Encephalitis in Thailand: A Neglected Disease Increasingly Caused by Enterovirus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. The BioFire FilmArray Meningitis/Encephalitis Panel
2.3. Nucleic Acid Extraction
2.4. Real-Time Reverse Transcription Polymerase Chain Reaction
2.5. Enteroviruses Family Polymerase Chain Reaction
2.6. Ethics Statement
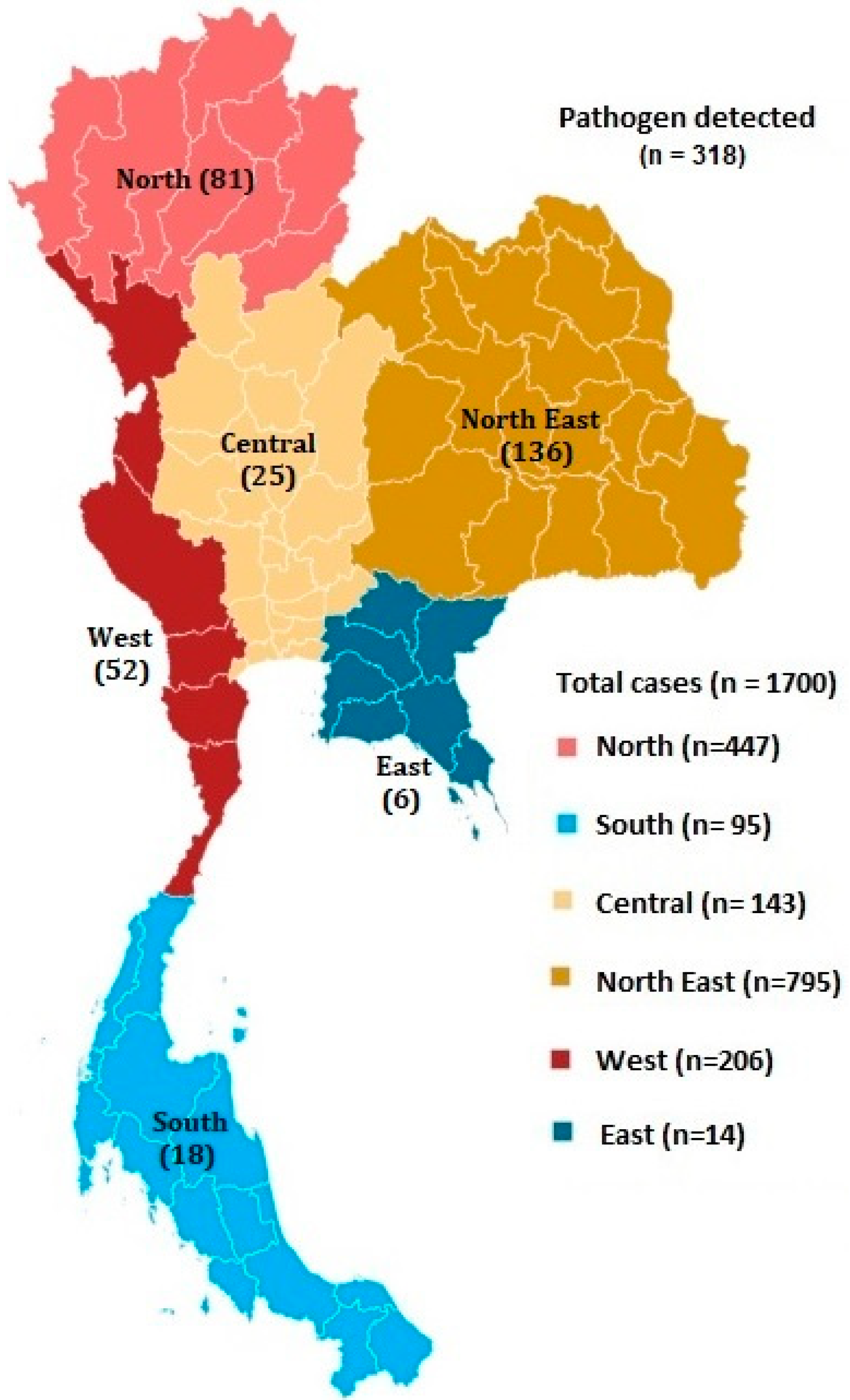
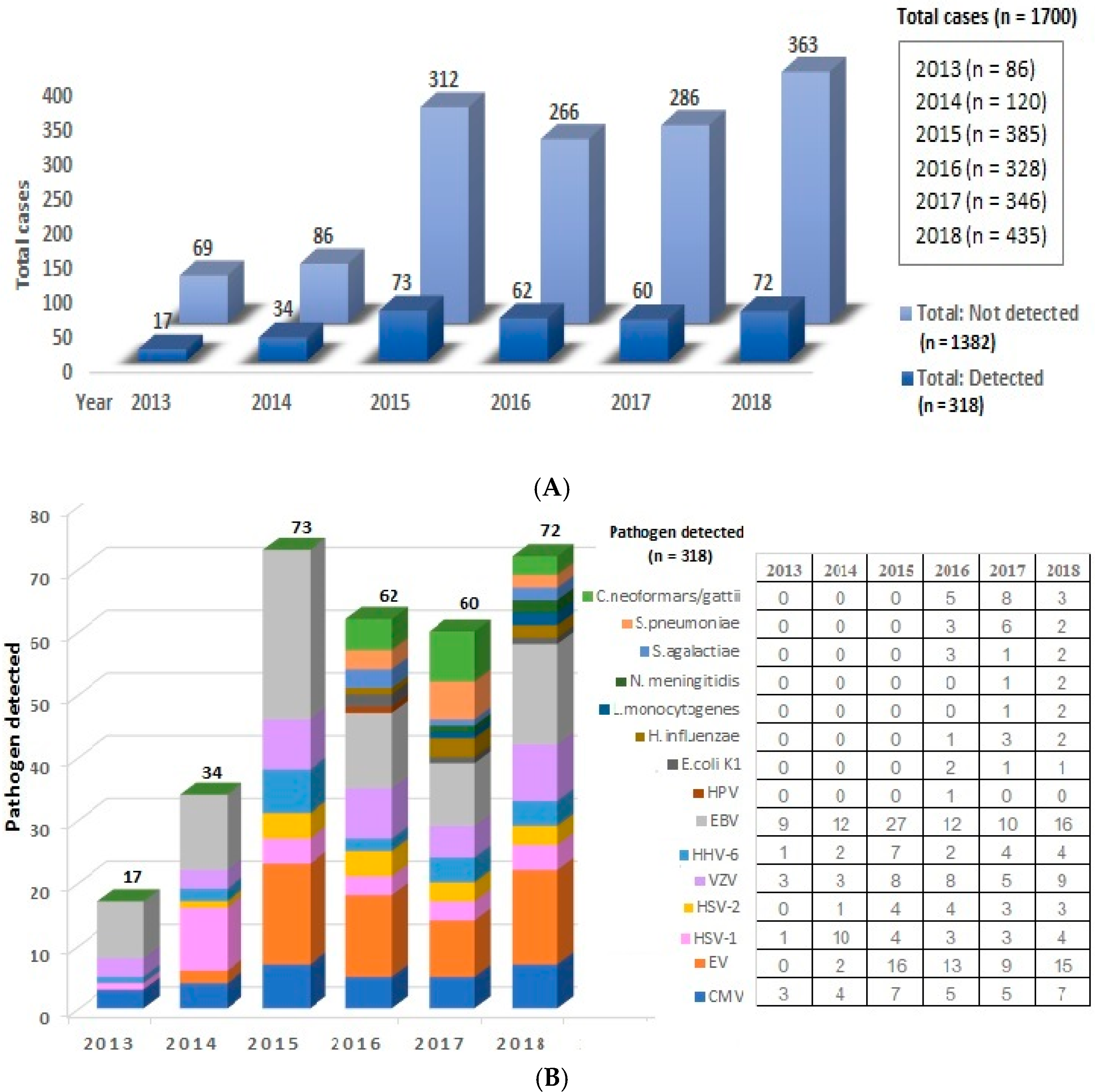
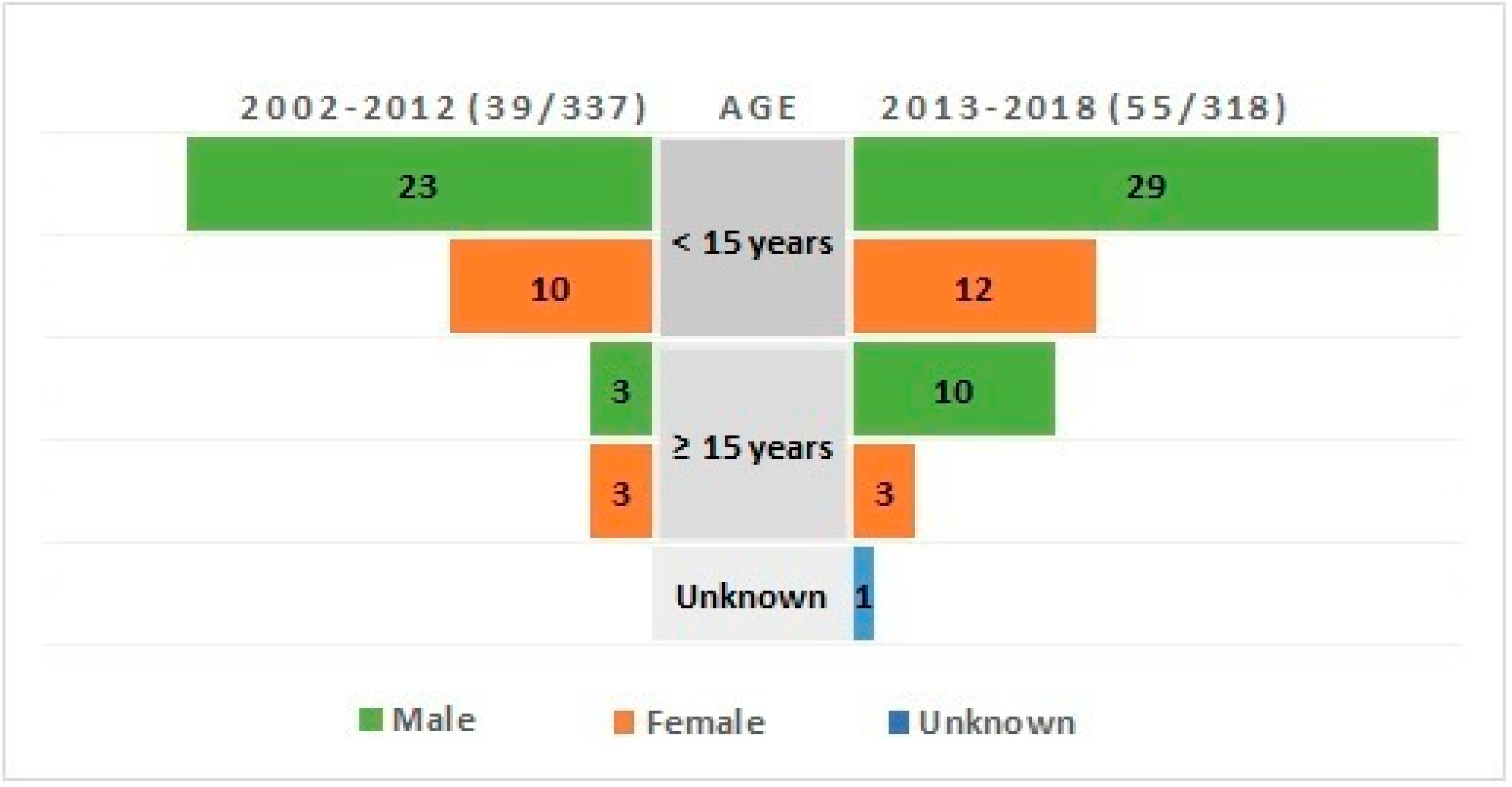
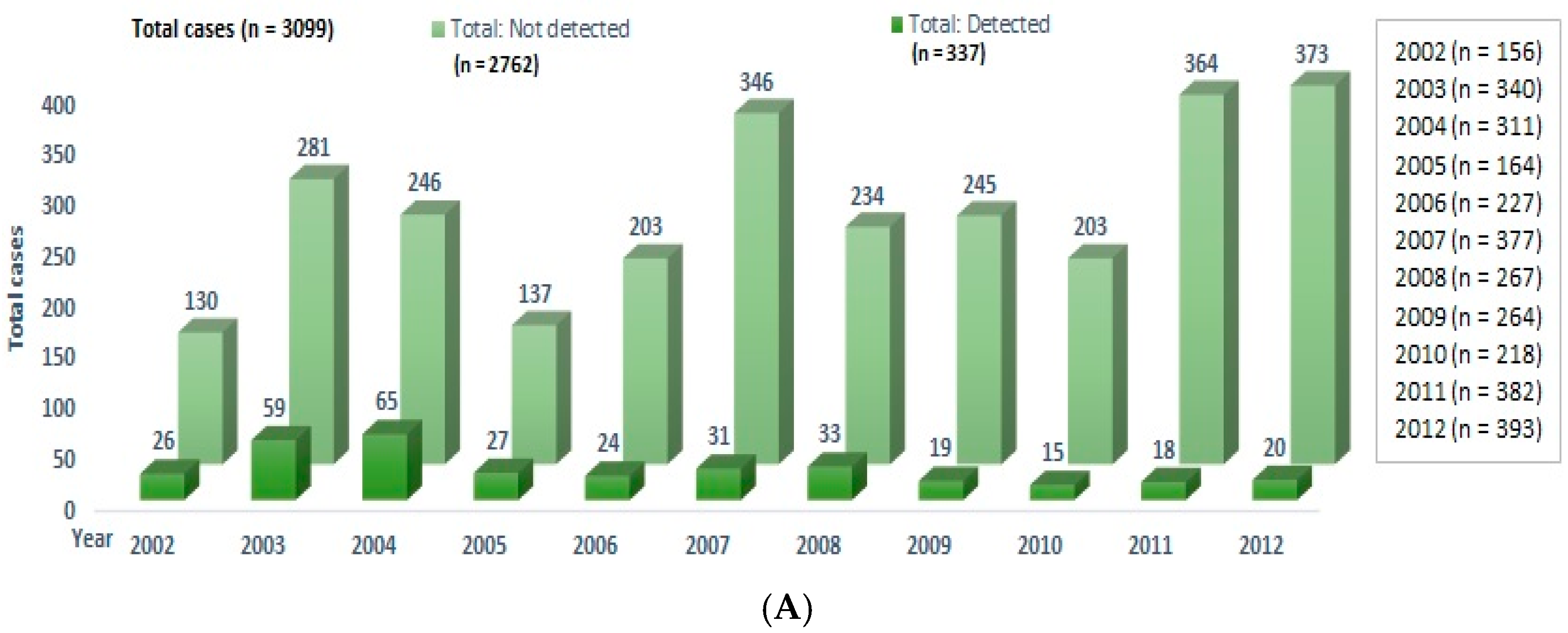
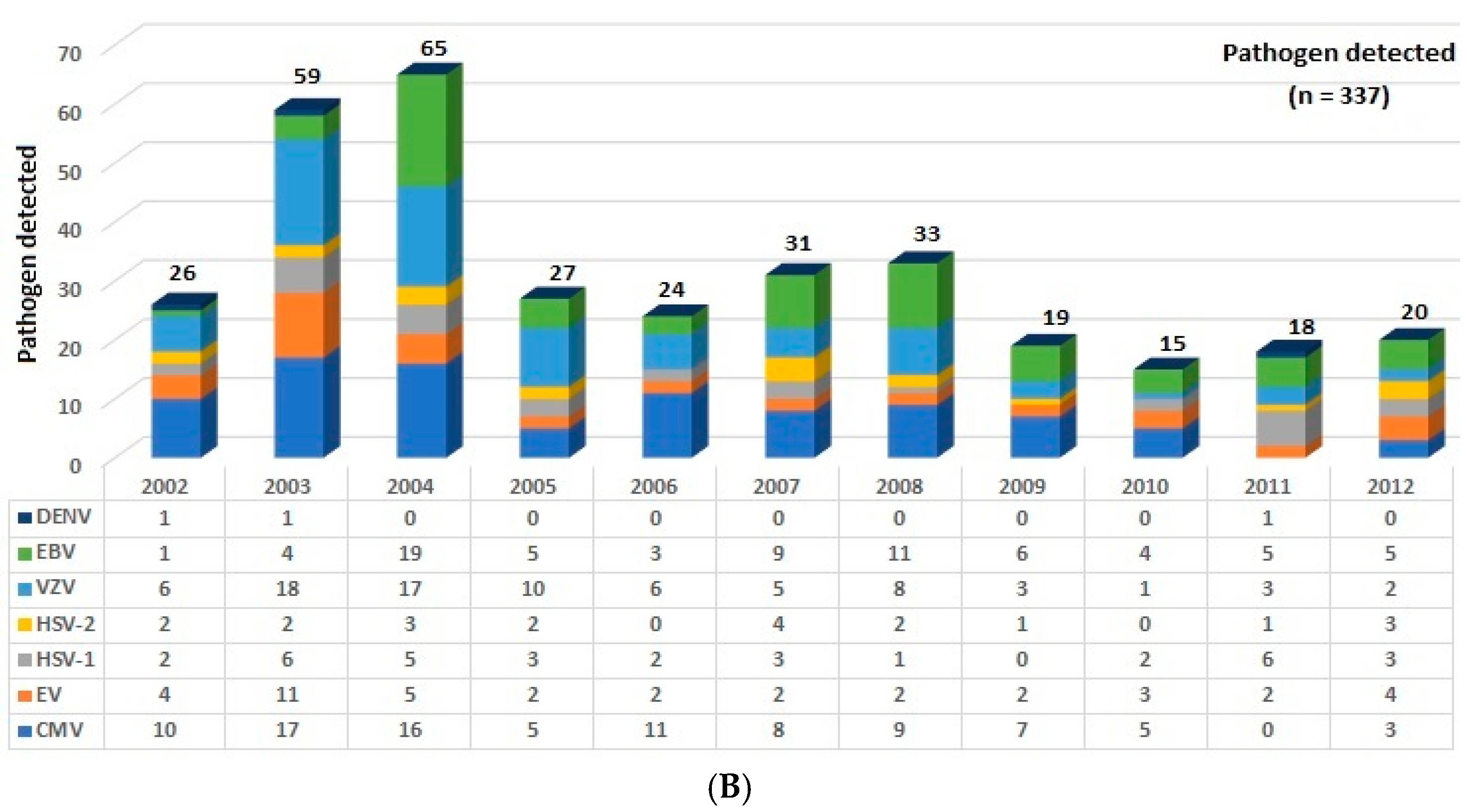
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Tunkel, A.R.; Glaser, C.A.; Bloch, K.C.; Sejvar, J.J.; Marra, C.M.; Roos, K.L.; Hartman, B.J.; Kaplan, S.L.; Scheld, W.M.; Whitley, R.J. The management of encephalitis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2008, 47, 303–327. [Google Scholar] [CrossRef] [PubMed]
- Koskiniemi, M.; Rantalaiho, T.; Piiparinen, H.; von Bonsdorff, C.H.; Färkkilä, M.; Järvinen, A.; Kinnunen, E.; Koskiniemi, S.; Mannonen, L.; Muttilainen, M.; et al. Infections of the central nervous system of suspected viral origin: A collaborative study from Finland. J. Neurovirol. 2001, 7, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Beig, F.K.; Malik, A.; Rizvi, M.; Acharya, D.; Khare, S. Etiology and clinico-epidemiological profile of acute viral encephalitis in children of western Uttar Pradesh, India. Int. J. Infect. Dis. 2010, 14, e141–e146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemachudha, P.; Hemachudha, T. Rabies: Presentation, case management and therapy. J. Neurol. Sci. 2021, 424, 117413. [Google Scholar] [CrossRef] [PubMed]
- Misra, U.K.; Tan, C.T.; Kalita, J. Viral encephalitis and epilepsy. Epilepsia 2008, 49, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Granerod, J.; Ambrose, H.E.; Davies, N.W.; Clewley, J.P.; Walsh, A.L.; Morgan, D.; Cunningham, R.; Zuckerman, M.; Mutton, K.J.; Solomon, T.; et al. Causes of encephalitis and differences in their clinical presentations in England: A multicentre, population-based prospective study. Lancet Infect. Dis. 2011, 10, 835–844. [Google Scholar] [CrossRef] [Green Version]
- De Ory, F.; Avellón, A.; Echevarría, J.E.; Sánchez-Seco, M.P.; Trallero, G.; Cabrerizo, M.; Casas, I.; Pozo, F.; Fedele, G.; Vicente, D.; et al. Viral infections of the central nervous system in Spain: A prospective study. J. Med. Virol. 2013, 85, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Zell, R.; Delwart, E.; Gorbalenya, A.E.; Hovi, T.; King, A.; Knowles, N.J.; Lindberg, A.M.; Pallansch, M.A.; Palmenberg, A.C.; Reuter, G.; et al. ICTV Virus Taxonomy Profile: Picornaviridae. J. Gen. Virol. 2017, 98, 2421–2422. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.W.; Holmes, C.W. Acute and chronic disease caused by enteroviruses. Virulence 2017, 8, 1062–1065. [Google Scholar] [CrossRef] [PubMed]
- Tapparel, C.; Siegrist, F.; Petty, T.J.; Kaiser, L. Picornavirus and enterovirus diversity with associated human diseases. Infect. Genet. Evol. 2013, 14, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; Forgie, S.; Robinson, J. Non-polio Enterovirus detection with acute flaccid paralysis: A systematic review. J. Med. Virol. 2018, 90, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Lowry, K.; Woodman, A.; Cook, J.; Evans, D.J. Recombination in enteroviruses is a biphasic replicative process involving the generation of greater-than genome length ’imprecise’ intermediates. PLoS Pathog. 2014, 10, e1004191. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, M.; Mimouli, K.; Kyriakopoulou, Z.; Tsimpidis, M.; Tsakogiannis, D.; Markoulatos, P.; Amoutzias, G.D. Large-scale genomic analysis reveals recurrent patterns of intertypic recombination in human enteroviruses. Virology 2019, 526, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Petwijit, T.; Desudchit, T.; Punnahitanonda, S.; Wacharapluesadee, S.; Hemachudha, T. A prospective study of enterovirus infection in Thai infants presenting as sepsis. Asian Biomed. 2007, 1, 59–65. [Google Scholar]
- Hasbun, R.; Rosenthal, N.; Balada-Llasat, J.M.; Chung, J.; Duff, S.; Bozzette, S.; Zimmer, L.; Ginocchio, C.C. Epidemiology of Meningitis and Encephalitis in the United States, 2011–2014. Clin. Infect. Dis. 2017, 65, 359–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Sun, J.; Wang, N.; Sun, Z.; Ma, Q.; Li, J.; Zhang, M.; Xu, J. Enterovirus A71 capsid protein VP1 increases blood-brain barrier permeability and virus receptor vimentin on the brain endothelial cells. J. Neurovirol. 2020, 26, 84–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, K.C.; Badmanathan, M.; Devi, S.; Leong, K.L.; Cardosa, M.J.; Wong, K.T. Pathologic characterization of a murine model of human enterovirus 71 encephalomyelitis. J. Neuropathol. Exp. Neurol. 2008, 67, 532–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabor-Godwin, J.M.; Ruller, C.M.; Bagalso, N.; An, N.; Pagarigan, R.R.; Harkins, S.; Gilbert, P.E.; Kiosses, W.B.; Gude, N.A.; Cornell, C.T.; et al. A novel population of myeloid cells responding to coxsackievirus infection assists in the dissemination of virus within the neonatal CNS. J. Neurosci. 2010, 30, 8676–8691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annual Report 2018, Vaccine Preventable Disease, Department of Disease Control. Available online: https://ddc.moph.go.th/uploads/files/c75162b7f21060ba6db46dd629b231f5.pdf (accessed on 21 July 2020).
- Saraya, A.; Mahavihakanont, A.; Shuangshoti, S.; Sittidetboripat, N.; Deesudchit, T.; Callahan, M.; Wacharapluesadee, S.; Wilde, H.; Hemachudha, T. Autoimmune causes of encephalitis syndrome in Thailand: Prospective study of 103 patients. BMC Neurol. 2013, 13, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hemachudha, P.; Petcharat, S.; Hinjoy, S.; Saraya, A.W.; Hemachudha, T. Encephalitis in Thailand: A Neglected Disease Increasingly Caused by Enterovirus. Trop. Med. Infect. Dis. 2021, 6, 117. https://doi.org/10.3390/tropicalmed6030117
Hemachudha P, Petcharat S, Hinjoy S, Saraya AW, Hemachudha T. Encephalitis in Thailand: A Neglected Disease Increasingly Caused by Enterovirus. Tropical Medicine and Infectious Disease. 2021; 6(3):117. https://doi.org/10.3390/tropicalmed6030117
Chicago/Turabian StyleHemachudha, Pasin, Sininat Petcharat, Soawapak Hinjoy, Abhinbhen W. Saraya, and Thiravat Hemachudha. 2021. "Encephalitis in Thailand: A Neglected Disease Increasingly Caused by Enterovirus" Tropical Medicine and Infectious Disease 6, no. 3: 117. https://doi.org/10.3390/tropicalmed6030117
APA StyleHemachudha, P., Petcharat, S., Hinjoy, S., Saraya, A. W., & Hemachudha, T. (2021). Encephalitis in Thailand: A Neglected Disease Increasingly Caused by Enterovirus. Tropical Medicine and Infectious Disease, 6(3), 117. https://doi.org/10.3390/tropicalmed6030117





