Emerging Trends in the West Nile Virus Epidemiology in Croatia in the ‘One Health’ Context, 2011–2020
Abstract
1. Introduction
2. WNV Infections in Humans
3. WNV Infections in Horses
4. WNV Infections in Birds
5. WNV Infections in Poultry
6. Mosquito Testing for WNV
7. Molecular Epidemiology of WNV in Croatia
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sambri, V.; Capobianchi, M.; Charrel, R.; Fyodorova, M.; Gaibani, P.; Gould, E.; Niedrig, M.; Papa, A.; Pierro, A.; Rossini, G.; et al. West Nile Virus in Europe: Emergence, Epidemiology, Diagnosis, Treatment, and Prevention. Clin. Microbiol. Infect. 2013, 19, 699–704. [Google Scholar] [CrossRef]
- Tsai, T.F.; Popovici, F.; Cernescu, C.; Campbell, G.L.; Nedelcu, N.I. West Nile Encephalitis Epidemic in Southeastern Romania. Lancet 1998, 352, 767–771. [Google Scholar] [CrossRef]
- Platonov, A.E.; Shipulin, G.A.; Shipulina, O.Y.; Tyutyunnik, E.N.; Frolochkina, T.I.; Lanciotti, R.S.; Yazyshina, S.; Platonova, O.V.; Obukhov, I.L.; Zhukov, A.N.; et al. Outbreak of West Nile Virus Infection, Volgograd Region, Russia, 1999. Emerg. Infect. Dis. 2001, 7, 128–132. [Google Scholar] [CrossRef]
- Vilibic-Cavlek, T.; Savic, V.; Petrovic, T.; Toplak, I.; Barbic, L.; Petric, D.; Tabain, I.; Hrnjakovic-Cvjetkovic, I.; Bogdanic, M.; Klobucar, A.; et al. Emerging Trends in the Epidemiology of West Nile and Usutu Virus Infections in Southern Europe. Front. Vet. Sci. 2019, 6, 437. [Google Scholar] [CrossRef] [PubMed]
- Bakonyi, T.; Haussig, J.M. West Nile Virus Keeps on Moving up in Europe. Euro Surveill. 2020, 25, 2001938. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (ECDC). Epidemiological Update: West Nile Virus Transmission Season in Europe. 2018. Available online: https://www.ecdc.europa.eu/en/news-events/epidemiological-update-west-nile-virus-transmission-season-europe-2018 (accessed on 25 June 2021).
- Young, J.J.; Haussig, J.M.; Aberle, S.W.; Pervanidou, D.; Riccardo, F.; Sekulić, N.; Bakonyi, T.; Gossner, C.M. Epidemiology of Human West Nile Virus Infections in the European Union and European Union Enlargement Countries, 2010 to 2018. Euro Surveill. 2021, 26. [Google Scholar] [CrossRef]
- Vogels, C.B.F.; Hartemink, N.; Koenraadt, C.J.M. Modelling West Nile Virus Transmission Risk in Europe: Effect of Temperature and Mosquito Biotypes on the Basic Reproduction Number. Sci. Rep. 2017, 7, 5022. [Google Scholar] [CrossRef]
- Barbić, L.; Listeš, E.; Katić, S.; Stevanović, V.; Madić, J.; Starešina, V.; Labrović, A.; Di Gennaro, A.; Savini, G. Spreading of West Nile Virus Infection in Croatia. Vet. Microbiol. 2012, 159, 504–508. [Google Scholar] [CrossRef]
- Selim, A.; Abdelhady, A. The First Detection of Anti-West Nile Virus Antibody in Domestic Ruminants in Egypt. Trop. Anim. Health Prod. 2020, 52, 3147–3151. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, A.P.; Prusinski, M.A.; Russell, A.; O’Connor, C.; Maffei, J.G.; Oliver, J.; Howard, J.J.; Sherwood, J.A.; Tober, K.; Rochlin, I.; et al. Serologic Survey of Mosquito-Borne Viruses in Hunter-Harvested White-Tailed Deer (Odocoileus virginianus), New York State. Am. J. Trop. Med. Hyg. 2020, 104, 593–603. [Google Scholar] [CrossRef]
- Bahnson, C.S.; Grove, D.M.; Maskey, J.J.; Smith, J.R. Exposure to Select Pathogens in an Expanding Moose (Alces alces) Population in North Dakota, USA. J. Wildl. Dis. 2021. [Google Scholar] [CrossRef]
- Bisanzio, D.; McMillan, J.R.; Barreto, J.G.; Blitvich, B.J.; Mead, D.G.; O’Connor, J.; Kitron, U. Evidence for West Nile Virus Spillover into the Squirrel Population in Atlanta, Georgia. Vector Borne Zoonotic Dis. 2015, 15, 303–310. [Google Scholar] [CrossRef]
- Rocheleau, J.P.; Michel, P.; Lindsay, L.R.; Drebot, M.; Dibernardo, A.; Ogden, N.H.; Fortin, A.; Arsenault, J. Characterizing Environmental Risk Factors for West Nile Virus in Quebec, Canada, Using Clinical Data in Humans and Serology in Pet Dogs. Epidemiol. Infect. 2017, 145, 2797–2807. [Google Scholar] [CrossRef][Green Version]
- García-Bocanegra, I.; Jurado-Tarifa, E.; Cano-Terriza, D.; Martínez, R.; Pérez-Marín, J.E.; Lecollinet, S. Exposure to West Nile Virus and Tick-Borne Encephalitis Virus in Dogs in Spain. Transbound. Emerg. Dis. 2018, 65, 765–772. [Google Scholar] [CrossRef]
- Egberink, H.; Addie, D.D.; Boucraut-Baralon, C.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Horzinek, M.C.; Hosie, M.J.; Marsilio, F.; Lloret, A.; et al. West Nile Virus Infection in Cats: ABCD Guidelines on Prevention and Management. J. Feline Med. Surg. 2015, 17, 617–619. [Google Scholar] [CrossRef] [PubMed]
- Currenti, L.; Tasca, P.; Díaz, M.D.P.; Contigiani, M.; Spinsanti, L. Serological Survey for Saint Louis Encephalitis Virus and West Nile Virus in Domestic Mammals in Córdoba, Argentina: Are Our Pets Potential Sentinels? Arch. Virol. 2020, 165, 2079–2082. [Google Scholar] [CrossRef] [PubMed]
- Sejvar, J.J. Clinical Manifestations and Outcomes of West Nile Virus Infection. Viruses 2014, 6, 606–623. [Google Scholar] [CrossRef]
- Jean, C.M.; Honarmand, S.; Louie, J.K.; Glaser, C.A. Risk Factors for West Nile Virus Neuroinvasive Disease, California, 2005. Emerg. Infect. Dis. 2007, 13, 1918–1920. [Google Scholar] [CrossRef] [PubMed]
- Mrzljak, A.; Dinjar-Kujundzic, P.; Santini, M.; Barbic, L.; Kosuta, I.; Savic, V.; Tabain, I.; Vilibic-Cavlek, T. West Nile Virus: An Emerging Threat in Transplant Population. Vector Borne Zoonotic Dis. 2020, 20, 613–618. [Google Scholar] [CrossRef] [PubMed]
- McDonald, E. Surveillance for West Nile Virus Disease—United States, 2009–2018. MMWR Surveill. Summ. 2021, 70. [Google Scholar] [CrossRef]
- Rizzoli, A.; Jimenez-Clavero, M.A.; Barzon, L.; Cordioli, P.; Figuerola, J.; Koraka, P.; Martina, B.; Moreno, A.; Nowotny, N.; Pardigon, N.; et al. The Challenge of West Nile Virus in Europe: Knowledge Gaps and Research Priorities. Euro Surveill. 2015, 20, 21135. [Google Scholar] [CrossRef]
- Zeller, H.G.; Schuffenecker, I. West Nile Virus: An Overview of Its Spread in Europe and the Mediterranean Basin in Contrast to Its Spread in the Americas. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 147–156. [Google Scholar] [CrossRef]
- Bakonyi, T.; Ivanics, E.; Erdélyi, K.; Ursu, K.; Ferenczi, E.; Weissenböck, H.; Nowotny, N. Lineage 1 and 2 Strains of Encephalitic West Nile Virus, Central Europe. Emerg. Infect. Dis. 2006, 12, 618–623. [Google Scholar] [CrossRef]
- Bakonyi, T.; Ferenczi, E.; Erdélyi, K.; Kutasi, O.; Csörgő, T.; Seidel, B.; Weissenböck, H.; Brugger, K.; Bán, E.; Nowotny, N. Explosive Spread of a Neuroinvasive Lineage 2 West Nile Virus in Central Europe, 2008/2009. Vet. Microbiol. 2013, 165, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Triana, L.M.; Jeffries, C.L.; Mansfield, K.L.; Carnell, G.; Fooks, A.R.; Johnson, N. Emergence of West Nile Virus Lineage 2 in Europe: A Review on the Introduction and Spread of a Mosquito-Borne Disease. Front. Public Health 2014, 2, 271. [Google Scholar] [CrossRef]
- Papa, A.; Bakonyi, T.; Xanthopoulou, K.; Vázquez, A.; Tenorio, A.; Nowotny, N. Genetic Characterization of West Nile Virus Lineage 2, Greece, 2010. Emerg. Infect. Dis. 2011, 17, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Vesenjak-Hirjan, J.; Galinovic-Weisglass, M.; Brudnjak, Z.; Calisher, C.H.; Tovornik, D.; Lazuick, J.S.; Rendic, Z. Island of Brač—Focus of Arbovirus Infections. In Arboviruses in the Mediterranean Countries; Vesenjak-Hirjan, J., Ed.; Gustav Fischer Verlag: Stuttgart, Germany, 1980; pp. 311–317. [Google Scholar]
- Vesenjak-Hirjan, J. Arboviruses in Yugoslavia. In Arboviruses in the Mediterranean Countries; Vesenjak-Hirjan, J., Ed.; ZbL. Bakt. Suppl 9; Gustav Fischer Verlag: Stuttgart, Germany, 1980; pp. 165–177. [Google Scholar]
- Golubić, D.; Dobler, G. Flaviviruses in the north-west Croatia. Infektol. Glasn. 2012, 32, 153–157. (In Croatian) [Google Scholar]
- Vilibić-Cavlek, T.; Barbić, L.; Ljubin-Sternak, S.; Pem-Novosel, I.; Stevanović, V.; Gjenero-Margan, I.; Mlinarić-Galinović, G. West nile virus infection: Re-emergent disease in Croatia. Lijec. Vjesn. 2013, 135, 156–161. (In Croatian) [Google Scholar] [PubMed]
- Pem-Novosel, I.; Vilibic-Cavlek, T.; Gjenero-Margan, I.; Pandak, N.; Peric, L.; Barbic, L.; Listes, E.; Cvitkovic, A.; Stevanovic, V.; Savini, G. First Outbreak of West Nile Virus Neuroinvasive Disease in Humans, Croatia, 2012. Vector Borne Zoonotic Dis. 2014, 14, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Vilibic-Cavlek, T.; Kaic, B.; Barbic, L.; Pem-Novosel, I.; Slavic-Vrzic, V.; Lesnikar, V.; Kurecic-Filipovic, S.; Babic-Erceg, A.; Listes, E.; Stevanovic, V.; et al. First Evidence of Simultaneous Occurrence of West Nile Virus and Usutu Virus Neuroinvasive Disease in Humans in Croatia during the 2013 Outbreak. Infection 2014, 42, 689–695. [Google Scholar] [CrossRef]
- Vilibic-Cavlek, T.; Savic, V.; Sabadi, D.; Peric, L.; Barbic, L.; Klobucar, A.; Miklausic, B.; Tabain, I.; Santini, M.; Vucelja, M.; et al. Prevalence and Molecular Epidemiology of West Nile and Usutu Virus Infections in Croatia in the “One Health” Context, 2018. Transbound. Emerg. Dis. 2019, 66, 1946–1957. [Google Scholar] [CrossRef] [PubMed]
- Vilibić-Čavlek, T.; Petrović, T.; Savić, V.; Šekler, M.; Klobučar, A.; Petrić, D.; Tabain, I.; Vidanović, D.; Bogdanić, M.; Lazić, G.; et al. Importance of Multidisciplinary and Regional Collaboration in Integrated West Nile Virus Surveillance—The “One Health” Concept. Infektol. Glasn. 2019, 39, 40–47. [Google Scholar] [CrossRef]
- Pervanidou, D.; Vakali, A.; Georgakopoulou, T.; Panagiotopoulos, T.; Patsoula, E.; Koliopoulos, G.; Politis, C.; Stamoulis, K.; Gavana, E.; Pappa, S.; et al. West Nile virus in humans, Greece, 2018: The largest seasonal number of cases, 9 years after its emergence in the country. Euro Surveill. 2020, 25, 1900543. [Google Scholar] [CrossRef]
- Riccò, M.; Peruzzi, S.; Balzarini, F. Public Perceptions on Non-Pharmaceutical Interventions for West Nile Virus Infections: A Survey from an Endemic Area in Northern Italy. Trop. Med. Infect. Dis. 2021, 6, 116. [Google Scholar] [CrossRef]
- Sabadi, D.; Peric, L.; Savic, V.; Rubil, I.; Baraban, V.; Tabain, I.; Barbic, L.; Duvnjak, M.; Bogdanic, M.; Stevanovic, V.; et al. Fatal Case of West Nile Encephalitis Associated with Acute Anteroseptal ST Elevation Myocardial Infarction (STEMI): A Case Report. New Microbiol. 2020, 43, 51–53. [Google Scholar]
- Konjevoda, S.; Dzelalija, B.; Canovic, S.; Pastar, Z.; Savic, V.; Tabain, I.; Barbic, L.; Peric, L.; Sabadi, D.; Stevanovic, V.; et al. West Nile Virus Retinitis in a Patient with Neuroinvasive Disease. Rev. Soc. Bras. Med. Trop. 2019, 52, e20190065. [Google Scholar] [CrossRef] [PubMed]
- Santini, M.; Zupetic, I.; Viskovic, K.; Krznaric, J.; Kutlesa, M.; Krajinovic, V.; Polak, V.L.; Savic, V.; Tabain, I.; Barbic, L.; et al. Cauda Equina Arachnoiditis—A Rare Manifestation of West Nile Virus Neuroinvasive Disease: A Case Report. World J. Clin. Cases 2020, 8, 3797–3803. [Google Scholar] [CrossRef]
- Sabadi, D.; Perić, L.J.; Duvnjak, M.; Rubil, I.; Grubišić, B.; Radočaj, V.; Jovanovac, I.; Adamović, Z.; Vilibić-Čavlek, T.; Savić, V.; et al. Cerebellitis: A rare clinical manifestation of West Nile infection. In Proceedings of the Symposium with International Participation—Diagnosis and Surveillance of West Nile Virus Infections in the “One Health” Context, Zagreb, Croatia, 7 June 2019; Croatian Institute of Public Health: Zagreb, Croatia, 2019; pp. 14–15. [Google Scholar]
- Zidovec-Lepej, S.; Vilibic-Cavlek, T.; Barbic, L.; Ilic, M.; Savic, V.; Tabain, I.; Ferenc, T.; Grgic, I.; Gorenec, L.; Bogdanic, M.; et al. Antiviral Cytokine Response in Neuroinvasive and Non-Neuroinvasive West Nile Virus Infection. Viruses 2021, 13, 342. [Google Scholar] [CrossRef] [PubMed]
- Madić, J.; Savini, G.; Di Gennaro, A.; Monaco, F.; Jukić, B.; Kovac, S.; Rudan, N.; Listes, E. Serological Evidence for West Nile Virus Infection in Horses in Croatia. Vet. Rec. 2007, 160, 772–773. [Google Scholar] [CrossRef]
- Savić, V.; Barbić, L.J.; Vilibić-Čavlek, T.; Balenović, M.; Stevanović, V.; Listeš, E.; Savini, G. Chickens and horses as sentinels for early warning system in prevention of human West Nile virus infections in Croatia. Slov. Vet. Res. 2016, 53 (Suppl. S17), 292–294. [Google Scholar]
- Merdić, E.; Perić, L.; Pandak, N.; Kurolt, I.C.; Turić, N.; Vignjević, G.; Stolfa, I.; Milas, J.; Bogojević, M.S.; Markotić, A. West Nile Virus Outbreak in Humans in Croatia, 2012. Coll. Antropol. 2013, 37, 943–947. [Google Scholar] [PubMed]
- Klobucar, A.; Benic, N.; Krajcar, D.; Kosanovic-Licina, M.L.; Tesic, V.; Merdic, E.; Vrucina, I.; Savic, V.; Barbic, L.; Stevanovic, V.; et al. An Overview of Mosquitoes and Emerging Arboviral Infections in the Zagreb Area, Croatia. J. Infect. Dev. Ctries. 2016, 10, 1286–1293. [Google Scholar] [CrossRef]
- Klobucar, A.; Savic, V.; Curman Posavec, M.; Petrinic, S.; Kuhar, U.; Toplak, I.; Madic, J.; Vilibic-Cavlek, T. Screening of Mosquitoes for West Nile Virus and Usutu Virus in Croatia, 2015–2020. Trop. Med. Infect. Dis. 2021, 6, 45. [Google Scholar] [CrossRef]
- Brdarić, D.; Fotakis, E.A.; Mavridis, K.; Vontas, J.; Bekina, H.; Sikora, M. Mosquito genotipization for species identification, insecticide resistance and pathogens detection in mosquito populations in Osijek-Baranja county. In Proceedings of the 32nd Scientific and Educational Seminar with International Participation Disinfection, Disinsection, Deratization and Protection of Stored Agricultural Products, Novi Vinodolski, Croatia, 31 March–3 April 2020. [Google Scholar]
- Kurolt, I.C.; Krajinović, V.; Topić, A.; Kuzman, I.; Baršić, B.; Markotić, A. First Molecular Analysis of West Nile Virus during the 2013 Outbreak in Croatia. Virus Res. 2014, 189, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Vilibic-Cavlek, T.; Barbic, L.; Mrzljak, A.; Brnic, D.; Klobucar, A.; Ilic, M.; Janev-Holcer, N.; Bogdanic, M.; Jemersic, L.; Stevanovic, V.; et al. Emerging and Neglected Viruses of Zoonotic Importance in Croatia. Pathogens 2021, 10, 73. [Google Scholar] [CrossRef] [PubMed]

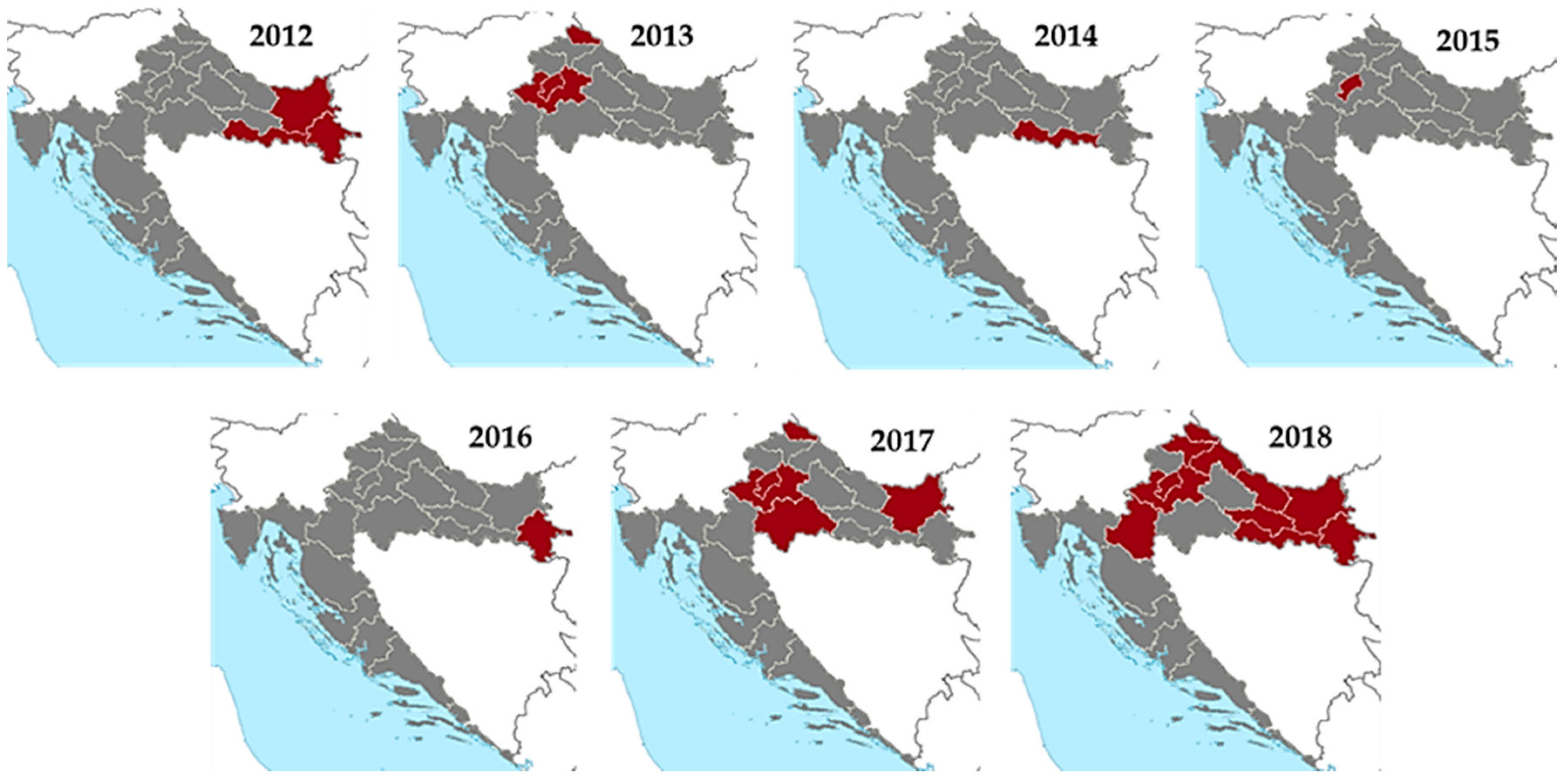



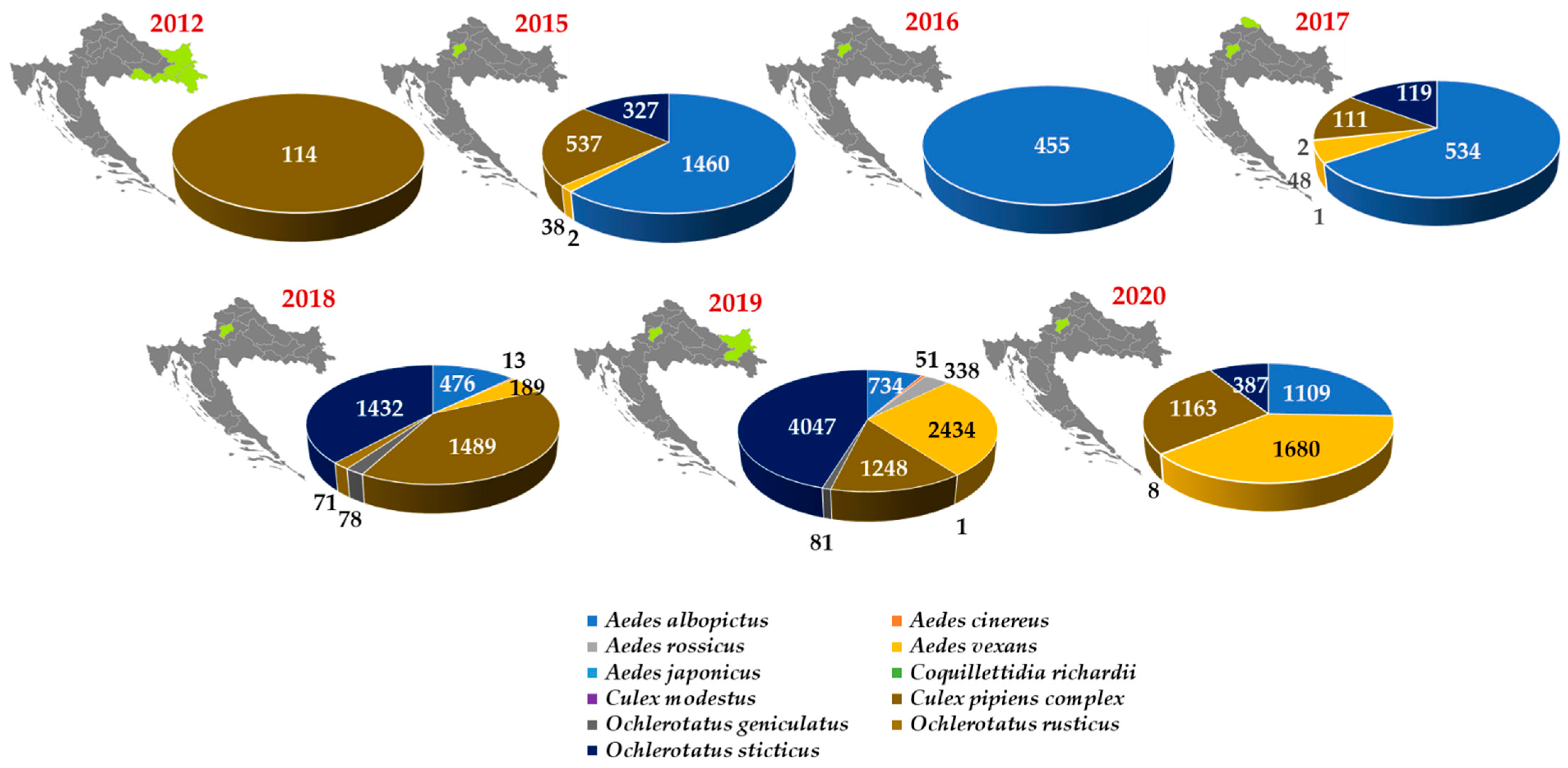

| Year | HUMANS | HORSES | BIRDS | POULTRY | ||
|---|---|---|---|---|---|---|
| Acute Infections | Seroprevalence | Acute Infections | Seroprevalence | Virus Detection/Serologic Evidence * | Seroprevalence | |
| 2011 | 0 | 0.33% | NT | 3.7% | NT | NT |
| 2012 | 7 | 1.01% | 12 | 8.7% | NT | NT |
| 2013 | 20 | 9 | 6.5% | NT | 22.9% | |
| 2014 | 1 | NT | 23 | 9.8% | NT | 8.8% |
| 2015 | 1 | NT | 10 | 7.1% | NT | 3.1% |
| 2016 | 2 | NT | 5 | 8.3% | NT | 1.8% |
| 2017 | 8 | 2.3% | 1 | 8.9% | NT | 10.3% |
| 2018 | 61 | 2.9% | 20 | 10.0% | Accipiter gentilis Butteo butteo * | 3.5% |
| 2019 | 0 | 1.6% | 3 | 21.4% | NT | 2.8% |
| 2020 | 0 | 3.2% | 14 | 18.6% | NT | 5.8% |
| Characteristic | WNND; N (%) | WNF; N (%) |
|---|---|---|
| Gender | ||
| Male | 54 (58.1) | 4 (57.1) |
| Female | 39 (41.9) | 3 (42.9) |
| Age group | ||
| <30 years | 4 (4.3) | 0 (0) |
| 30–39 years | 4 (4.3) | 1 (14.3) |
| 40–49 years | 11 (11.8) | 0 (0) |
| 50–59 years | 25 (26.9) | 4 (57.1) |
| 60–69 years | 9 (9.7) | 2 (28.6) |
| 70+ years | 40 (43.0) | 0 (0) |
| Clinical diagnosis (WNND) | NA | |
| Meningitis | 44 (47.4) | |
| Meningoencephalitis | 34 (36.5) | |
| Meningitis/encephalitis, acute flaccid paralysis | 12 (12.9) | |
| Other (cerebellitis, polyradiculitis, cauda equina arachnoiditis) | 3 (3.2) | |
| TOTAL | 93 (100%) | 7 (100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilibic-Cavlek, T.; Savic, V.; Klobucar, A.; Ferenc, T.; Ilic, M.; Bogdanic, M.; Tabain, I.; Stevanovic, V.; Santini, M.; Curman Posavec, M.; et al. Emerging Trends in the West Nile Virus Epidemiology in Croatia in the ‘One Health’ Context, 2011–2020. Trop. Med. Infect. Dis. 2021, 6, 140. https://doi.org/10.3390/tropicalmed6030140
Vilibic-Cavlek T, Savic V, Klobucar A, Ferenc T, Ilic M, Bogdanic M, Tabain I, Stevanovic V, Santini M, Curman Posavec M, et al. Emerging Trends in the West Nile Virus Epidemiology in Croatia in the ‘One Health’ Context, 2011–2020. Tropical Medicine and Infectious Disease. 2021; 6(3):140. https://doi.org/10.3390/tropicalmed6030140
Chicago/Turabian StyleVilibic-Cavlek, Tatjana, Vladimir Savic, Ana Klobucar, Thomas Ferenc, Maja Ilic, Maja Bogdanic, Irena Tabain, Vladimir Stevanovic, Marija Santini, Marcela Curman Posavec, and et al. 2021. "Emerging Trends in the West Nile Virus Epidemiology in Croatia in the ‘One Health’ Context, 2011–2020" Tropical Medicine and Infectious Disease 6, no. 3: 140. https://doi.org/10.3390/tropicalmed6030140
APA StyleVilibic-Cavlek, T., Savic, V., Klobucar, A., Ferenc, T., Ilic, M., Bogdanic, M., Tabain, I., Stevanovic, V., Santini, M., Curman Posavec, M., Petrinic, S., Benvin, I., Ferencak, I., Rozac, V., & Barbic, L. (2021). Emerging Trends in the West Nile Virus Epidemiology in Croatia in the ‘One Health’ Context, 2011–2020. Tropical Medicine and Infectious Disease, 6(3), 140. https://doi.org/10.3390/tropicalmed6030140










