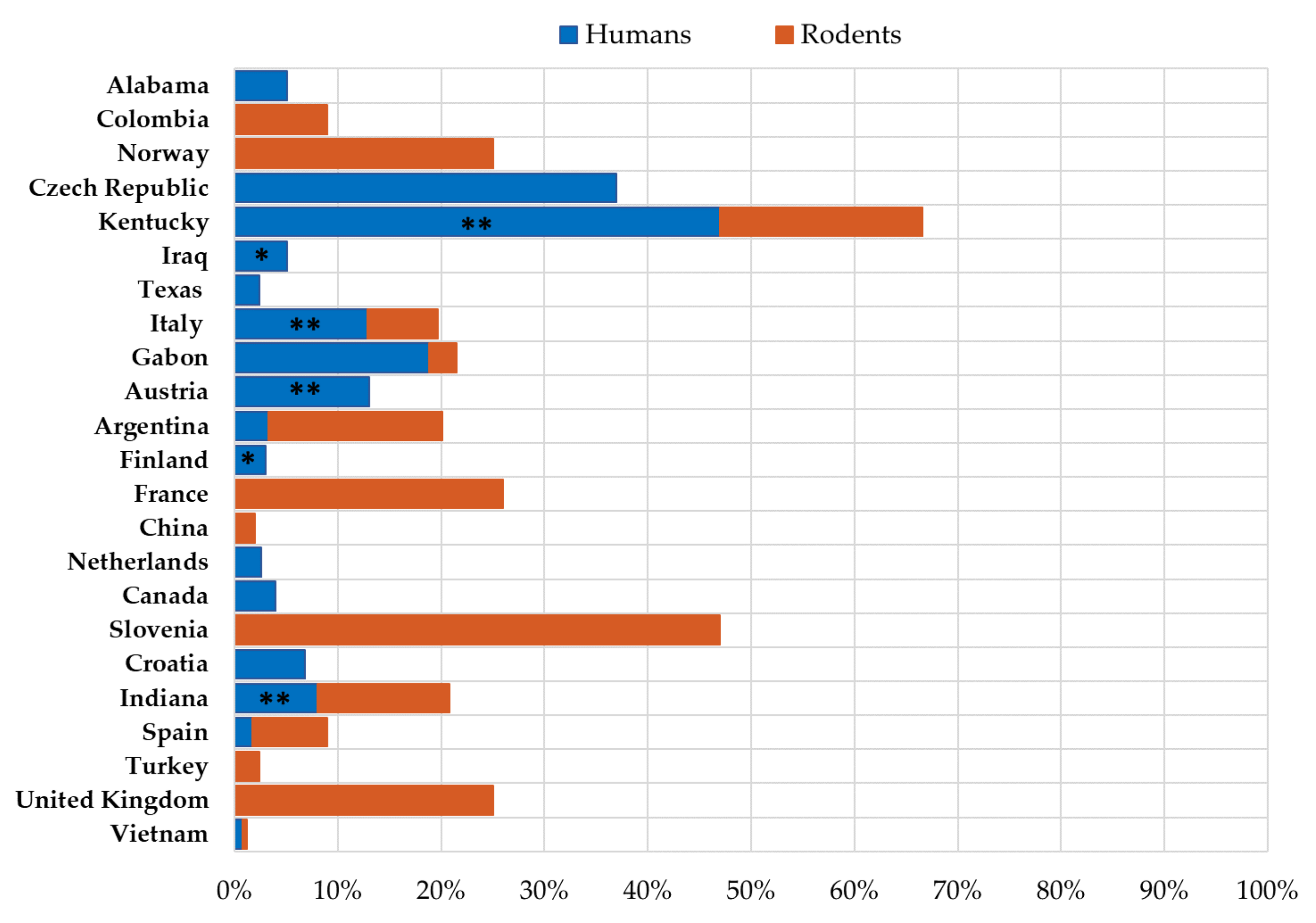
3.1. LCMV Prevalence in Humans
3.2. LCMV Prevalence in Rodents
3.3. Molecular Epidemiology of LCMV
4. Clinical Characteristics of LCMV Infection
4.1. LCMV Infection in Immunocompetent Individuals
4.2. LCMV Infection in Immunocompromised Patients/Transplantation Associated LCMV Infection
4.3. Congenital LCMV Infection
5. Diagnosis of LCMV Infection
6. Therapy of LCMV Infection
7. LCMV Infection in Mice as an Immunological Model
8. LCMV in Vaccine Research
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Traub, E. A filterable virus recovered from white mice. Science 1935, 81, 298–299. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.M.; Johnson, R.T.; Crawford, I.P.; Dascomb, H.E.; Rogers, N.G. Central nervous system syndromes of “viral” etiology. A study of 713 cases. Am. J. Med. 1960, 29, 334–347. [Google Scholar] [CrossRef]
- Ackermann, R.; Stille, W.; Blumenthal, W.; Helm, E.B.; Keller, K.; Baldus, O. Syrische goldhamster als uberträger von lymphozytärer choriomeningitis. Deutsche Medizinische Wochenschrift 1972, 97, 1725–1731. [Google Scholar] [CrossRef]
- Gregg, M.B. Recent outbreaks of lymphocytic choriomeningitis in the United States of America. Bull. World Health Org. 1975, 52, 549–553. [Google Scholar]
- Centers for Diseases Control and Prevention. Lymphocytic Choriomeningitis Virus (LCM). Available online: https://www.cdc.gov/vhf/lcm/index.html (accessed on 10 April 2021).
- Barakat, A.M.; Lapahat, O.; Hasoni, H.J. Lymphocytic choriomeningitis virus (LCMV) in Southern Iraq. Int. J. Sci. Eng. Res. 2015, 6, 1523–1535. [Google Scholar]
- Fevola, C.; Kuivanen, S.; Smura, T.; Vaheri, A.; Kallio-Kokko, H.; Hauffe, H.C.; Vapalahti, O.; Jääskeläinen, A.J. Seroprevalence of lymphocytic choriomeningitis virus and Ljungan virus in Finnish patients with suspected neurological infections. J. Med. Virol. 2018, 90, 429–435. [Google Scholar] [CrossRef]
- Komrower, G.M.; Williams, B.L.; Stones, P.B. Lymphocytic choriomeningitis in the newborn; Probable transplacental infection. Lancet 1955, 268, 697–698. [Google Scholar] [CrossRef]
- Ackermann, R.; Körver, G.; Turss, R.; Wönne, R.; Hochgesand, P. Pränatale infektion mit dem virus der lymphozytären choriomeningitis: Bericht über zwei fälle. Deutsche Medizinische Wochenschrift 1974, 99, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Sheinbergas, M. Hydrocephalus due to prenatal infection with the lymphocytic choriomeningitis virus. Infection 1976, 4, 185–191. [Google Scholar] [CrossRef]
- Chastel, C.; Bosshard, S.; Le Goff, F.; Quillien, M.C.; Gilly, R.; Aymard, M. Infection transplacentaire par le virus de la chorioméningite lymphocytaire. Résultats d’une enquête sérologique rétrospective en France. Nouvelle Presse Medicale 1978, 7, 1089–1092. [Google Scholar]
- Larsen, P.D.; Chartrand, S.A.; Tomashek, K.M.; Hauser, L.G.; Ksiazek, T.G. Hydrocephalus complicating lymphocytic choriomeningitis virus infection. Pediatr. Infect. Dis. J. 1993, 12, 528–531. [Google Scholar] [CrossRef]
- Wright, R.; Johnson, D.; Neumann, M.; Ksiazek, T.G.; Rollin, P.; Keech, R.V.; Bonthius, D.J.; Hitchon, P.; Grose, C.F.; Bell, W.E.; et al. Congenital lymphocytic choriomeningitis virus syndrome: A disease that mimics congenital toxoplasmosis or cytomegalovirus infection. Pediatrics 1997, 100, e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention (CDC). Lymphocytic choriomeningitis virus infection in organ transplant recipients-Massachusetts, Rhode Island, 2005. Morb. Mortal. Wkly. Rep. 2005, 54, 537–539. [Google Scholar]
- MacNeil, A.; Ströher, U.; Farnon, E.; Campbell, S.; Cannon, D.; Paddock, C.D.; Drew, C.P.; Kuehnert, M.; Knust, B.; Gruenenfelder, R.; et al. Solid organ transplant-associated lymphocytic choriomeningitis, United States, 2011. Emerg. Infect. Dis. 2012, 18, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Rollin, P.E.; Nichol, S.T.; Zaki, S.; Ksiazek, T.G. Arenaviruses and Filoviruses. Manual of Clinical Microbiology, 11th ed.; Jorgensen, J.H., Phaller, M.A., Carroll, K.C., Landry, M.L., Funke, G., Richter, S.S., Warnock, D.W., Eds.; ASM Press: Washington, DC, USA, 2015; pp. 1669–1686. [Google Scholar]
- Buchmeier, M.J.; Welsh, R.M.; Dutko, F.J.; Oldstone, M.B. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv. Immunol. 1980, 30, 275–331. [Google Scholar] [PubMed]
- Takagi, T.; Ohsawa, M.; Morita, C.; Sato, H.; Ohsawa, K. Genomic analysis and pathogenic characteristics of lymphocytic choriomeningitis virus strains isolated in Japan. Comp. Med. 2012, 62, 185–192. [Google Scholar]
- Takagi, T.; Ohsawa, M.; Yamanaka, H.; Matsuda, N.; Sato, H.; Ohsawa, K. Difference of two new LCMV strains in lethality and viral genome load in tissues. Exp. Anim. 2017, 66, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Lan, S.; Ou, R.; Price, G.E.; Jiang, H.; de la Torre, J.C.; Moskophidis, D. Genomic and biological characterization of aggressive and docile strains of lymphocytic choriomeningitis virus rescued from a plasmid-based reverse-genetics system. J. Gen. Virol. 2008, 89, 1421–1433. [Google Scholar] [CrossRef]
- Lapošová, K.; Pastoreková, S.; Tomášková, J. Lymphocytic choriomeningitis virus: Invisible but not innocent. Acta Virol. 2013, 57, 160–170. [Google Scholar] [CrossRef] [Green Version]
- Hotchin, J.; Sikora, E.; Kinch, W.; Hinman, A.; Woodall, J. Lymphocytic choriomeningitis in a hamster colony causes infection of hospital personnel. Science 1974, 185, 1173–1174. [Google Scholar] [CrossRef]
- Ackermann, R.; Stille, W.; Blumenthal, K. Syrische hamster als unbertrager von lymphozytarer chorio-meningitis. Deutsche Medizinische Wochenschrift 1972, 97, 1725–1731. [Google Scholar] [CrossRef] [PubMed]
- Montali, R.J.; Connolly, B.M.; Armstrong, D.L.; Scanga, C.A.; Holmes, K.V. Pathology and immunohistochemistry of callitrichid hepatitis, an emerging disease of captive New World primates caused by lymphocytic choriomeningitis virus. Am. J. Pathol. 1995, 147, 1441–1449. [Google Scholar] [PubMed]
- Adair, C.V.; Gauld, R.L.; Smadel, J.E. Aseptic meningitis, a disease of diverse etiology: Clinical and etiologic studies on 854 cases. Ann. Intern. Med. 1953, 39, 675–704. [Google Scholar] [CrossRef] [PubMed]
- Alburkat, H.; Jääskeläinen, A.J.; Barakat, A.M.; Hasony, H.J.; Sironen, T.; Al-Hello, H.; Smura, T.; Vapalahti, O. Lymphocytic choriomeningitis virus infections and seroprevalence, Southern Iraq. Emerg. Infect. Dis. 2020, 26, 3002–3006. [Google Scholar] [CrossRef] [PubMed]
- Lledó, L.; Gegúndez, M.I.; Saz, J.V.; Bahamontes, N.; Beltrán, M. Lymphocytic choriomeningitis virus infection in a province of Spain: Analysis of sera from the general population and wild rodents. J. Med. Virol. 2003, 70, 273–275. [Google Scholar] [CrossRef]
- Riera, L.; Castillo, E.; del Carmen, M.S.; Priotto, J.; Sottosanti, J.; Polop, J.; Ambrosio, A.M. Serological study of the lymphochoriomeningitis virus (LCMV) in an inner city of Argentina. J. Med. Virol. 2005, 76, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Kallio-Kokko, H.; Laakkonen, J.; Rizzoli, A.; Tagliapietra, V.; Cattadori, I.; Perkins, S.E.; Hudson, P.J.; Cristofolini, A.; Versini, W.; Vapalahti, O.; et al. Hantavirus and arenavirus antibody prevalence in rodents and humans in Trentino, Northern Italy. Epidemiol. Infect. 2006, 134, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Van Cuong, N.; Carrique-Mas, J.; Be, H.V.; An, N.N.; Tue, N.T.; Anh, N.L.; Anh, P.H.; Phuc, N.T.; Baker, S.; Voutilainen, L.; et al. Rodents and risk in the Mekong delta of Vietnam: Seroprevalence of selected zoonotic viruses in rodents and humans. Vector Borne Zoonotic Dis. 2015, 15, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrosio, A. Ecological and epidemiological features of lymphocytic choriomeningitis virus activity in Argentina. Curr. Top. Virol. 2014, 12, 53–63. [Google Scholar]
- Stephensen, C.B.; Blount, S.R.; Lanford, R.E.; Holmes, K.V.; Montali, R.J.; Fleenor, M.E.; Shaw, J.F. Prevalence of serum antibodies against lymphocytic choriomeningitis virus in selected populations from two, U.S. cities. J. Med. Virol. 1992, 38, 27–31. [Google Scholar] [CrossRef]
- Marrie, T.J.; Saron, M.F. Seroprevalence of lymphocytic choriomeningitis virus in Nova Scotia. Am. J. Trop. Med. Hyg. 1998, 58, 47–49. [Google Scholar] [CrossRef]
- Ushijima, Y.; Abe, H.; Ozeki, T.; Ondo, G.N.; Mbadinga, M.J.V.M.; Bikangui, R.; Nze-Nkogue, C.; Akomo-Okoue, E.F.; Ella, G.W.E.; Koumba, L.B.M.; et al. Identification of potential novel hosts and the risk of infection with lymphocytic choriomeningitis virus in humans in Gabon, Central Africa. Int. J. Infect. Dis. 2021, 105, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Vilibic-Cavlek, T.; Oreski, T.; Korva, M.; Kolaric, B.; Stevanovic, V.; Zidovec-Lepej, S.; Tabain, I.; Jelicic, P.; Miklausic-Pavic, B.; Savic, V.; et al. Prevalence and risk factors for lymphocytic choriomeningitis virus infection in continental Croatian regions. Trop. Med. Infect. Dis. 2021, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- De Lamballerie, X.; Fulhorst, C.F.; Charrel, R.N. Prevalence of antibodies to lymphocytic choriomeningitis virus in blood donors in Southeastern France. Transfusion 2007, 47, 172–173. [Google Scholar] [CrossRef]
- Knust, B.; MacNeil, A.; Wong, S.J.; Backenson, P.B.; Gibbons, A.; Rollin, P.E.; Nichol, S.T. Exposure to lymphocytic choriomeningitis virus, New York, USA. Emerg. Infect. Dis. 2011, 17, 1324–1325. [Google Scholar] [CrossRef] [PubMed]
- Juncker-Voss, M.; Prosl, H.; Lussy, H.; Enzenberg, U.; Auer, H.; Lassnig, H.; Müller, M.; Nowotny, N. Screening for antibodies against zoonotic agents among employees of the Zoological garden of Vienna, Schönbrunn, Austria. Berliner und Münchener Tierärztliche Wochenschrift 2004, 117, 404–409. [Google Scholar]
- Tagliapietra, V.; Rosà, R.; Rossi, C.; Rosso, F.; Hauffe, H.C.; Tommasini, M.; Versini, W.; Cristallo, A.F.; Rizzoli, A. Emerging rodent-borne viral zoonoses in Trento, Italy. Ecohealth 2018, 15, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Knust, B.; Ströher, U.; Edison, L.; Albariño, C.G.; Lovejoy, J.; Armeanu, E.; House, J.; Cory, D.; Horton, C.; Fowler, K.L.; et al. Lymphocytic choriomeningitis virus in employees and mice at multipremises feeder-rodent operation, United States, 2012. Emerg. Infect. Dis. 2014, 20, 240–247. [Google Scholar] [CrossRef]
- Reiserova, L.; Kaluzova, M.; Kaluz, S.; Willis, A.C.; Zavada, J.; Zavodsk, E.; Zavadova, Z.; Ciampor, F.; Pastorek, J.; Pastorekova, S. Identification of MaTu-MX agent as a new strain of lymphocytic choriomeningitis virus (LCMV) and serological indication of horizontal spread of LCMV in human population. Virology 1999, 257, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Dobec, M.; Dzelalija, B.; Punda-Polic, V.; Zoric, I. High prevalence of antibodies to lymphocytic choriomeningitis virus in a murine typhus endemic region in Croatia. J. Med. Virol. 2006, 78, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Vilibic-Cavlek, T.; Barbic, L.; Mrzljak, A.; Brnic, D.; Klobucar, A.; Ilic, M.; Janev-Holcer, N.; Bogdanic, M.; Jemersic, L.; Stevanovic, V.; et al. Emerging and neglected viruses of zoonotic importance in Croatia. Pathogens 2021, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Morita, C.; Tsuchiya, K.; Ueno, H.; Muramatsu, Y.; Kojimahara, A.; Suzuki, H.; Miyashita, N.; Moriwaki, K.; Jin, M.L.; Wu, X.L.; et al. Seroepidemiological survey of lymphocytic choriomeningitis virus in wild house mice in China with particular reference to their subspecies. Microbiol. Immunol. 1996, 40, 313–315. [Google Scholar] [CrossRef] [Green Version]
- Stuart, A.M.; Prescott, C.V.; MacIntyre, S.; Sethar, A.; Neuman, B.W.; McCarthy, N.D.; Wimalarathna, H.; Maiden, M.C.J. The role of rodents as carriers of disease on UK farms: A preliminary investigation. In Proceedings of the 8th European Vertebrate Pest Management Conference, Berlin, Germany, 26–30 September 2011; Julius-Kühn-Archiv: Quedlinburg, Germany, 2011; Volume 432, pp. 198–199. [Google Scholar] [CrossRef]
- Tagliapietra, V.; Rosà, R.; Hauffe, H.C.; Laakkonen, J.; Voutilainen, L.; Vapalahti, O.; Vaheri, A.; Henttonen, H.; Rizzoli, A. Spatial and temporal dynamics of lymphocytic choriomeningitis virus in wild rodents, Northern Italy. Emerg. Infect. Dis. 2009, 15, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Laakkonen, J.; Kallio-Kokko, H.; Oktem, M.A.; Blasdell, K.; Plyusnina, A.; Niemimaa, J.; Karataş, A.; Plyusnin, A.; Vaheri, A.; Henttonen, H. Serological survey for viral pathogens in Turkish rodents. J. Wildl. Dis. 2006, 42, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Castellar, A.; Guevara, M.; Rodas, J.D.; Londoño, A.F.; Arroyave, E.; Díaz, F.J.; Levis, S.; Blanco, P.J. First evidence of lymphocytic choriomeningitis virus (Arenavirus) infection in Mus musculus rodents captured in the urban area of the municipality of Sincelejo, Sucre, Colombia. Biomedica 2017, 37, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Duh, D.; Hasic, S.; Buzan, E. The impact of illegal waste sites on a transmission of zoonotic viruses. Virol. J. 2017, 14, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albariño, C.G.; Palacios, G.; Khristova, M.L.; Erickson, B.R.; Carroll, S.A.; Comer, J.A.; Hui, J.; Briese, T.; St. George, K.; Ksiazek, T.G.; et al. High diversity and ancient common ancestry of lymphocytic choriomeningitis virus. Emerg. Infect. Dis. 2010, 16, 1093–1100. [Google Scholar] [CrossRef] [Green Version]
- Lavergne, A.; de Thoisy, B.; Tirera, S.; Donato, D.; Bouchier, C.; Catzeflis, F.; Lacoste, V. Identification of lymphocytic choriomeningitis mammarenavirus in house mouse (Mus musculus, Rodentia) in French Guiana. Infect. Genet. Evol. 2016, 37, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Yama, I.N.; Cazaux, B.; Britton-Davidian, J.; Moureau, G.; Thirion, L.; de Lamballerie, X.; Dobigny, G.; Charrel, R.N. Isolation and characterization of a new strain of lymphocytic choriomeningitis virus from rodents in southwestern France. Vector Borne Zoonotic Dis. 2012, 12, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, S.; Huang, S.J.; Wang, Z.D.; Wei, F.; Feng, X.M.; Jiang, D.X.; Liu, Q. Isolation and genomic characterization of lymphocytic choriomeningitis virus in ticks from northeastern China. Transbound. Emerg. Dis. 2018, 65, 1733–1739. [Google Scholar] [CrossRef]
- Bonthius, D.J. The arenaviruses. In Neurotropic Viral Infections; Reiss, C.S., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 149–174. [Google Scholar] [CrossRef]
- Fischer, S.A.; Graham, M.B.; Kuehnert, M.J.; Kotton, C.N.; Srinivasan, A.; Marty, F.M.; Comer, J.A.; Guarner, J.; Paddock, C.D.; DeMeo, D.L.; et al. LCMV in transplant recipients investigation team. Transmission of lymphocytic choriomeningitis virus by organ transplantation. N. Engl. J. Med. 2006, 354, 2235–2249. [Google Scholar] [CrossRef]
- Palacios, G.; Druce, J.; Du, L.; Tran, T.; Birch, C.; Briese, T.; Conlan, S.; Quan, P.L.; Hui, J.; Marshall, J.; et al. A new arenavirus in a cluster of fatal transplant-associated diseases. N. Engl. J. Med. 2008, 358, 991–998. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention (CDC). Brief report: Lymphocytic choriomeningitis virus transmitted through solid organ transplantation—Massachusetts, 2008. Morb. Mortal. Wkly. Rep. 2008, 57, 799–801. [Google Scholar]
- Schafer, I.J.; Miller, R.; Ströher, U.; Knust, B.; Nichol, S.T.; Rollin, P.E.; Centers for Disease Control and Prevention (CDC). Notes from the field: A cluster of lymphocytic choriomeningitis virus infections transmitted through organ transplantation—Iowa, 2013. Morb. Mortal. Wkly. Rep. 2014, 63, 249. [Google Scholar]
- Mathur, G.; Yadav, K.; Ford, B.; Schafer, I.J.; Basavaraju, S.V.; Knust, B.; Shieh, W.-J.; Hill, S.; Locke, G.D.; Quinlisk, P.; et al. High clinical suspicion of donor-derived disease leads to timely recognition and early intervention to treat solid organ transplant-transmitted lymphocytic choriomeningitis virus. Transpl. Infect. Dis. 2017, 19, e12707. [Google Scholar] [CrossRef]
- Bonthius, D.J.; Mahoney, J.; Buchmeier, M.J.; Karacay, B.; Taggard, D. Critical role for glial cells in the propagation and spread of lymphocytic choriomeningitis virus in the developing rat brain. J. Virol. 2002, 76, 6618–6635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonthius, D.J. Lymphocytic choriomeningitis virus: An underrecognized cause of neurologic disease in the fetus, child, and adult. Semin. Pediatr. Neurol. 2012, 19, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Folk, S.; Steinbecker, S.; Windmeyer, J.; Macneil, A.; Campbell, S.; Rollin, P.E. Lymphocytic choriomeningitis with severe manifestations, Missouri, USA. Emerg. Infect. Dis. 2011, 17, 1973–1974. [Google Scholar] [CrossRef] [PubMed]
- Anesi, J.A.; Silveira, F.P.; AST infectious diseases community of practice. Arenaviruses and west Nile virus in solid organ transplant recipients: Guidelines from the American society of transplantation infectious diseases community of practice. Clin. Transplant. 2019, 33, e13576. [Google Scholar] [CrossRef] [PubMed]
- Tanveer, F.; Younas, M.; Fishbain, J. Lymphocytic choriomeningitis virus meningoencephalitis in a renal transplant recipient following exposure to mice. Transpl. Infect. Dis. 2018, 20, e13013. [Google Scholar] [CrossRef] [PubMed]
- Barton, L.L.; Mets, M.B. Congenital lymphocytic choriomeningitis virus infection: Decade of rediscovery. Clin. Infect. Dis. 2001, 33, 370–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamieson, D.J.; Kourtis, A.P.; Bell, M.; Rasmussen, S.A. Lymphocytic choriomeningitis virus: An emerging obstetric pathogen? Am. J. Obstet. Gynecol. 2006, 194, 1532–1536. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.L.; Levy, P.T.; Leonard, K.B.; Smyser, C.D.; Tychsen, L.; Cole, F. Sessions. Congenital lymphocytic choriomeningitis virus: When to consider the diagnosis. J. Child. Neurol. 2014, 29, 837–842. [Google Scholar] [CrossRef] [Green Version]
- Enders, G.; Varho-Göbel, M.; Löhler, J.; Terletskaia-Ladwig, E.; Eggers, M. Congenital lymphocytic choriomeningitis virus infection: An underdiagnosed disease. Pediatr. Infect. Dis. J. 1999, 18, 652–655. [Google Scholar] [CrossRef]
- Sheinbergas, M.M.; Lewis, V.J.; Thacker, W.L.; Verikiene, V.V. Serological diagnosis in children infected prenatally with lymphocytic choriomeningitis virus. Infect. Immun. 1981, 31, 837–838. [Google Scholar] [CrossRef] [Green Version]
- Brézin, A.P.; Thulliez, P.; Cisneros, B.; Mets, M.B.; Saron, M.F. Lymphocytic choriomeningitis virus chorioretinitis mimicking ocular toxoplasmosis in two otherwise normal children. Am. J. Ophthalmol. 2000, 130, 245–247. [Google Scholar] [CrossRef]
- Mets, M.B.; Barton, L.L.; Khan, A.S.; Ksiazek, T.G. Lymphocytic choriomeningitis virus: An underdiagnosed cause of congenital chorioretinitis. Am. J. Ophthalmol. 2000, 130, 209–215. [Google Scholar] [CrossRef]
- Delaine, M.; Weingertner, A.-S.; Nougairede, A.; Lepiller, Q.; Fafi-Kremer, S.; Favre, R.; Charrel, R. Microcephaly caused by lymphocytic choriomeningitis virus. Emerg. Infect. Dis. 2017, 23, 1548–1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barton, L. Lymphocytic choriomeningitis virus: An unrecognized teratogenic pathogen. Emerg. Infect. Dis. 1995, 1, 152–153. [Google Scholar] [CrossRef]
- Barton, L.L.; Peters, C.J.; Seaver, L.H.; Chartrand, S.A. Congenital lymphocytic choriomeningitis virus infection. Arch. Pediatr. Adolesc. Med. 1996, 150, 440. [Google Scholar] [CrossRef]
- Bechtel, R.T.; Haught, K.A.; Mets, M.B. Lymphocytic choriomeningitis virus: A new addition to the TORCH evaluation. Arch. Ophthalmol. 1997, 115, 680–681. [Google Scholar] [CrossRef]
- Barton, L.L.; Mets, M.B.; Beauchamp, C.L. Lymphocytic choriomeningitis virus: Emerging fetal teratogen. Am. J. Obstet. Gynecol. 2002, 187, 1715–1716. [Google Scholar] [CrossRef]
- Greenhow, T.L.; Weintrub, P.S. Your diagnosis, please. Neonate with hydrocephalus. Pediatr. Infect. Dis. J. 2003, 22, 1111–1112. [Google Scholar] [CrossRef]
- Schulte, D.J.; Comer, J.A.; Erickson, B.R.; Rollin, P.E.; Nichol, S.T.; Ksiazek, T.G.; Lehman, D. Congenital lymphocytic choriomeningitis virus: An underdiagnosed cause of neonatal hydrocephalus. Pediatr. Infect. Dis. J. 2006, 25, 560–562. [Google Scholar] [CrossRef]
- Yu, J.T.; Culican, S.M.; Tychsen, L. Aicardi-like chorioretinitis and maldevelopment of the corpus callosum in congenital lymphocytic choriomeningitis virus. J. AAPOS 2006, 10, 58–60. [Google Scholar] [CrossRef]
- Bonthius, D.J.; Wright, R.; Tseng, B.; Barton, L.; Marco, E.; Karacay, B.; Larsen, P.D. Congenital lymphocytic choriomeningitis virus infection: Spectrum of disease. Ann. Neurol. 2007, 62, 347–355. [Google Scholar] [CrossRef]
- El Feghaly, R.E.; Hunstad, D.A. A newborn with hydrops, hydrocephalus, and ophthalmologic abnormalities. J. Pediatr. Infect. Dis. Soc. 2013, 2, 391–393. [Google Scholar] [CrossRef] [Green Version]
- Meritet, J.F.; Krivine, A.; Lewin, F.; Poissonnier, M.H.; Poizat, R.; Loget, P.; Rozenberg, F.; Lebon, P. A case of congenital lymphocytic choriomeningitis virus (LCMV) infection revealed by hydrops fetalis. Prenat. Diagn. 2009, 29, 626–627. [Google Scholar] [CrossRef] [PubMed]
- Kinori, M.; Schwartzstein, H.; Zeid, J.L.; Kurup, S.P.; Mets, M.B. Congenital lymphocytic choriomeningitis virus—An underdiagnosed fetal teratogen. J. AAPOS 2018, 22, 79.e1–81.e1. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.R.; Peters, C.J. Diseases of the central nervous system caused by lymphocytic choriomeningitis virus and other arenaviruses. In Handbook of Clinical Neurology, 3rd ed.; Tselis, A.C., Booss, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 123, pp. 671–681. [Google Scholar]
- Cordey, S.; Sahli, R.; Moraz, M.L.; Estrade, C.; Morandi, L.; Cherpillod, P.; Charrel, R.N.; Kunz, S.; Kaiser, L. Analytical validation of a lymphocytic choriomeningitis virus real-time RT-PCR assay. J. Virol. Methods 2011, 177, 118–122. [Google Scholar] [CrossRef] [Green Version]
- Takimoto, K.; Taharaguchi, M.; Morikawa, S.; Ike, F.; Yamada, Y.K. Detection of the antibody to lymphocytic choriomeningitis virus in sera of laboratory rodents infected with viruses of laboratory and newly isolated strains by ELISA using purified recombinant nucleoprotein. Exp. Anim. 2008, 57, 357–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapošová, K.; Lukáčiková, L.; Ovečková, I.; Pastoreková, S.; Rosocha, J.; Kuba, D.; Beňa, L.; Tomášková, J. Development and application of ELISA for the detection of IgG antibodies to lymphocytic choriomeningitis virus. Acta Virol. 2016, 60, 143–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez-Olivencia, G.; Estébanez, M.; Membrillo, F.J.; Ybarra, M.D.C. Use of ribavirin in viruses other than hepatitis C. A review of the evidence. Enferm. Infecc. Microbiol. Clin. 2019, 37, 602–608. [Google Scholar] [CrossRef]
- Tian, D.; Liu, Y.; Liang, C.; Xin, L.; Xie, X.; Zhang, D.; Wan, M.; Li, H.; Fu, X.; Liu, H.; et al. An update review of emerging small-molecule therapeutic options for COVID-19. Biomed. Pharmacother. 2021, 137, 111313. [Google Scholar] [CrossRef]
- Hickerson, B.T.; Westover, J.B.; Jung, K.H.; Komeno, T.; Furuta, Y.; Gowen, B.B. Effective treatment of experimental lymphocytic choriomeningitis virus infection: Consideration of Favipiravir for use with infected organ transplant recipients. J. Infect. Dis. 2018, 218, 522–527. [Google Scholar] [CrossRef] [Green Version]
- Indari, O.; Jakhmola, S.; Manivannan, E.; Jha, H.C. An update on antiviral therapy against SARS-CoV-2: How far have we come? Front. Pharmacol. 2021, 12, 632677. [Google Scholar] [CrossRef]
- Herring, S.; Oda, J.M.; Wagoner, J.; Kirchmeier, D.; O’Connor, A.; Nelson, E.A.; Huang, Q.; Liang, Y.; DeWald, L.E.; Johansen, L.M.; et al. Inhibition of arenaviruses by combinations of orally available approved drugs. Antimicrob. Agents Chemother. 2021, 65, e01146. [Google Scholar] [CrossRef]
- Wan, W.; Zhu, S.; Li, S.; Shang, W.; Zhang, R.; Li, H.; Liu, W.; Xiao, G.; Peng, K.; Zhang, L. High-throughput screening of an FDA-approved drug library identifies inhibitors against arenaviruses and SARS-CoV-2. ACS Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Kim, Y.J.; Cubitt, B.; Chen, E.; Hull, M.V.; Chatterjee, A.K.; Cai, Y.; Kuhn, J.H.; de la Torre, J.C. The ReFRAME library as a comprehensive drug repurposing library to identify mammarenavirus inhibitors. Antivir. Res. 2019, 169, 104558. [Google Scholar] [CrossRef]
- Bösch, N.M.; Borsa, M.; Greczmiel, U.; Morinaka, B.I.; Gugger, M.; Oxenius, A.; Vagstad, A.L.; Piel, J. Landornamides: Antiviral ornithine-containing ribosomal peptides discovered through genome mining. Angewandte Chemie 2020, 59, 11763–11768. [Google Scholar] [CrossRef]
- Mire, C.E.; Cross, R.W.; Geisbert, J.B.; Borisevich, V.; Agans, K.N.; Deer, D.J.; Heinrich, M.L.; Rowland, M.M.; Goba, A.; Momoh, M.; et al. Human-monoclonal-antibody therapy protects nonhuman primates against advanced Lassa fever. Nat. Med. 2017, 23, 1146–1149. [Google Scholar] [CrossRef] [Green Version]
- Robinson, J.E.; Hastie, K.M.; Cross, R.W.; Yenni, R.; Elliott, D.H.; Rouelle, J.A.; Kannadka, C.B.; Smira, A.A.; Garry, C.E.; Bradley, B.T.; et al. Most neutralizing human monoclonal antibodies target novel epitopes requiring both Lassa virus glycoprotein subunits. Nat. Commun. 2016, 7, 11544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cross, R.W.; Hastie, K.M.; Mire, C.E.; Robinson, J.E.; Geisbert, T.W.; Branco, L.M.; Saphire, E.O.; Garry, R.F. Antibody therapy for Lassa fever. Curr. Opin. Virol. 2019, 37, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ramachandran, S.; Mann, M.; Popkin, D.L. Role of lymphocytic choriomeningitis virus (LCMV) in understanding viral immunology: Past, present and future. Viruses 2012, 4, 2650–2669. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Hakeem, M.S. Viruses teaching immunology: Role of LCMV model and human viral infections in immunological discoveries. Viruses 2019, 11, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dangi, T.; Chung, Y.R.; Palacio, N.; Penaloza-MacMaster, P. Interrogating adaptive immunity using LCMV. Curr. Protoc. Immunol. 2020, 130, 99. [Google Scholar] [CrossRef]
- Suprunenko, T.; Hofer, M.J. Complexities of type I interferon biology: Lessons from LCMV. Viruses 2019, 11, 172. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.R.; Ashhurst, T.M.; West, P.K.; Viengkhou, B.; King, N.J.C.; Campbell, I.L.; Hofer, M.J. Contribution of STAT1 to innate and adaptive immunity during type I interferon-mediated lethal virus infection. PLoS Pathog. 2020, 16, e1008525. [Google Scholar] [CrossRef] [Green Version]
- Daniels, K.A.; O’Donnell, C.L.; Castonguay, C.; Strutt, T.M.; McKinstry, K.K.; Swain, S.L.; Welsh, R.M. Virus-induced natural killer cell lysis of T cell subsets. Virology 2020, 539, 26–37. [Google Scholar] [CrossRef]
- Elsaesser, H.J.; Mohtashami, M.; Osokine, I.; Snell, L.M.; Cunningham, C.R.; Boukhaled, G.M.; McGavern, D.B.; Zúñiga-Pflücker, J.C.; Brooks, D.G. Chronic virus infection drives CD8 T cell-mediated thymic destruction and impaired negative selection. Proc. Natl. Acad. Sci. USA 2020, 117, 5420–5429. [Google Scholar] [CrossRef] [PubMed]
- Schorer, M.; Lambert, K.; Rakebrandt, N.; Rost, F.; Kao, K.C.; Yermanos, A.; Spörri, R.; Oderbolz, J.; Raeber, M.E.; Keller, C.W.; et al. Rapid expansion of Treg cells protects from collateral colitis following a viral trigger. Nat. Commun. 2020, 23, 1522. [Google Scholar] [CrossRef] [PubMed]
- Lercher, A.; Bhattacharya, A.; Popa, A.M.; Caldera, M.; Schlapansky, M.F.; Baazim, H.; Agerer, B.; Gürtl, B.; Kosack, L.; Májek, P.; et al. Type I interferon signaling disrupts the hepatic urea cycle and alters systemic metabolism to suppress T cell function. Immunity 2019, 51, 1074–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoycheva, D.; Sandu, I.; Gräbnitz, F.; Amorim, A.; Borsa, M.; Weber, S.; Becher, B.; Oxenius, A. Non-neutralizing antibodies protect against chronic LCMV infection by promoting infection of inflammatory monocytes in mice. Eur. J. Immunol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Krolik, M.; Csepregi, L.; Hartmann, F.; Engetschwiler, C.; Flatz, L. Recombinant lymphocytic choriomeningitis virus-based vaccine vector protects type I interferon receptor deficient mice from viral challenge. Vaccine 2021, 39, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Bonilla, W.V.; Reiter, A.; Stemeseder, F.; Kleissner, T.; Oeler, D.; Berka, U.; El-Gazzar, A.; Kiefmann, B.; Schulha, S.C.; et al. Live-attenuated lymphocytic choriomeningitis virus-based vaccines for active immunotherapy of HPV16-positive cancer. Oncoimmunology 2020, 9, 1809960. [Google Scholar] [CrossRef] [PubMed]



| Population | Clinical Symptoms | Outcome | Reference |
|---|---|---|---|
| Immunocompetent individuals | Asymptomatic infection, flu-like disease, aseptic meningitis/meningoencephalitis | Recovery in most cases; mortality < 1% | [54] |
| Organ transplant recipients | Fatal hemorrhagic fever-like disease | Mortality > 70% | [15,55,56,57,58,59] |
| Pregnant women | Asymptomatic infection, flu-like disease, aseptic meningitis/meningoencephalitis; symptoms may be severe | Depending on the time of infection: spontaneous abortion, congenital LCMV infection | [60,61] |
| Newborns (congenital infection) | Hydrocephalus, periventricular calcifications, chorioretinitis | Mortality ~ 35%; persistent neurologic sequelae ~ 70% |
| Country/Year | N Cases | Clinical Features | CT/MRI Imaging | Reference |
|---|---|---|---|---|
| England 1955 | 1 | Convulsion, opisthotonos, subarachnoid and intracerebral haemorrhage, petechiae, ventriculomegaly | ND * | [8] |
| Germany 1974, 1999 | 8 (2-twins) | Hydrocephalus, microcephaly, intracranial calcifications, chorioretinitis, chorioretinal scars, blindness, conjuctivitis, developmental delay, myocarditis, congestive heart failure, psychomotor retardation, meningitis | ND | [9,68] |
| Lithuania 1976, 1981 | 22 | Hydrocephalus, microcephaly, spastic tetraparesis, epilepsy-like attacks, chorioretinal degeneration, optic disc subatrophy, microphtalmy, cataract, psychomotor retardation | ND | [10,69] |
| France 1978, 2000, 2009, 2017 | 6 (2-twins) | Hydrocephalus, ventriculomegaly, microcephaly, periventricular calcifications, chorioretinitis chorioretinal scars, fetal hydrops, hepatosplenomegaly, cardiomegaly, ascites, pericardial and pleural effusion | MRI: normal (2 cases) | [11,70,71,72] |
| USA 1993, 1995, 1996, 1997, 2000, 2002, 2003, 2006, 2007, 2013, 2014, 2018 | 45 (2-twins) | Hydrocephalus, microcephaly, dolichocephaly, chorioretinitis, optic nerve atrophy, retinal colobomas, microphtalmia, blindness, hearing loss, developmental delay, pyschomotor retardation, spastic quadriplegia, seizures, heart abnormalities, ataxia, fetal hydrops, cataracts | CT: periventricular and intracranial calcifications, diffuse and periventricular brain substance loss, gyral malformations, shizencephaly, cerebellar hypoplasia, calcification of the lens MRI: ventriculomegaly, cerebral atrophy, corpus callosum atrophy and agenesis, encephalomalacia, cerebellar hypoplasia, intracranial hemorrhage, periventricular cysts | [12,13,67,71,73,74,75,76,77,78,79,80,81,82,83] |
| Immune Responses | Areas of Immunological Research with a Major Contribution of LCMV Mouse Experimental Model |
|---|---|
| Innate immunity | |
| Recognition of pathogen-associated molecular patterns by pattern-recognition receptors | Recognition of LCMV single-stranded RNA by TLR-7 and -8; recognition of double-stranded RNA and 5′-triphosphate RNA that are synthesized during the LCMV replication cycle by MDA-5 and RIG-I; role of TLR-2 in the immune response to LCMV |
| Innate immunity signal-transduction pathways and transcription factors | Signaling pathways leading to the activation of transcription factors IRF-3, AP-1 and NF-κB that induce the synthesis of IFN-β, IFN-α4 and other pro-inflammatory cytokines |
| Biology of type I IFNs | Role of interferons in regulating the activity of innate immune cells; differential regulation of interferon-stimulated genes during infection with various LCMV strains; relative contribution of STAT1 on innate and adaptive immunity during LCMV infection; role of IFN-mediated signals in CD8+ T-cell responses |
| The role of NK-cells in the pathogenesis of viral infections | Cytolytic effect of NK-cells on activated CD4+ and CD8+ T-cells in viral infection; Treg, Th17 and Th2 cells are more sensitive to lysis by LCMV-induced activated NK-cells |
| Specific immunity | |
| Thymic depletion in chronic viral infections | Chronic LCMV infection induces severe thymic depletion, mediated by CD8+ T cell-intrinsic type I IFNs and STAT-2 signaling pathway |
| Viral infection as a trigger of Treg cell impairment and associated immune-mediated pathology in autoimmunity | LCMV infection leads to the loss of IFN type I-dependent Treg cells, which is subsequently compensated by the conversion of Vβ5+ conventional T cells into iTreg cells; delayed replenishment of Treg cells in Vβ5-deficient mice compromises suppression of microbiota-dependent activation of CD8+ T-cells leading to the development of colitis |
| Metabolic alterations in the liver during chronic viral infections | Type I interferon-mediated suppression of the hepatic urea cycle and subsequent suppression of virus-specific CD8+ T-cell responses and ameliorated liver pathology |
| Indirect protective role of non-neutralizing antibodies in viral infections | LCMV-specific monoclonal Abs can prevent the establishment of chronic infection in an Fc-receptor-independent manner by inducing the differentiation of Ly6Chi inflammatory monocytes into antigen-presenting cells leading to an early activation of virus-specific CD8+ T-cells |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilibic-Cavlek, T.; Savic, V.; Ferenc, T.; Mrzljak, A.; Barbic, L.; Bogdanic, M.; Stevanovic, V.; Tabain, I.; Ferencak, I.; Zidovec-Lepej, S. Lymphocytic Choriomeningitis—Emerging Trends of a Neglected Virus: A Narrative Review. Trop. Med. Infect. Dis. 2021, 6, 88. https://doi.org/10.3390/tropicalmed6020088
Vilibic-Cavlek T, Savic V, Ferenc T, Mrzljak A, Barbic L, Bogdanic M, Stevanovic V, Tabain I, Ferencak I, Zidovec-Lepej S. Lymphocytic Choriomeningitis—Emerging Trends of a Neglected Virus: A Narrative Review. Tropical Medicine and Infectious Disease. 2021; 6(2):88. https://doi.org/10.3390/tropicalmed6020088
Chicago/Turabian StyleVilibic-Cavlek, Tatjana, Vladimir Savic, Thomas Ferenc, Anna Mrzljak, Ljubo Barbic, Maja Bogdanic, Vladimir Stevanovic, Irena Tabain, Ivana Ferencak, and Snjezana Zidovec-Lepej. 2021. "Lymphocytic Choriomeningitis—Emerging Trends of a Neglected Virus: A Narrative Review" Tropical Medicine and Infectious Disease 6, no. 2: 88. https://doi.org/10.3390/tropicalmed6020088
APA StyleVilibic-Cavlek, T., Savic, V., Ferenc, T., Mrzljak, A., Barbic, L., Bogdanic, M., Stevanovic, V., Tabain, I., Ferencak, I., & Zidovec-Lepej, S. (2021). Lymphocytic Choriomeningitis—Emerging Trends of a Neglected Virus: A Narrative Review. Tropical Medicine and Infectious Disease, 6(2), 88. https://doi.org/10.3390/tropicalmed6020088









