Apicortin, a Constituent of Apicomplexan Conoid/Apical Complex and Its Tentative Role in Pathogen—Host Interaction
Abstract
:1. Name
2. Occurrence
3. Domains
4. Primary, Secondary and Tertiary Structure
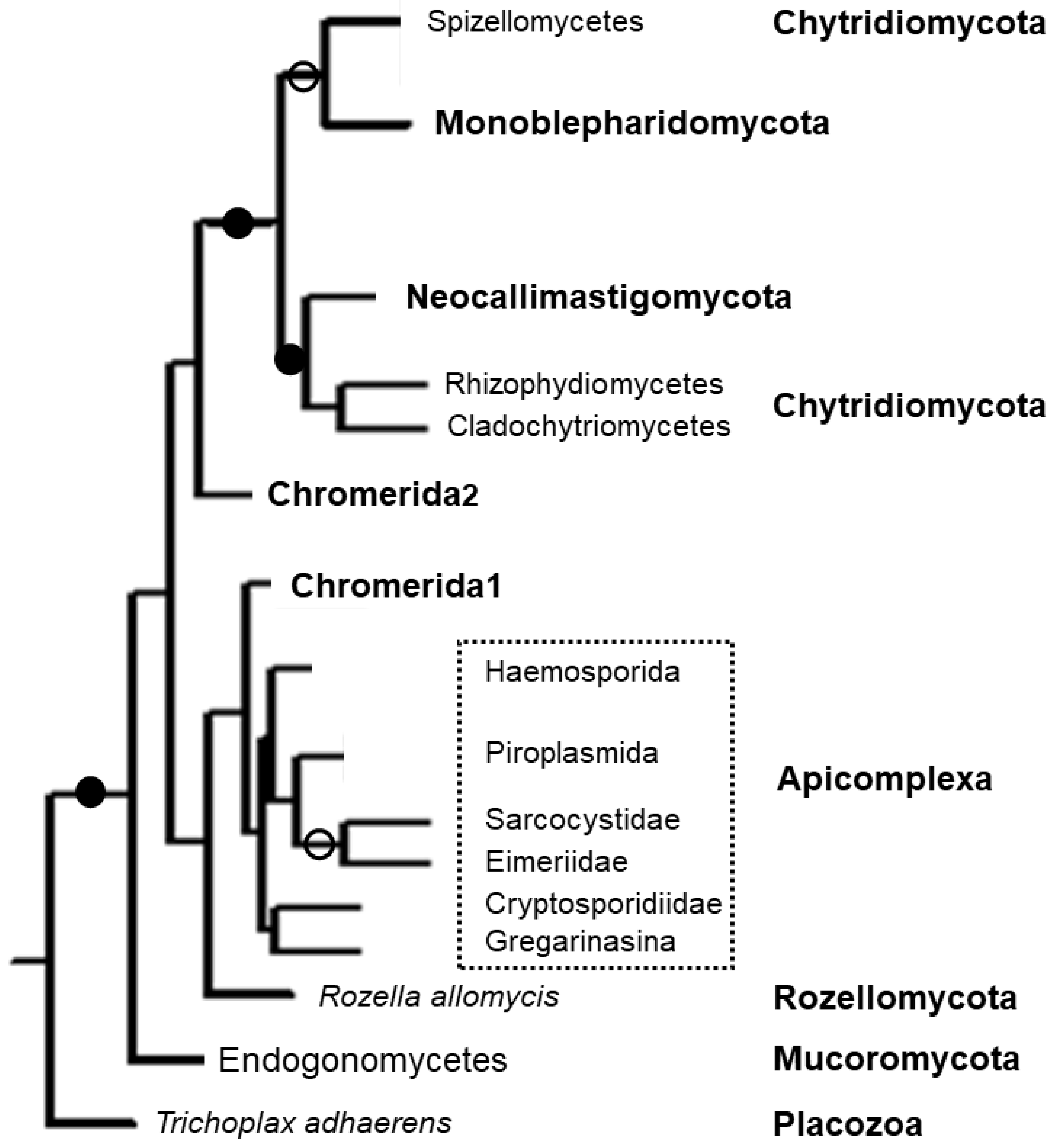
5. Phylogenetics
6. Apicomplexan Apicortins
7. Life Stages of Apicomplexans
8. Structural Features of Apicomplexa: The Apical Complex and the Apicomplexan Cytoskeleton
9. The Role of Apicortin
9.1. The Role of Apicortin in Toxoplasma gondii
9.1.1. Structure
9.1.2. Function
9.2. The Role of Apicortin in Plasmodium falciparum
9.2.1. Localization
9.2.2. In Silico and In Vitro Studies
9.2.3. Effect of Human Erythrocyte microRNAs on Apicortin Expression and on Parasite–Host Interaction
10. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Orosz, F. Apicortin, a unique protein, with a putative cytoskeletal role, shared only by apicomplexan parasites and the placozoan Trichoplax adhaerens. Infect. Genet. Evol. 2009, 9, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Cornillot, E.; Hadj-Kaddour, K.; Dassouli, A.; Noel, B.; Ranwez, V.; Vacherie, B.; Augagneur, Y.; Brès, V.; Duclos, A.; Randazzo, S.; et al. Sequencing of the smallest Apicomplexan genome from the human pathogen Babesia microti. Nucleic Acids Res. 2012, 40, 9102–9114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, R.B.; Oborník, M.; Janouškovec, J.; Chrudimský, T.; Vancová, M.; Green, D.H.; Wright, S.W.; Davies, N.W.; Bolch, C.J.; Heimann, K.; et al. A photosynthetic alveolate closely related to apicomplexan parasites. Nature 2008, 451, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Oborník, M.; Modrý, D.; Lukeš, M.; Cernotíková-Stříbrná, E.; Cihlář, J.; Tesařová, M.; Kotabová, E.; Vancová, M.; Prášil, O.; Lukeš, J. Morphology ultrastructure and life cycle of Vitrella brassicaformis n. sp., n. gen., a novel chromerid from the Great Barrier Reef. Protist 2012, 163, 306–323. [Google Scholar] [CrossRef] [PubMed]
- Orosz, F. Apicomplexan apicortins possess a long disordered N-terminal extension. Infect. Genet. Evol. 2011, 11, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Bogema, D.R.; Yam, J.; Micallef, M.L.; Gholipourkanani, H.; Go, J.; Jenkins, C.; Dang, C. Draft genomes of Perkinsus olseni and Perkinsus chesapeaki reveal polyploidy and regional differences in heterozygosity. Genomics 2021, 113, 677–688. [Google Scholar] [CrossRef]
- Orosz, F. Wider than thought phylogenetic occurrence of apicortin, a characteristic protein of apicomplexan parasites. J. Mol. Evol. 2016, 82, 303–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orosz, F. On the TPPP-like proteins of flagellated fungi. Fungal Biol. 2021, 125, 357–367. [Google Scholar] [CrossRef]
- Ringrose, J.H.; van den Toorn, H.W.P.; Eitel, M.; Post, H.; Neerincx, P.; Schierwater, B.; Altelaar, A.F.M.; Heck, A.J.R. Deep proteome profiling of Trichoplax adhaerens reveals remarkable features at the origin of metazoan multicellularity. Nat. Commun. 2013, 4, 1408. [Google Scholar] [CrossRef] [Green Version]
- Orosz, F. A new protein superfamily: TPPP-like proteins. PLoS ONE 2012, 7, e49276. [Google Scholar] [CrossRef] [Green Version]
- Orosz, F. Two recently sequenced vertebrate genomes are contaminated with apicomplexan species of the Sarcocystidae family. Int. J. Parasitol. 2015, 45, 871–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orosz, F. Does apicortin, a characteristic protein of apicomplexan parasites and placozoa, occur in Eumetazoa? Acta Parasitol. 2018, 63, 617–633. [Google Scholar] [CrossRef] [PubMed]
- Borner, J.; Burmester, T. Parasite infection of public databases: A data mining approach to identify apicomplexan contaminations in animal genome and transcriptome assemblies. BMC Genom. 2017, 18, 100. [Google Scholar] [CrossRef] [Green Version]
- Lopes, R.J.; Mérida, A.M.; Carneiro, M. Unleashing the potential of public genomic resources to find parasite genetic data. Trends Parasitol. 2017, 33, 750–753. [Google Scholar] [CrossRef]
- Orosz, F. On the benefit of publishing uncurated genome assembly data. J. Bacteriol. Parasitol. 2017, 8, 317. [Google Scholar] [CrossRef]
- Orosz, F.; Ovádi, J. TPPP orthologs are ciliary proteins. FEBS Lett. 2008, 582, 3757–3764. [Google Scholar] [CrossRef] [Green Version]
- Vincze, O.; Tőkési, N.; Oláh, J.; Hlavanda, E.; Zotter, Á.; Horváth, I.; Lehotzky, A.; Tirián, L.; Medzihradszky, K.F.; Kovács, J.; et al. Tubulin polymerization promoting proteins (TPPPs): Members of a new family with distinct structures and functions. Biochemistry 2006, 45, 13818–13826. [Google Scholar] [CrossRef]
- Hlavanda, E.; Kovács, J.; Oláh, J.; Orosz, F.; Medzihradszky, K.F.; Ovádi, J. Brain-specific p25 protein binds to tubulin and microtubules and induces aberrant microtubule assemblies at substoichiometric concentrations. Biochemistry 2002, 41, 8657–8664. [Google Scholar] [CrossRef]
- Tirián, L.; Hlavanda, E.; Oláh, J.; Horváth, I.; Orosz, F.; Szabó, B.; Kovács, J.; Szabad, J.; Ovádi, J. TPPP/p25 promotes tubulin assemblies and blocks mitotic spindle formation. Proc. Natl. Acad. Sci. USA 2003, 100, 13976–13981. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, M.; Tomizawa, K.; Ishiguro, K.; Sato, K.; Omori, A.; Sato, S.; Shiratsuchi, A.; Uchida, T.; Imahori, K. A novel brainspecific 25 kDa protein (p25) is phosphorylated by a Ser/Thr-Pro kinase (TPK II) from tau protein kinase fractions. FEBS Lett. 1991, 289, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Hlavanda, E.; Klement, E.; Kókai, E.; Kovács, J.; Vincze, O.; Tőkési, N.; Orosz, F.; Medzihradszky, K.F.; Dombrádi, V.; Ovádi, J. Phosphorylation blocks the activity of tubulin polymerization-promoting protein TPPP: Identification of sites targeted by different kinases. J. Biol. Chem. 2007, 282, 29531–29539. [Google Scholar] [CrossRef] [Green Version]
- Tőkési, N.; Oláh, J.; Hlavanda, E.; Szunyogh, S.; Szabó, A.; Babos, F.; Magyar, A.; Lehotzky, A.; Vass, E.; Ovádi, J. Identification of motives mediating alternative functions of the neomorphic moonlighting TPPP/p25. Biochim. Biophys. Acta 2014, 1842, 547–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeBonis, S.; Neumann, E.; Skoufias, D. Self protein-protein interactions are involved in TPPP/p25 mediated microtubule bundling. Sci. Rep. 2015, 5, 13242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oláh, J.; Szénási, T.; Szabó, A.; Kovács, K.; Lőw, P.; Štifanić, M.; Orosz, F. Tubulin binding and polymerization promoting properties of TPPP proteins are evolutionarily conserved. Biochemistry 2017, 56, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Sapir, T.; Horesh, D.; Caspi, M.; Atlas, R.; Burgess, H.A. Doublecortin mutations cluster in evolutionarily conserved functional domains. Hum. Mol. Genet. 2000, 9, 703–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.H.; Cierpicki, T.; Derewenda, U.; Krowarsch, D.; Feng, Y.; Devedjiev, Y.; Dauter, Z.; Walsh, C.A.; Otlewski, J.; Bushweller, J.H.; et al. The DCX-domain tandems of doublecortin and doublecortin-like kinase. Nat. Struct. Biol. 2003, 10, 324–333. [Google Scholar] [CrossRef]
- Reiner, O.; Coquelle, F.M.; Peter, B.; Levy, T.; Kaplan, A.; Sapir, T.; Orr, I.; Barkai, N.; Eichele, G.; Bergmann, S. The evolving doublecortin (DCX) superfamily. BMC Genome 2006, 7, 188. [Google Scholar] [CrossRef] [Green Version]
- Leung, J.M.; Nagayasu, E.; Hwang, Y.C.; Liu, J.; Pierce, P.G.; Phan, I.Q.; Prentice, R.A.; Murray, J.M.; Hu, K. A doublecortin-domain protein of Toxoplasma and its orthologues bind to and modify the structure and organization of tubulin polymers. BMC Mol. Cell Biol. 2020, 21, 8. [Google Scholar] [CrossRef] [Green Version]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Wiser, M.F. Apicomplexa. Available online: https://www.tulane.edu/~wiser/protozoology/notes/api.html (accessed on 23 March 2021).
- World Health Organization. Malaria. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 23 March 2021).
- Krotoski, W.A.; Garnham, P.C.; Bray, R.S.; Krotoski, D.M.; Killick-Kendrick, R.; Draper, C.C.; Targett, G.A.; Guy, M.W. Observations on early and late post-sporozoite tissue stages in primate malaria. I. Discovery of a new latent form of Plasmodium cynomolgi (the hypnozoite), and failure to detect hepatic forms within the first 24 hours after infection. Am. J. Trop. Med. Hyg. 1982, 31, 24–35. [Google Scholar] [CrossRef]
- Baton, L.A.; Ranford-Cartwright, L.C. Spreading the seeds of million-murdering death: Metamorphoses of malaria in the mosquito. Trends Parasitol. 2005, 21, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Pacheco, N.; Tosetti, N.; Koreny, L.; Waller, R.F.; Soldati-Favre, D. Evolution, composition, assembly, and function of the conoid in Apicomplexa. Trends Parasitol. 2020, 36, 688–704. [Google Scholar] [CrossRef] [PubMed]
- Morrissette, N.S.; Sibley, L.D. Cytoskeleton of apicomplexan parasites. Microbiol. Mol. Biol. Rev. 2002, 66, 21–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, K.; Roos, D.S.; Murray, J.M. A novel polymer of tubulin forms the conoid of Toxoplasma gondii. J. Cell Biol. 2002, 156, 1039–1050. [Google Scholar] [CrossRef] [Green Version]
- Morrissette, N. Targeting Toxoplasma tubules: Tubulin, microtubules, and associated proteins in a human pathogen. Eukaryot. Cell 2015, 14, 2–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portman, N.; Šlapeta, J. The flagellar contribution to the apical complex: A new tool for the eukaryotic Swiss Army knife? Trends Parasitol. 2014, 30, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Johnson, J.; Florens, L.; Fraunholz, M.; Suravajjala, S.; DiLullo, C.; Yates, J.; Roos, D.S.; Murray, J.M. Cytoskeletal components of an invasion machine--the apical complex of Toxoplasma gondii. PLoS Pathog. 2006, 2, 13. [Google Scholar] [CrossRef] [Green Version]
- Nagayasu, E.; Hwang, Y.C.; Liu, J.; Murray, J.M.; Hu, K. Loss of a doublecortin (DCX)-domain protein causes structural defects in a tubulin-based organelle of Toxoplasma gondii and impairs host-cell invasion. Mol. Biol. Cell 2017, 28, 411–428. [Google Scholar] [CrossRef]
- Long, S.; Anthony, B.; Drewry, L.L.; Sibley, L.D. A conserved ankyrin repeat-containing protein regulates conoid stability, motility and cell invasion in Toxoplasma gondii. Nat. Commun. 2017, 8, 2236. [Google Scholar] [CrossRef]
- Chen, Y.; Maxwell, A.; Westerhoff, H.V. Co-operativity and enzymatic activity in polymer-activated enzymes. A one-dimensional piggy-back binding model and its application to the DNA-dependent ATPase of DNA gyrase. J. Mol. Biol. 1986, 190, 201–214. [Google Scholar] [CrossRef]
- Díaz-Martín, R.D.; Mercier, C.; Gómez de León, C.T.; González, R.M.; Pozos, S.G.; Ríos-Castro, E.; García, R.A.; Fox, B.A.; Bzik, D.J.; Flores, R.M. The dense granule protein 8 (GRA8) is a component of the sub-pellicular cytoskeleton in Toxoplasma gondii. Parasitol. Res. 2019, 118, 1899–1918. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Joshi, M.; Kumari, G.; Singh, P.; Shoaib, R.; Munjal, A.; Kumar, V.; Behl, A.; Abid, M.; Garg, S.; et al. Interaction of Plasmodium falciparum apicortin with α- and β-tubulin is critical for parasite growth and survival. Sci. Rep. 2021, 11, 4688. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Garg, S.; Rajagopal, A.; Pati, S.; Singh, S. Targeted repression of Plasmodium apicortin by host microRNA impairs malaria parasite growth and invasion. Dis. Models Mech. 2020, 13, dmm042820. [Google Scholar] [CrossRef] [PubMed]
- Patra, K.P.; Vinetz, J.M. New ultrastructural analysis of the invasive apparatus of the Plasmodium ookinete. Am. J. Trop. Med. Hyg. 2012, 87, 412–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brockley Paterson, W.; Desser, S.S. The polar ring complex in ookinetes of Leucocytozoon simondi (Apicomplexa: Haemosporina) and evidence for a conoid in haemosporidian ookinetes. Eur. J. Protistol. 1989, 24, 244–251. [Google Scholar] [CrossRef]
- Wall, R.J.; Roques, M.; Katris, N.J.; Koreny, L.; Stanway, R.R.; Brady, D.; Waller, R.F.; Tewari, R. SAS6-like protein in Plasmodium indicates that conoid-associated apical complex proteins persist in invasive stages within the mosquito vector. Sci. Rep. 2016, 6, 28604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamonte, G.; Philip, N.; Reardon, J.; Lacsina, J.R.; Majoros, W.; Chapman, L.; Thornburg, C.D.; Telen, M.J.; Ohler, U.; Nicchitta, C.V.; et al. Translocation of sickle cell erythrocyte microRNAs into Plasmodium falciparum inhibits parasite translation and contributes to malaria resistance. Cell Host Microbe 2012, 12, 187–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dandewad, V.; Vindu, A.; Joseph, J.; Seshadri, V. Import of human miRNA-RISC complex into Plasmodium falciparum and regulation of the parasite gene expression. J. Biosci. 2019, 44, 50. [Google Scholar] [CrossRef]
- Swapna, L.S.; Parkinson, J. Genomics of apicomplexan parasites. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 254–273. [Google Scholar] [CrossRef]


| Partner 1 | Short Name | Type | Method | Reference |
|---|---|---|---|---|
| α- and β-tubulin | protein | immunofluorescence, immunoprecipitation, ELISA, surface plasmon resonance, tubulin polymerization, in silico docking | [44] | |
| fluorescence microscopy, co-expression in cell line model | [28] | |||
| Conoid protein hub 1 | TgCPH1 | protein | fluorescence microscopy, co-expression in cell line model | [28] |
| merozoite surface protein 1 | MSP1 | protein | immunofluorescence | [45] |
| myosin A tail interacting protein | MTIP | protein | immunofluorescence | [45] |
| dense granule protein 8 | TgGRA8 | protein | co-precipitation | [43] |
| tamoxifen | TMX | small molecule (drug) | surface plasmon resonance, cellular thermal shift assay, in silico docking, ELISA | [44] |
| miR-150-3p | microRNA | in silico hybridization, co-expression in cell line model | [45] | |
| miR-197-5p | microRNA | in silico hybridization, co-expression in cell line model | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orosz, F. Apicortin, a Constituent of Apicomplexan Conoid/Apical Complex and Its Tentative Role in Pathogen—Host Interaction. Trop. Med. Infect. Dis. 2021, 6, 118. https://doi.org/10.3390/tropicalmed6030118
Orosz F. Apicortin, a Constituent of Apicomplexan Conoid/Apical Complex and Its Tentative Role in Pathogen—Host Interaction. Tropical Medicine and Infectious Disease. 2021; 6(3):118. https://doi.org/10.3390/tropicalmed6030118
Chicago/Turabian StyleOrosz, Ferenc. 2021. "Apicortin, a Constituent of Apicomplexan Conoid/Apical Complex and Its Tentative Role in Pathogen—Host Interaction" Tropical Medicine and Infectious Disease 6, no. 3: 118. https://doi.org/10.3390/tropicalmed6030118
APA StyleOrosz, F. (2021). Apicortin, a Constituent of Apicomplexan Conoid/Apical Complex and Its Tentative Role in Pathogen—Host Interaction. Tropical Medicine and Infectious Disease, 6(3), 118. https://doi.org/10.3390/tropicalmed6030118






