Genetic Diversity of Dengue Virus in Clinical Specimens from Bangkok, Thailand, during 2018–2020: Co-Circulation of All Four Serotypes with Multiple Genotypes and/or Clades
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Sample Collection
2.2. DENV Serotype Determination
2.3. Whole Genome Sequencing
2.4. Sanger Sequencing
2.5. Phylogenetic Analysis of DENV Envelope Regions
3. Results
3.1. Dengue Infection and Serotype Distribution
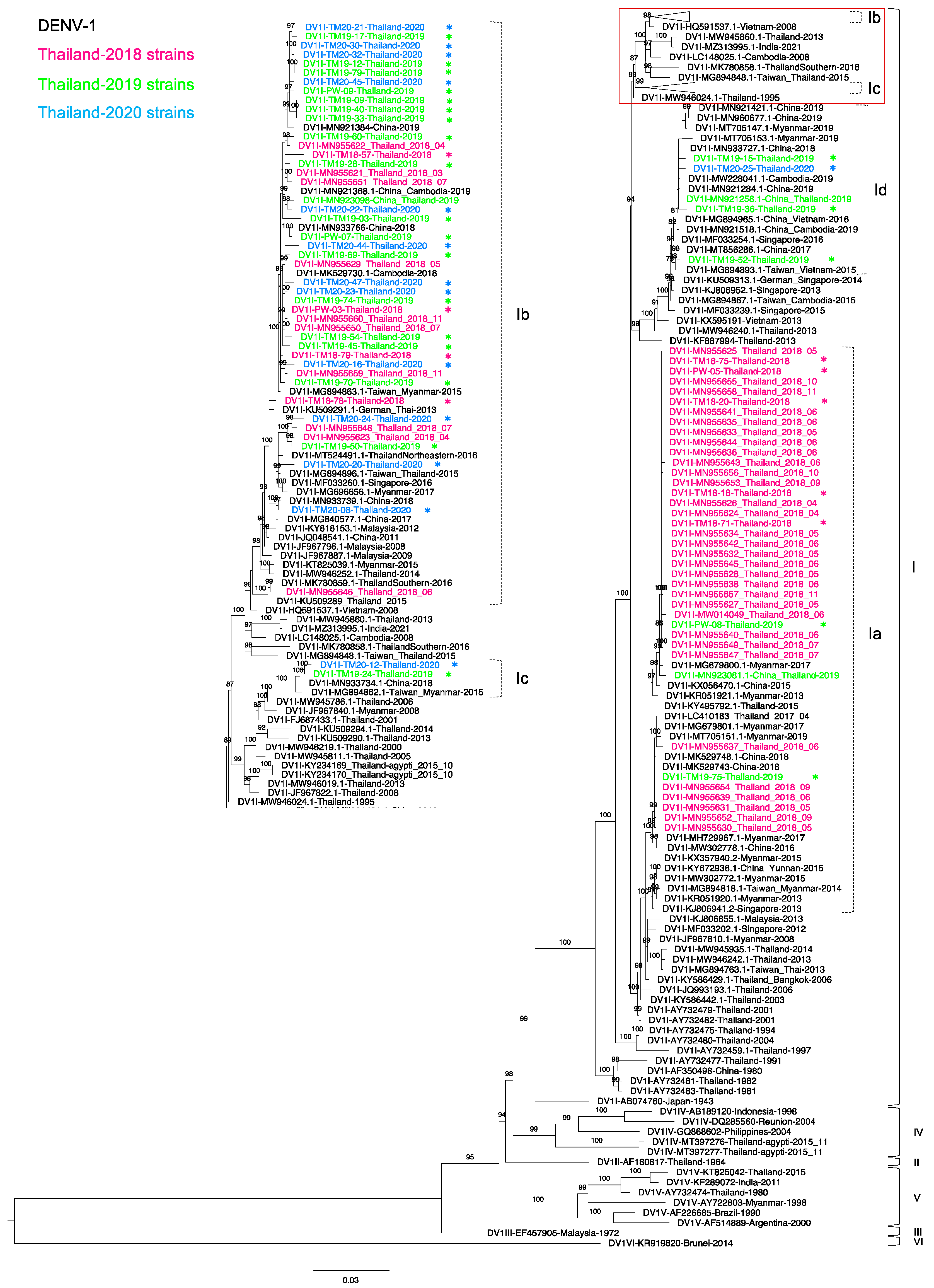
3.2. Phylogenetic Analysis of DENV-1
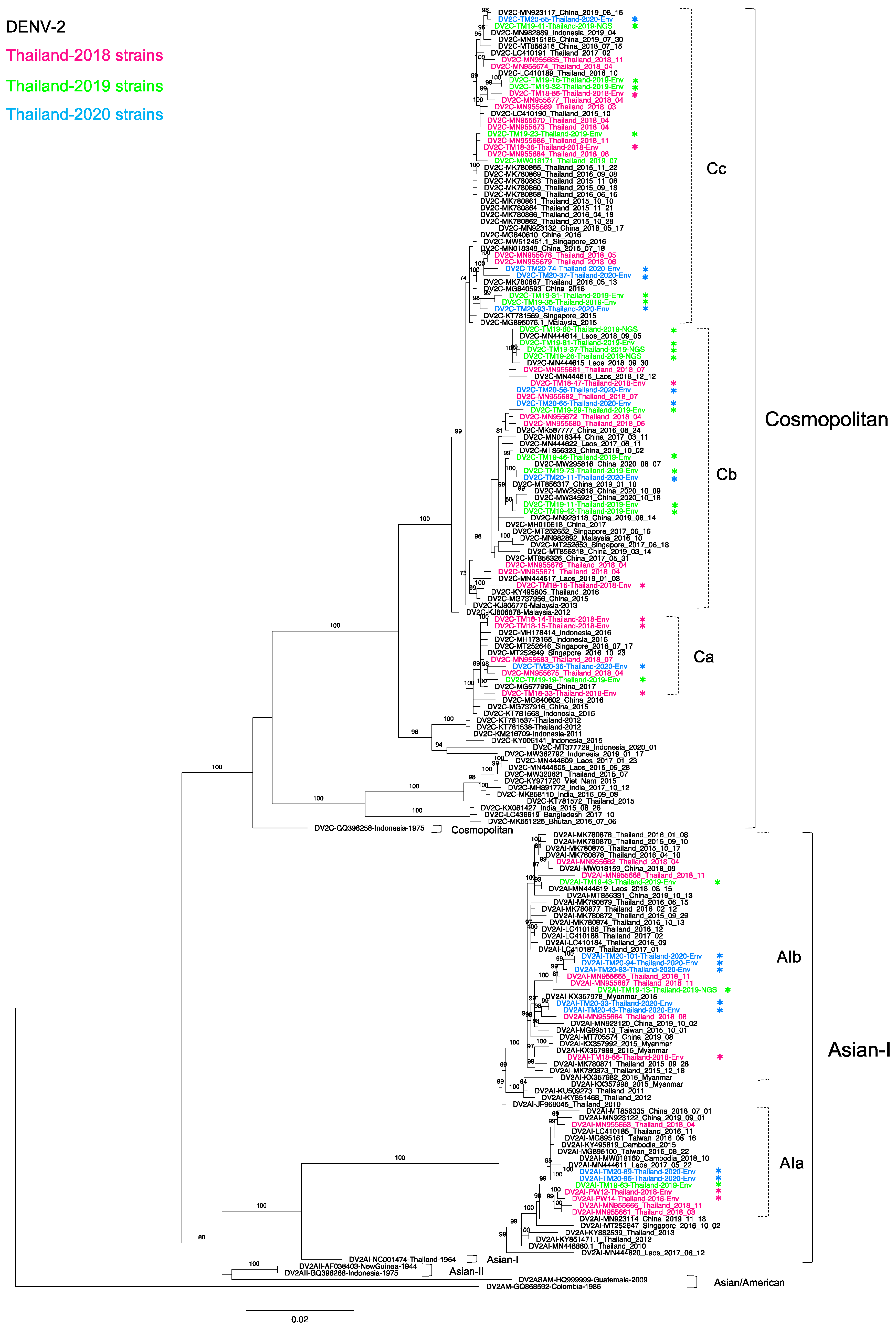
3.3. Phylogenetic Analysis of Thailand DENV-2 in 2015–2020
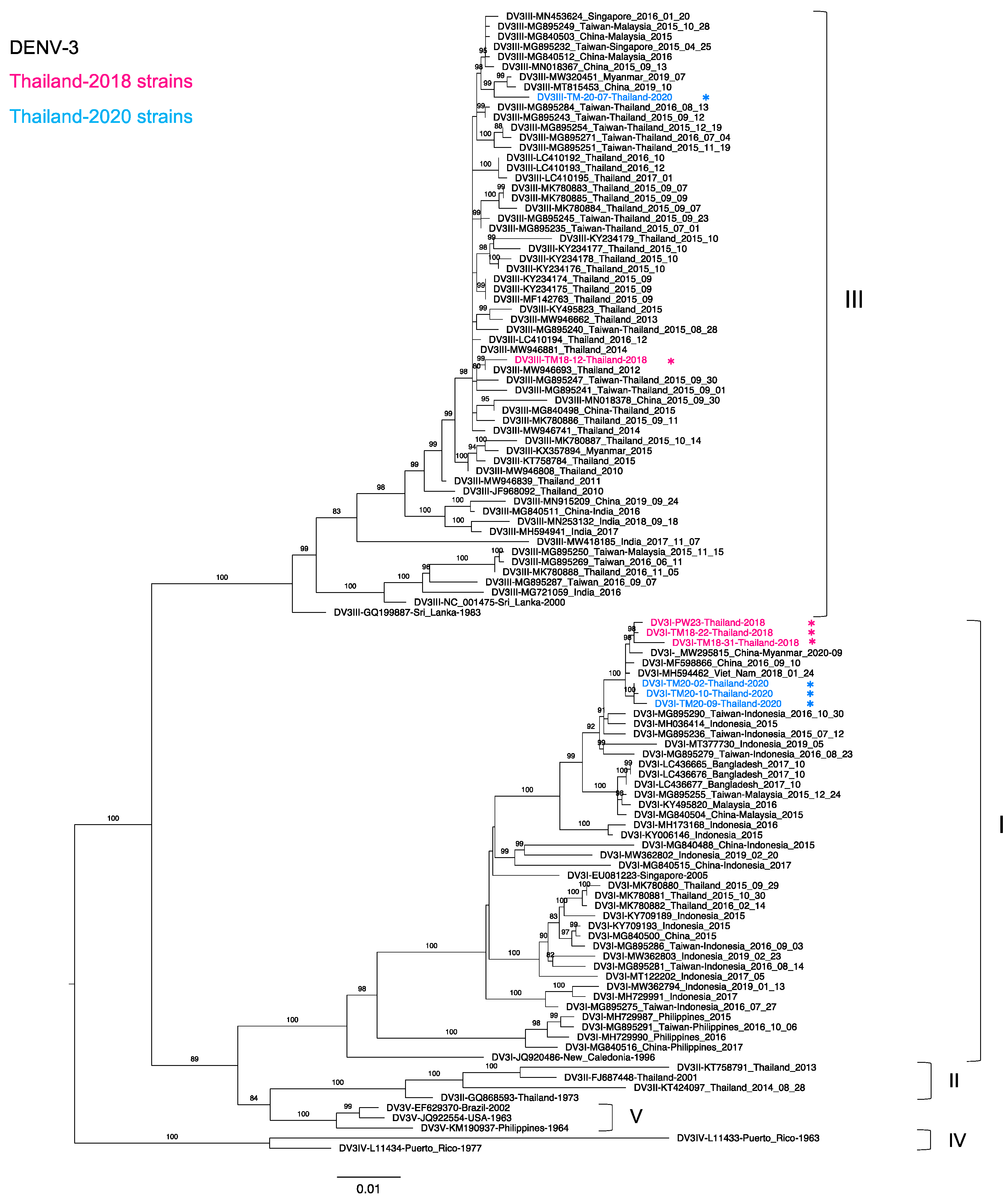
3.4. Phylogenetic Analysis of Thailand DENV-3 Collected in 2015–2020
3.5. Phylogenetic Analysis of Thailand DENV-4 in 2015–2020
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Ooi, E.E.; Gubler, D.J. Dengue in Southeast Asia: Epidemiological characteristics and strategic challenges in disease prevention. Cad. Saude Publica 2009, 25 (Suppl. S1), S115–S124. [Google Scholar] [CrossRef]
- Okanurak, K.; Sornmani, S.; Indaratna, K. The cost of dengue hemorrhagic fever in Thailand. Southeast Asian J. Trop. Med. Public Health 1997, 28, 711–717. [Google Scholar] [PubMed]
- World Health Organization. Global Strategy for Dengue Prevention and Control 2012–2020; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Medeiros, A.S.; Costa, D.M.P.; Branco, M.S.D.; Sousa, D.M.C.; Monteiro, J.D.; Galvao, S.P.M.; Azevedo, P.R.M.; Fernandes, J.V.; Jeronimo, S.M.B.; Araujo, J.M.G. Dengue virus in Aedes aegypti and Aedes albopictus in urban areas in the state of Rio Grande do Norte, Brazil: Importance of virological and entomological surveillance. PLoS ONE 2018, 13, e0194108. [Google Scholar] [CrossRef]
- Wilson, M.E.; Schlagenhauf, P. Aedes and the triple threat of DENV, CHIKV, ZIKV--Arboviral risks and prevention at the 2016 Rio Olympic games. Travel Med. Infect. Dis. 2016, 14, 1–4. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- World Health Organization. Regional Office for South-East, A. Dengue Bulletin, Vol-41; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Rigau-Perez, J.G.; Clark, G.G.; Gubler, D.J.; Reiter, P.; Sanders, E.J.; Vorndam, A.V. Dengue and dengue haemorrhagic fever. Lancet 1998, 352, 971–977. [Google Scholar] [CrossRef]
- Kurane, I. Dengue hemorrhagic fever with special emphasis on immunopathogenesis. Comp. Immunol. Microbiol. Infect. Dis. 2007, 30, 329–340. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Dengue Guidelines for Diagnosis, Treatment, Prevention and Control; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Division of Vector Born Disease. Annual Epidemiological Surveillance Report. 2020; MoPH: Nonthaburi, Thailand, 2020; Available online: https://drive.google.com/file/d/1yBscj4RirjIaBszSBhcib9gu8aRKIuqc/view (accessed on 16 July 2021).
- Division of Vector Born Disease. Annual Epidemiological Surveillance Report. 2019; MoPH: Nonthaburi, Thailand, 2019; Available online: https://apps.doe.moph.go.th/boeeng/download/MIX_AESR_2562.pdf (accessed on 16 July 2021).
- Xu, Z.; Bambrick, H.; Pongsumpun, P.; Ming Tang, I.; Yakob, L.; Devine, G.; Frentiu, F.D.; Williams, G.; Hu, W. Does Bangkok have a central role in the dengue dynamics of Thailand? Parasites Vectors 2020, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Hammon, W.M. Dengue hemorrhagic fever--do we know its cause? Am. J. Trop. Med. Hyg. 1973, 22, 82–91. [Google Scholar] [CrossRef]
- Halstead, S.B. Dengue haemorrhagic fever—A public health problem and a field for research. Bull. World Health Organ. 1980, 58, 1–21. [Google Scholar]
- Cummings, D.A.; Irizarry, R.A.; Huang, N.E.; Endy, T.P.; Nisalak, A.; Ungchusak, K.; Burke, D.S. Travelling waves in the occurrence of dengue haemorrhagic fever in Thailand. Nature 2004, 427, 344–347. [Google Scholar] [CrossRef]
- Perera, R.; Kuhn, R.J. Structural proteomics of dengue virus. Curr. Opin. Microbiol. 2008, 11, 369–377. [Google Scholar] [CrossRef]
- Guzman, M.G.; Halstead, S.B.; Artsob, H.; Buchy, P.; Farrar, J.; Gubler, D.J.; Hunsperger, E.; Kroeger, A.; Margolis, H.S.; Martinez, E.; et al. Dengue: A continuing global threat. Nat. Rev. Microbiol. 2010, 8, S7–S16. [Google Scholar] [CrossRef]
- Heinz, F.X.; Stiasny, K. Flaviviruses and flavivirus vaccines. Vaccine 2012, 30, 4301–4306. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.C.; Twiddy, S.S. The origin, emergence and evolutionary genetics of dengue virus. Infect. Genet. Evol. 2003, 3, 19–28. [Google Scholar] [CrossRef]
- Chen, R.; Vasilakis, N. Dengue—Quo tu et quo vadis? Viruses 2011, 3, 1562–1608. [Google Scholar] [CrossRef]
- Goncalvez, A.P.; Escalante, A.A.; Pujol, F.H.; Ludert, J.E.; Tovar, D.; Salas, R.A.; Liprandi, F. Diversity and evolution of the envelope gene of dengue virus type 1. Virology 2002, 303, 110–119. [Google Scholar] [CrossRef]
- Yamashita, A.; Sasaki, T.; Kurosu, T.; Yasunaga, T.; Ikuta, K. Origin and distribution of divergent dengue virus: Novel database construction and phylogenetic analyses. Future Virol. 2013, 8, 1061–1083. [Google Scholar] [CrossRef]
- Yenamandra, S.P.; Koo, C.; Chiang, S.; Lim, H.S.J.; Yeo, Z.Y.; Ng, L.C.; Hapuarachchi, H.C. Evolution, heterogeneity and global dispersal of cosmopolitan genotype of Dengue virus type 2. Sci. Rep. 2021, 11, 13496. [Google Scholar] [CrossRef] [PubMed]
- Twiddy, S.S.; Farrar, J.J.; Vinh Chau, N.; Wills, B.; Gould, E.A.; Gritsun, T.; Lloyd, G.; Holmes, E.C. Phylogenetic relationships and differential selection pressures among genotypes of dengue-2 virus. Virology 2002, 298, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Waman, V.P.; Kale, M.M.; Kulkarni-Kale, U. Genetic diversity and evolution of dengue virus serotype 3: A comparative genomics study. Infect. Genet. Evol. 2017, 49, 234–240. [Google Scholar] [CrossRef]
- Klungthong, C.; Zhang, C.; Mammen, M.P., Jr.; Ubol, S.; Holmes, E.C. The molecular epidemiology of dengue virus serotype 4 in Bangkok, Thailand. Virology 2004, 329, 168–179. [Google Scholar] [CrossRef]
- Nisalak, A.; Clapham, H.E.; Kalayanarooj, S.; Klungthong, C.; Thaisomboonsuk, B.; Fernandez, S.; Reiser, J.; Srikiatkhachorn, A.; Macareo, L.R.; Lessler, J.T.; et al. Forty Years of Dengue Surveillance at a Tertiary Pediatric Hospital in Bangkok, Thailand, 1973–2012. Am. J. Trop. Med. Hyg. 2016, 94, 1342–1347. [Google Scholar] [CrossRef]
- Yamanaka, A.; Imad, H.A.; Phumratanaprapin, W.; Phadungsombat, J.; Konishi, E.; Shioda, T. Antibody-dependent enhancement representing in vitro infective progeny virus titer correlates with the viremia level in dengue patients. Sci. Rep. 2021, 11, 12354. [Google Scholar] [CrossRef]
- Uttayamakul, S.; Reawrang, S.; Nitiyanontakij, R.; Phadungsombat, J.; Nakayama, E.; Suttha, P.; Moolasart, V.; Shioda, T. Molecular Characteristics of Dengue Viruses in Patients Hospitalized at the Bamrasnaradura Infectious Diseases Institute, Thailand. Jpn. J. Infect. Dis. 2020, 73, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Phadungsombat, J.; Nakayama, E.E.; Saito, A.; Egawa, A.; Sato, T.; Rahim, R.; Hasan, A.; Lin, M.Y.; Takasaki, T.; et al. Genotype replacement of dengue virus type 3 and clade replacement of dengue virus type 2 genotype Cosmopolitan in Dhaka, Bangladesh in 2017. Infect. Genet. Evol. 2019, 75, 103977. [Google Scholar] [CrossRef] [PubMed]
- Phadungsombat, J.; Lin, M.Y.; Srimark, N.; Yamanaka, A.; Nakayama, E.E.; Moolasart, V.; Suttha, P.; Shioda, T.; Uttayamakul, S. Emergence of genotype Cosmopolitan of dengue virus type 2 and genotype III of dengue virus type 3 in Thailand. PLoS ONE 2018, 13, e0207220. [Google Scholar] [CrossRef]
- Christenbury, J.G.; Aw, P.P.; Ong, S.H.; Schreiber, M.J.; Chow, A.; Gubler, D.J.; Vasudevan, S.G.; Ooi, E.E.; Hibberd, M.L. A method for full genome sequencing of all four serotypes of the dengue virus. J. Virol. Methods 2010, 169, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Kurosu, T.; Khamlert, C.; Phanthanawiboon, S.; Ikuta, K.; Anantapreecha, S. Highly efficient rescue of dengue virus using a co-culture system with mosquito/mammalian cells. Biochem. Biophys. Res. Commun. 2010, 394, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Hamel, R.; Surasombatpattana, P.; Wichit, S.; Dauve, A.; Donato, C.; Pompon, J.; Vijaykrishna, D.; Liegeois, F.; Vargas, R.M.; Luplertlop, N.; et al. Phylogenetic analysis revealed the co-circulation of four dengue virus serotypes in Southern Thailand. PLoS ONE 2019, 14, e0221179. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, B.P.; Manakkadan, A.; Das Manandhar, K.; Sreekumar, E. Phylogenetic study reveals co-circulation of Asian II and Cosmopolitan genotypes of Dengue virus serotype 2 in Nepal during 2013. Infect. Genet. Evol. 2015, 34, 402–409. [Google Scholar] [CrossRef]
- Luo, L.; Liang, H.Y.; Jing, Q.L.; He, P.; Yuan, J.; Di, B.; Bai, Z.J.; Wang, Y.L.; Zheng, X.L.; Yang, Z.C. Molecular characterization of the envelope gene of dengue virus type 3 newly isolated in Guangzhou, China, during 2009–2010. Int. J. Infect. Dis. 2013, 17, e498–e504. [Google Scholar] [CrossRef]
- Phommanivong, V.; Kanda, S.; Shimono, T.; Lamaningao, P.; Darcy, A.W.; Mishima, N.; Phaytanavanh, B.; Nishiyama, T. Co-circulation of the dengue with chikungunya virus during the 2013 outbreak in the southern part of Lao PDR. Trop. Med. Health 2016, 44, 24. [Google Scholar] [CrossRef]
- Larsson, A. AliView: A fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree v. 1.4.4. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 1 March 2021).
- Wen, S.; Ma, D.; Lin, Y.; Li, L.; Hong, S.; Li, X.; Wang, X.; Xi, J.; Qiu, L.; Pan, Y.; et al. Complete Genome Characterization of the 2017 Dengue Outbreak in Xishuangbanna, a Border City of China, Burma and Laos. Front. Cell. Infect. Microbiol. 2018, 8, 148. [Google Scholar] [CrossRef]
- Zhang, C.; Mammen, M.P., Jr.; Chinnawirotpisan, P.; Klungthong, C.; Rodpradit, P.; Monkongdee, P.; Nimmannitya, S.; Kalayanarooj, S.; Holmes, E.C. Clade replacements in dengue virus serotypes 1 and 3 are associated with changing serotype prevalence. J. Virol. 2005, 79, 15123–15130. [Google Scholar] [CrossRef]
- Salje, H.; Lessler, J.; Maljkovic Berry, I.; Melendrez, M.C.; Endy, T.; Kalayanarooj, S.; Atchareeya, A.; Chanama, S.; Sangkijporn, S.; Klungthong, C.; et al. Dengue diversity across spatial and temporal scales: Local structure and the effect of host population size. Science 2017, 355, 1302–1306. [Google Scholar] [CrossRef]
- Huang, J.H.; Su, C.L.; Yang, C.F.; Liao, T.L.; Hsu, T.C.; Chang, S.F.; Lin, C.C.; Shu, P.Y. Molecular characterization and phylogenetic analysis of dengue viruses imported into Taiwan during 2008–2010. Am. J. Trop. Med. Hyg. 2012, 87, 349–358. [Google Scholar] [CrossRef]
- Yang, C.F.; Chang, S.F.; Hsu, T.C.; Su, C.L.; Wang, T.C.; Lin, S.H.; Yang, S.L.; Lin, C.C.; Shu, P.Y. Molecular characterization and phylogenetic analysis of dengue viruses imported into Taiwan during 2011–2016. PLoS Negl. Trop. Dis. 2018, 12, e0006773. [Google Scholar] [CrossRef]
- Koo, C.; Tien, W.P.; Xu, H.; Ong, J.; Rajarethinam, J.; Lai, Y.L.; Ng, L.C.; Hapuarachchi, H.C. Highly Selective Transmission Success of Dengue Virus Type 1 Lineages in a Dynamic Virus Population: An Evolutionary and Fitness Perspective. iScience 2018, 6, 38–51. [Google Scholar] [CrossRef]
- Shihada, S.; Emmerich, P.; Thome-Bolduan, C.; Jansen, S.; Gunther, S.; Frank, C.; Schmidt-Chanasit, J.; Cadar, D. Genetic Diversity and New Lineages of Dengue Virus Serotypes 3 and 4 in Returning Travelers, Germany, 2006–2015. Emerg. Infect. Dis. 2017, 23, 272–275. [Google Scholar] [CrossRef]
- Berry, I.M.; Melendrez, M.C.; Pollett, S.; Figueroa, K.; Buddhari, D.; Klungthong, C.; Nisalak, A.; Panciera, M.; Thaisomboonsuk, B.; Li, T.; et al. Precision Tracing of Household Dengue Spread Using Inter- and Intra-Host Viral Variation Data, Kamphaeng Phet, Thailand. Emerg. Infect. Dis. 2021, 27, 1637–1644. [Google Scholar] [CrossRef]
- Kyaw, A.K.; Ngwe Tun, M.M.; Moi, M.L.; Nabeshima, T.; Soe, K.T.; Thwe, S.M.; Myint, A.A.; Maung, K.T.T.; Aung, W.; Hayasaka, D.; et al. Clinical, virological and epidemiological characterization of dengue outbreak in Myanmar, 2015. Epidemiol. Infect. 2017, 145, 1886–1897. [Google Scholar] [CrossRef]
- Chang, S.F.; Yang, C.F.; Hsu, T.C.; Su, C.L.; Lin, C.C.; Shu, P.Y. Laboratory-Based Surveillance and Molecular Characterization of Dengue Viruses in Taiwan, 2014. Am. J. Trop. Med. Hyg. 2016, 94, 804–811. [Google Scholar] [CrossRef]
- Kotaki, T.; Yamanaka, A.; Mulyatno, K.C.; Churrotin, S.; Sucipto, T.H.; Labiqah, A.; Ahwanah, N.L.; Soegijanto, S.; Kameoka, M.; Konishi, E. Divergence of the dengue virus type 2 Cosmopolitan genotype associated with two predominant serotype shifts between 1 and 2 in Surabaya, Indonesia, 2008–2014. Infect. Genet. Evol. 2016, 37, 88–93. [Google Scholar] [CrossRef]
- Salda, L.T.; Parquet, M.D.; Matias, R.R.; Natividad, F.F.; Kobayashi, N.; Morita, K. Molecular epidemiology of dengue 2 viruses in the Philippines: Genotype shift and local evolution. Am. J. Trop. Med. Hyg. 2005, 73, 796–802. [Google Scholar] [CrossRef]
- Sasmono, R.T.; Wahid, I.; Trimarsanto, H.; Yohan, B.; Wahyuni, S.; Hertanto, M.; Yusuf, I.; Mubin, H.; Ganda, I.J.; Latief, R.; et al. Genomic analysis and growth characteristic of dengue viruses from Makassar, Indonesia. Infect. Genet. Evol. 2015, 32, 165–177. [Google Scholar] [CrossRef]
- Holmes, E.C.; Tio, P.H.; Perera, D.; Muhi, J.; Cardosa, J. Importation and co-circulation of multiple serotypes of dengue virus in Sarawak, Malaysia. Virus Res. 2009, 143, 1–5. [Google Scholar] [CrossRef]
- Lee, K.S.; Lo, S.; Tan, S.S.; Chua, R.; Tan, L.K.; Xu, H.; Ng, L.C. Dengue virus surveillance in Singapore reveals high viral diversity through multiple introductions and in situ evolution. Infect. Genet. Evol. 2012, 12, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Megawati, D.; Masyeni, S.; Yohan, B.; Lestarini, A.; Hayati, R.F.; Meutiawati, F.; Suryana, K.; Widarsa, T.; Budiyasa, D.G.; Budiyasa, N.; et al. Dengue in Bali: Clinical characteristics and genetic diversity of circulating dengue viruses. PLoS Negl. Trop. Dis. 2017, 11, e0005483. [Google Scholar] [CrossRef]
- Masyeni, S.; Yohan, B.; Somia, I.K.A.; Myint, K.S.A.; Sasmono, R.T. Dengue infection in international travellers visiting Bali, Indonesia. J. Travel Med. 2018, 25, tay061. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.R.; van den Hurk, A.F.; Mackenzie, J.S.; Pyke, A.T. Dengue viruses in Papua New Guinea: Evidence of endemicity and phylogenetic variation, including the evolution of new genetic lineages. Emerg. Microbes Infect. 2017, 6, e114. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.H.; Yip, J.T.; Chen, Y.L.; Liu, W.; Harun, S.; Lystiyaningsih, E.; Heriyanto, B.; Beckett, C.G.; Mitchell, W.P.; Hibberd, M.L.; et al. Periodic re-emergence of endemic strains with strong epidemic potential-a proposed explanation for the 2004 Indonesian dengue epidemic. Infect. Genet. Evol. 2008, 8, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Ding, Z.; Yan, J.; Yao, W.; Pan, J.; Yang, Z.; Lou, X.; Mao, H.; Lin, J.; Sun, J.; et al. Epidemiological Characterization of the 2017 Dengue Outbreak in Zhejiang, China and Molecular Characterization of the Viruses. Front. Cell. Infect. Microbiol. 2018, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.C.; Chem, Y.K.; Koo, C.; Mudin, R.N.B.; Amin, F.M.; Lee, K.S.; Kheong, C.C. 2013 dengue outbreaks in Singapore and Malaysia caused by different viral strains. Am. J. Trop. Med. Hyg. 2015, 92, 1150–1155. [Google Scholar] [CrossRef]
- Calvez, E.; Pommelet, V.; Somlor, S.; Pompon, J.; Viengphouthong, S.; Bounmany, P.; Chindavong, T.A.; Xaybounsou, T.; Prasayasith, P.; Keosenhom, S.; et al. Trends of the Dengue Serotype-4 Circulation with Epidemiological, Phylogenetic, and Entomological Insights in Lao PDR between 2015 and 2019. Pathogens 2020, 9, 728. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.; Holmes, E.C.; Zhang, C.; Mammen, M.P., Jr.; Nimmannitya, S.; Kalayanarooj, S.; Boots, M. Cross-protective immunity can account for the alternating epidemic pattern of dengue virus serotypes circulating in Bangkok. Proc. Natl. Acad. Sci. USA 2006, 103, 14234–14239. [Google Scholar] [CrossRef]
- Wartel, T.A.; Prayitno, A.; Hadinegoro, S.R.; Capeding, M.R.; Thisyakorn, U.; Tran, N.H.; Moureau, A.; Bouckenooghe, A.; Nealon, J.; Taurel, A.F. Three Decades of Dengue Surveillance in Five Highly Endemic South East Asian Countries. Asia Pac. J. Public Health 2017, 29, 7–16. [Google Scholar] [CrossRef]
- Guzman, M.G.; Kouri, G.; Halstead, S.B. Do escape mutants explain rapid increases in dengue case-fatality rates within epidemics? Lancet 2000, 355, 1902–1903. [Google Scholar] [CrossRef]
- Thu, H.M.; Lowry, K.; Myint, T.T.; Shwe, T.N.; Han, A.M.; Khin, K.K.; Thant, K.Z.; Thein, S.; Aaskov, J. Myanmar dengue outbreak associated with displacement of serotypes 2, 3, and 4 by dengue 1. Emerg. Infect. Dis. 2004, 10, 593–597. [Google Scholar] [CrossRef]
- Division of Vector Born Disease. Annual Epidemiological Surveillance Report. 2010; MoPH: Nonthaburi, Thailand, 2010; Available online: http://www.boe.moph.go.th/Annual/aesr2553/Open.html (accessed on 16 July 2021).
- Division of Vector Born Disease. Annual Epidemiological Surveillance Report. 2005; MoPH: Nonthaburi, Thailand, 2005; Available online: http://www.boe.moph.go.th/Annual/Annual48/home_001.htm (accessed on 16 July 2021).
- Huang, C.Y.; Butrapet, S.; Pierro, D.J.; Chang, G.J.; Hunt, A.R.; Bhamarapravati, N.; Gubler, D.J.; Kinney, R.M. Chimeric dengue type 2 (vaccine strain PDK-53)/dengue type 1 virus as a potential candidate dengue type 1 virus vaccine. J. Virol. 2000, 74, 3020–3028. [Google Scholar] [CrossRef][Green Version]
- Punpapong, V.; Sittivicharpinyo, T.; Wonnapinij, P.; Surat, W. Phylogenetic and recombinant analyses of complete coding sequences of DENV-1 from field-caught mosquitoes in Thailand. Virus Res. 2020, 286, 198041. [Google Scholar] [CrossRef]
- Ngwe Tun, M.M.; Kyaw, A.K.; Nabeshima, T.; Soe, A.M.; Nwe, K.M.; Htet, K.K.K.; Aung, T.H.; Htwe, T.T.; Aung, T.; Myaing, S.S.; et al. Detection of genotype-1 of dengue virus serotype 3 for the first time and complete genome analysis of dengue viruses during the 2018 epidemic in Mandalay, Upper Myanmar. PLoS ONE 2021, 16, e0251314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shu, Y.; Shan, X.; Li, D.; Ma, D.; Li, T.; Long, S.; Wang, X.; Pan, Y.; Chen, J.; et al. Co-circulation of three dengue virus serotypes led to a severe dengue outbreak in Xishuangbanna, a border area of China, Myanmar, and Laos, in 2019. Int. J. Infect. Dis. 2021, 107, 15–17. [Google Scholar] [CrossRef]
- Lee, S.H.; Nam, K.W.; Jeong, J.Y.; Yoo, S.J.; Koh, Y.S.; Lee, S.; Heo, S.T.; Seong, S.Y.; Lee, K.H. The effects of climate change and globalization on mosquito vectors: Evidence from Jeju Island, South Korea on the potential for Asian tiger mosquito (Aedes albopictus) influxes and survival from Vietnam rather than Japan. PLoS ONE 2013, 8, e68512. [Google Scholar] [CrossRef]
- Waman, V.P.; Kolekar, P.; Ramtirthkar, M.R.; Kale, M.M.; Kulkarni-Kale, U. Analysis of genotype diversity and evolution of Dengue virus serotype 2 using complete genomes. PeerJ 2016, 4, e2326. [Google Scholar] [CrossRef]
- van Panhuis, W.G.; Choisy, M.; Xiong, X.; Chok, N.S.; Akarasewi, P.; Iamsirithaworn, S.; Lam, S.K.; Chong, C.K.; Lam, F.C.; Phommasak, B.; et al. Region-wide synchrony and traveling waves of dengue across eight countries in Southeast Asia. Proc. Natl. Acad. Sci. USA 2015, 112, 13069–13074. [Google Scholar] [CrossRef] [PubMed]
- Kerdpanich, P.; Kongkiatngam, S.; Buddhari, D.; Simasathien, S.; Klungthong, C.; Rodpradit, P.; Thaisomboonsuk, B.; Wongstitwilairoong, T.; Hunsawong, T.; Anderson, K.B.; et al. Comparative Analyses of Historical Trends in Confirmed Dengue Illnesses Detected at Public Hospitals in Bangkok and Northern Thailand, 2002–2018. Am. J. Trop. Med. Hyg. 2020, 104, 1058. [Google Scholar] [CrossRef]
- Messina, J.P.; Brady, O.J.; Scott, T.W.; Zou, C.; Pigott, D.M.; Duda, K.A.; Bhatt, S.; Katzelnick, L.; Howes, R.E.; Battle, K.E.; et al. Global spread of dengue virus types: Mapping the 70 year history. Trends Microbiol. 2014, 22, 138–146. [Google Scholar] [CrossRef]
- Sang, S.; Liu-Helmersson, J.; Quam, M.B.M.; Zhou, H.; Guo, X.; Wu, H.; Liu, Q. The evolutionary dynamics of DENV 4 genotype I over a 60-year period. PLoS Negl. Trop. Dis. 2019, 13, e0007592. [Google Scholar] [CrossRef]

| Year | No. of DENV Suspected Patient | No. of Participants | % of Positive (No. of Positive) | |||
|---|---|---|---|---|---|---|
| DENV-1 | DENV-2 | DENV-3 | DENV-4 | |||
| 2018 | 540 | 88 | 40.9% (36) | 17.0% (15) | 9.1% (8) | 20.5% (18) |
| 2019 | 424 | 81 | 44.4% (36) | 27.2% (22) | 0% | 2.5% (2) |
| 2020 | 136 | 107 | 45.8% (49) | 14.0% (15) | 3.7% (4) | 11.2% (12) |
| Total | 1100 | 276 | 43.8% (121) | 18.8% (52) | 4.3% (12) | 11.6% (32) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poltep, K.; Phadungsombat, J.; Nakayama, E.E.; Kosoltanapiwat, N.; Hanboonkunupakarn, B.; Wiriyarat, W.; Shioda, T.; Leaungwutiwong, P. Genetic Diversity of Dengue Virus in Clinical Specimens from Bangkok, Thailand, during 2018–2020: Co-Circulation of All Four Serotypes with Multiple Genotypes and/or Clades. Trop. Med. Infect. Dis. 2021, 6, 162. https://doi.org/10.3390/tropicalmed6030162
Poltep K, Phadungsombat J, Nakayama EE, Kosoltanapiwat N, Hanboonkunupakarn B, Wiriyarat W, Shioda T, Leaungwutiwong P. Genetic Diversity of Dengue Virus in Clinical Specimens from Bangkok, Thailand, during 2018–2020: Co-Circulation of All Four Serotypes with Multiple Genotypes and/or Clades. Tropical Medicine and Infectious Disease. 2021; 6(3):162. https://doi.org/10.3390/tropicalmed6030162
Chicago/Turabian StylePoltep, Kanaporn, Juthamas Phadungsombat, Emi E. Nakayama, Nathamon Kosoltanapiwat, Borimas Hanboonkunupakarn, Witthawat Wiriyarat, Tatsuo Shioda, and Pornsawan Leaungwutiwong. 2021. "Genetic Diversity of Dengue Virus in Clinical Specimens from Bangkok, Thailand, during 2018–2020: Co-Circulation of All Four Serotypes with Multiple Genotypes and/or Clades" Tropical Medicine and Infectious Disease 6, no. 3: 162. https://doi.org/10.3390/tropicalmed6030162
APA StylePoltep, K., Phadungsombat, J., Nakayama, E. E., Kosoltanapiwat, N., Hanboonkunupakarn, B., Wiriyarat, W., Shioda, T., & Leaungwutiwong, P. (2021). Genetic Diversity of Dengue Virus in Clinical Specimens from Bangkok, Thailand, during 2018–2020: Co-Circulation of All Four Serotypes with Multiple Genotypes and/or Clades. Tropical Medicine and Infectious Disease, 6(3), 162. https://doi.org/10.3390/tropicalmed6030162







