Identification of Novel Reassortant Shuni Virus Strain in Clinical Cases of Israeli Ruminants, 2020–2021
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Samples
2.2. Viral RNA/DNA Extraction
2.3. Laboratory Tests
2.4. Sequencing and Phylogenetic Analyses
3. Results
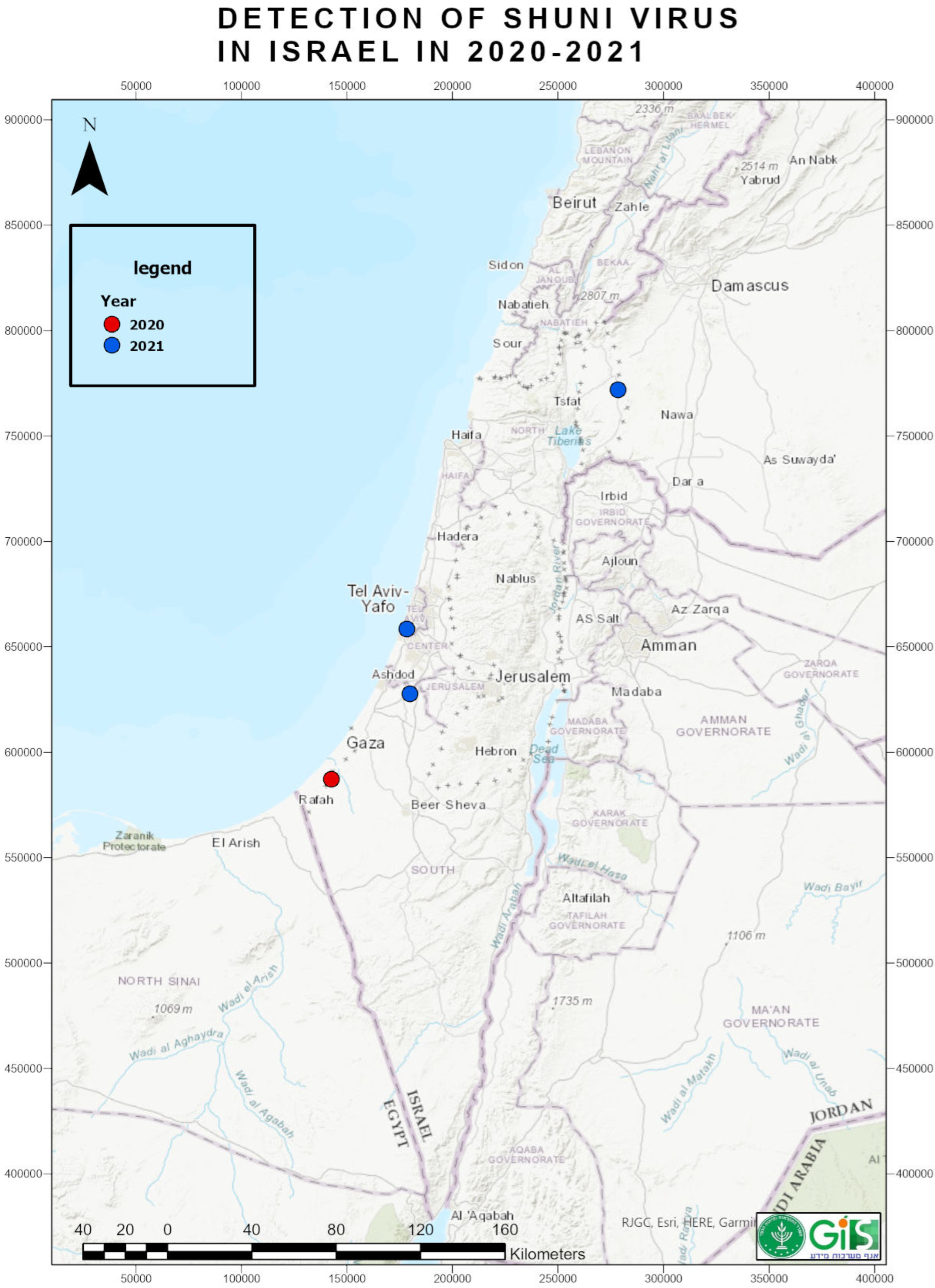
3.1. Clinical Cases and Laboratory Diagnosis
3.1.1. Cattle Abortions
3.1.2. Beef Cattle
3.1.3. Fattening Calf
3.1.4. Goat Abortion
3.2. Total PanSimbu RT-qPCR and Identification of Simbuviruses from Field Samples
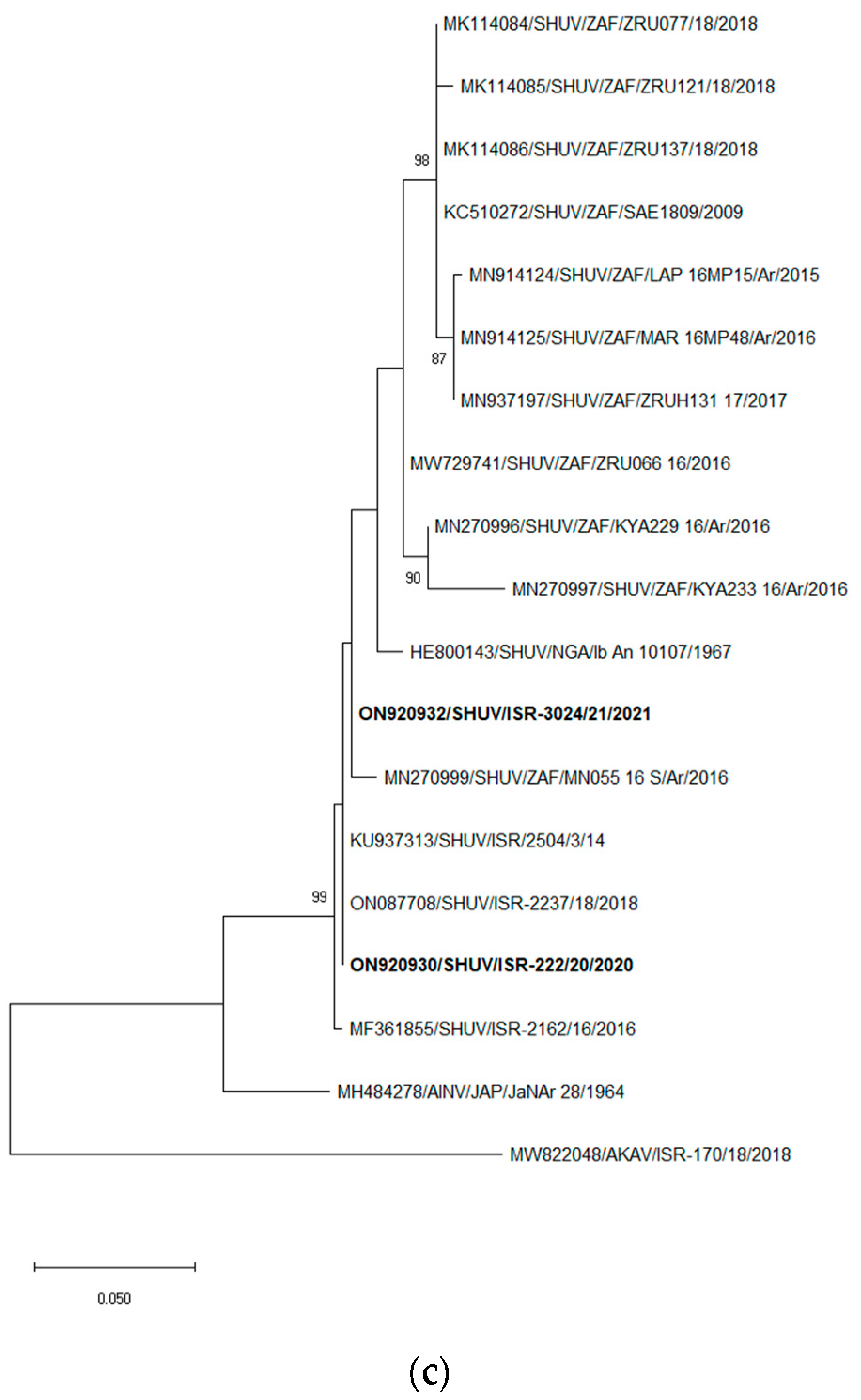
3.3. Sequencing and Pairwise and Phylogenetic Analyses
3.3.1. M Segment Analyses
3.3.2. L Segment Analyses
3.3.3. S Segment Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sick, F.; Beer, M.; Kampen, H.; Wernike, K. Culicoides Biting Midges-Underestimated Vectors for Arboviruses of Public Health and Veterinary Importance. Viruses 2019, 24, 376. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.M. Orthobunyaviruses: Recent genetic and structural insights. Nat. Rev. Microbiol. 2014, 12, 673–685. [Google Scholar] [CrossRef]
- Plyusnin, A.; Elliott, R.M. Bunyaviridae: Molecular and Cellular Biology; Plyusnin, A., Elliott, R.M., Eds.; Caister Academic Press: Norfolk, UK, 2011; pp. 1–40. [Google Scholar]
- Beer, M.; Wernike, K. Akabane virus and Schmallenberg virus (Peribunyaviridae). In Virology, 4th ed.; Bamford, D.H., Zuckerman, M., Eds.; Academic Press: Oxford, UK, 2021; Volume 2, pp. 34–39. [Google Scholar] [CrossRef]
- Kirkland, P.D. Akabane virus infection. Rev. Sci. Tech. 2015, 34, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Golender, N.; Bumbarov, V.; Eldar, A.; Zamir, L.; Even-Tov, B.; Gabriel, K.; Eitan, T. Isolation of Sango viruses from Israeli symptomatic cattle. Int. J. Veter Sci. Res. 2021, 7, 069–072. [Google Scholar]
- Golender, N.; Bumbarov, V.; Assis, I.; Beer, M.; Khinich, Y.; Koren, O.; Edery, N.; Eldar, A.; Wernike, K. Shuni virus in Israel: Neurological disease and fatalities in cattle. Transbound. Emerg. Dis. 2019, 66, 1126–1131. [Google Scholar] [CrossRef]
- Sick, F.; Breithaupt, A.; Golender, N.; Bumbarov, V.; Beer, M.; Wernike, K. Shuni virus-induced meningoencephalitis after experimental infection of cattle. Transbound. Emerg. Dis. 2021, 68, 1531–1540. [Google Scholar] [CrossRef]
- Kono, R.; Hirata, M.; Kaji, M.; Goto, Y.; Ikeda, S.; Yanase, T.; Kato, T.; Tanaka, S.; Tsutsui, T.; Imada, T.; et al. Bovine epizootic encephalomyelitis caused by Akabane virus in southern Japan. BMC Vet. Res. 2008, 4, 20. [Google Scholar] [CrossRef]
- van Eeden, C.; Williams, J.H.; Gerdes, T.G.; van Wilpe, E.; Viljoen, A.; Swanepoel, R.; Venter, M. Shuni virus as cause of neurologic disease in horses. Emerg. Infect. Dis. 2012, 18, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Yanase, T.; Kato, T.; Aizawa, M.; Shuto, Y.; Shirafuji, H.; Yamakawa, M.; Tsuda, T. Genetic reassortment between Sathuperi and Shamonda viruses of the genus Orthobunyavirus in nature: Implications for their genetic relationship to Schmallenberg virus. Arch. Virol. 2012, 157, 1611–1616. [Google Scholar] [CrossRef]
- Garigliany, M.M.; Bayrou, C.; Kleijnen, D.; Cassart, D.; Jolly, S.; Linden, A.; Desmecht, D. Schmallenberg virus: A new Shamonda/Sathuperi-like virus on the rise in Europe. Antiviral Res. 2012, 95, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Bowen, M.D.; Trappier, S.G.; Sanchez, A.J.; Meyer, R.F.; Goldsmith, C.S.; Zaki, S.R.; Dunster, L.M.; Peters, C.J.; Ksiazek, T.G.; Nichol, S.T.; et al. A reassortant bunyavirus isolated from acute hemorrhagic fever cases in Kenya and Somalia. Virology 2001, 291, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Yanase, T.; Yamakawa, M.; Kato, T.; Yoshida, K.; Tsuda, T. Genetic diversity and reassortments among Akabane virus field isolates. Virus Res. 2007, 130, 162–171. [Google Scholar] [CrossRef]
- Oymans, J.; Wichgers Schreur, P.J.; van Oort, S.; Vloet, R.; Venter, M.; Pijlman, G.P.; van Oers, M.M.; Kortekaas, J. Reverse Genetics System for Shuni Virus, an Emerging Orthobunyavirus with Zoonotic Potential. Viruses 2020, 12, 455. [Google Scholar] [CrossRef] [PubMed]
- Causey, O.R.; Kemp, G.E.; Causey, C.E.; Lee, V.H. Isolations of Simbu-group viruses in Ibadan, Nigeria 1964–69, including the new types Sango, Shamonda, Sabo and Shuni. Ann. Trop. Med. Parasitol. 1972, 66, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Golender, N.; Brenner, J.; Valdman, M.; Khinich, Y.; Bumbarov, V.; Panshin, A.; Edery, N.; Pismanik, S.; Behar, A. Malformations Caused by Shuni Virus in Ruminants, Israel, 2014–2015. Emerg. Infect. Dis. 2015, 21, 2267–2268. [Google Scholar] [CrossRef]
- Steyn, J.; Motlou, P.; van Eeden, C.; Pretorius, M.; Stivaktas, V.I.; Williams, J.; Snyman, L.P.; Buss, P.E.; Beechler, B.; Jolles, A.; et al. Shuni Virus in Wildlife and Nonequine Domestic Animals, South Africa. Emerg. Infect. Dis. 2020, 26, 1521–1525. [Google Scholar] [CrossRef]
- Golender, N.; Bumbarov, V.; Kovtunenko, A.; David, D.; Guini-Rubinstein, M.; Sol, A.; Beer, M.; Eldar, A.; Wernike, K. Identification and Genetic Characterization of Viral Pathogens in Ruminant Gestation Abnormalities, Israel, 2015–2019. Viruses 2021, 13, 2136. [Google Scholar] [CrossRef]
- Moore, D.L.; Causey, O.R.; Carey, D.E.; Reddy, S.; Cooke, A.R.; Akinkugbe, F.M.; David-West, T.S.; Kemp, G.E. Arthropod-borne viral infections of man in Nigeria, 1964–1970. Ann. Trop. Med. Parasitol. 1975, 69, 49–64. [Google Scholar] [CrossRef]
- Motlou, T.P.; Venter, M. Shuni Virus in Cases of Neurologic Disease in Humans, South Africa. Emerg. Infect. Dis. 2021, 27, 565–569. [Google Scholar] [CrossRef]
- Golender, N.; Bumbarov, V.Y.; Erster, O.; Beer, M.; Khinich, Y.; Wernike, K. Development and validation of a universal S-segment-based real-time RT-PCR assay for the detection of Simbu serogroup viruses. J. Virol. Methods 2018, 261, 80–85. [Google Scholar] [CrossRef]
- Wernike, K.; Hoffmann, B.; Beer, M. Simultaneous detection of five notifiable viral diseases of cattle by single-tube multiplex real-time RT-PCR. J. Virol. Methods 2015, 217, 28–35. [Google Scholar] [CrossRef]
- Smith, J.S. Rabies virus. In Manual of Clinical Microbiology, 6th ed.; Murray, P.R., Baron, E.J., Pfaller, M.A., Tenover, F.C., Yolken, R.H., Eds.; American Society for Microbiology: Washington, DC, USA, 1995; pp. 907–1003. [Google Scholar]
- Cunha, C.W.; Otto, L.; Taus, N.S.; Knowles, D.P.; Li, H. Development of a multiplex real-time PCR for detection and differentiation of malignant catarrhal fever viruses in clinical samples. J. Clin. Microbiol. 2009, 47, 2586–2589. [Google Scholar] [CrossRef] [PubMed]
- Erster, O.; Stram, R.; Menasherow, S.; Rubistein-Giuni, M.; Sharir, B.; Kchinich, E.; Stram, Y. High-resolution melting (HRM) for genotyping bovine ephemeral fever virus (BEFV). Virus Res. 2017, 229, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Schirrmeier, H.; Wernike, K.; Wegelt, A.; Beer, M.; Hoffmann, B. Development of a pan-Simbu real-time reverse transcriptase PCR for the detection of Simbu serogroup viruses and comparison with SBV diagnostic PCR systems. Virol. J. 2013, 10, 327. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]

| Aborted Fetus and Newborn | Other Age Categories | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Brain | Placenta | Mixed | Blood | Total Samples | Total Cases | Brain | Internal Organs | Blood | Total Samples | Total | |
| 2020 | cattle | 4/42 | 1/18 | 0/1 | 2/13 | 7/74 | 7/65 | 0/11 | 0/7 | 0/240 | 0/258 | 7/332 |
| sheep | 18/85 | 15/56 | 0/5 | 0/2 | 33/148 | 20/101 | 0/11 | 0/1 | 1/35 | 1/47 | 34/195 | |
| goat | 4/9 | 0/4 | 0 | 0 | 4/13 | 4/10 | 0/4 | 0 | 0/2 | 0/6 | 4/19 | |
| wild/zoo | 0/6 | 0/2 | 0 | 0 | 0/8 | 0/6 | 0/12 | 0 | 0/3 | 0/15 | 0/23 | |
| total | 26/142 | 16/80 | 0/6 | 2/15 | 44/243 | 31/182 | 0/38 | 0/8 | 1/280 | 1/326 | 45/569 | |
| 2021 | cattle | 2/45 | 1/16 | 0/1 | 0/13 | 3/75 | 3/68 | 1/5 | 0 | 1/136 | 2/141 | 5/216 |
| sheep | 6/60 | 6/34 | 0 | 0/1 | 12/95 | 10/72 | 0/4 | 0 | 0/23 | 0/27 | 12/122 | |
| goat | 2/7 | 2/4 | 0 | 0 | 4/11 | 3/10 | 0/1 | 0/1 | 0/4 | 0/6 | 4/17 | |
| wild/zoo | 0 | 0 | 0 | 0 | 0 | 0 | 0/8 | 0 | 0 | 0/8 | 0/8 | |
| total | 10/112 | 9/54 | 0/1 | 0/14 | 19/181 | 16/150 | 1/18 | 0/1 | 1/163 | 2/182 | 21/363 | |
| total | 36/254 | 26/134 | 0/7 | 2/29 | 63/424 | 45/332 | 1/56 | 0/9 | 2/443 | 3/508 | 66/932 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golender, N.; Varsano, J.S.; Nissimyan, T.; Tiomkin, E. Identification of Novel Reassortant Shuni Virus Strain in Clinical Cases of Israeli Ruminants, 2020–2021. Trop. Med. Infect. Dis. 2022, 7, 297. https://doi.org/10.3390/tropicalmed7100297
Golender N, Varsano JS, Nissimyan T, Tiomkin E. Identification of Novel Reassortant Shuni Virus Strain in Clinical Cases of Israeli Ruminants, 2020–2021. Tropical Medicine and Infectious Disease. 2022; 7(10):297. https://doi.org/10.3390/tropicalmed7100297
Chicago/Turabian StyleGolender, Natalia, Joseph Seffi Varsano, Tomer Nissimyan, and Eitan Tiomkin. 2022. "Identification of Novel Reassortant Shuni Virus Strain in Clinical Cases of Israeli Ruminants, 2020–2021" Tropical Medicine and Infectious Disease 7, no. 10: 297. https://doi.org/10.3390/tropicalmed7100297
APA StyleGolender, N., Varsano, J. S., Nissimyan, T., & Tiomkin, E. (2022). Identification of Novel Reassortant Shuni Virus Strain in Clinical Cases of Israeli Ruminants, 2020–2021. Tropical Medicine and Infectious Disease, 7(10), 297. https://doi.org/10.3390/tropicalmed7100297









