Prevalence and Risk Factors of Opisthorchis viverrini Infection in Sakon Nakhon Province, Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Statistical Analysis
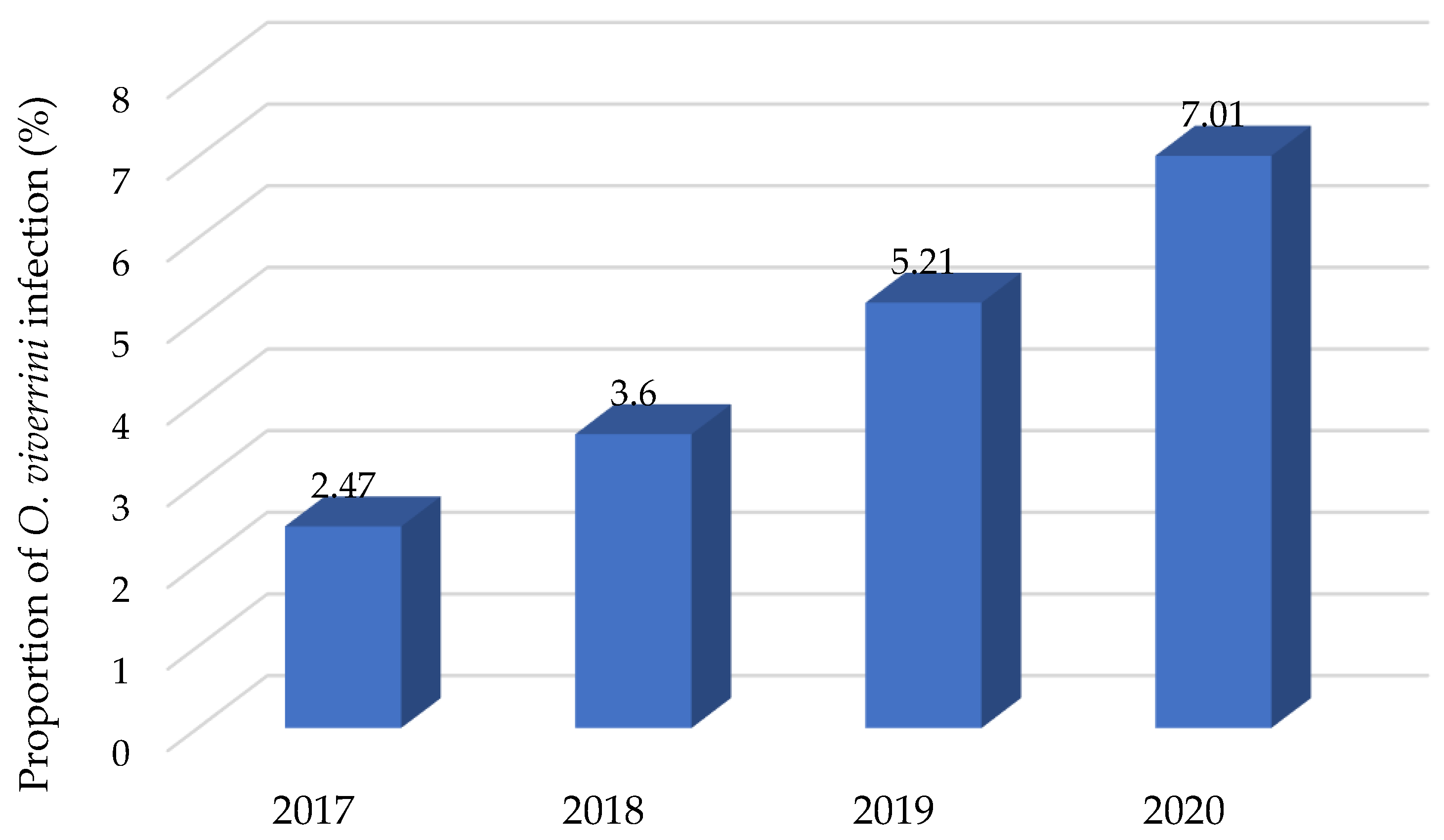
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Infection with liver flukes (Opisthorchis viverrini, Opisthorchis felineus and Clonorchis sinensis). IARC Monogr. Eval. Carcinog. Risks Hum. 1994, 61, 121–175. [Google Scholar]
- Chai, J.Y.; Darwin Murrell, K.; Lymbery, A.J. Fish-borne parasitic zoonoses: Status and issues. Int. J. Parasitol. 2005, 35, 1233–1254. [Google Scholar] [CrossRef]
- Mairiang, E.; Haswell-Elkins, M.R.; Mairiang, P.; Sithithaworn, P.; Elkins, D.B. Reversal of biliary tract abnormalities associated with Opisthorchis viverrini infection following praziquantel treatment. Trans. R. Soc. Trop. Med. Hyg. 1993, 87, 194–197. [Google Scholar] [CrossRef]
- Hughes, T.; O’Connor, T.; Techasen, A.; Namwat, N.; Loilome, W.; Andrews, R.H.; Khuntikeo, N.; Yongvanit, P.; Sithithaworn, P.; Taylor-Robinson, S.D. Opisthorchiasis and cholangiocarcinoma in Southeast Asia: An unresolved problem. Int. J. Gen. Med. 2017, 10, 227. [Google Scholar] [CrossRef]
- Labony, S.S.; Alim, M.A.; Hasan, M.M.; Hossain, M.S.; Islam, A.; Alam, M.Z.; Tsuji, N. Anisuzzaman. Fish-borne trematode infections in wild fishes in Bangladesh. Pathog. Glob. 2020, 114, 91–98. [Google Scholar] [CrossRef]
- Jongsuksuntigul, P.; Imsomboon, T. Opisthorchiasis control in Thailand. Acta Trop. 2003, 88, 229–232. [Google Scholar] [CrossRef]
- Sithithaworn, P.; Andrews, R.H.; Nguyen, V.D.; Wongsaroj, T.; Sinuon, M.; Odermatt, P.; Nawa, Y.; Liang, S.; Brindley, P.J.; Sripa, B. The current status of opisthorchiasis and clonorchiasis in the Mekong basin. Parasitol. Int. 2012, 61, 10–16. [Google Scholar] [CrossRef]
- Sripa, B.; Bethony, J.M.; Sithithaworn, P.; Kaewkes, S.; Mairiang, E.; Loukas, A.; Mulvenna, J.; Laha, T.; Hotez, P.J.; Brindley, P.J. Opisthorchiasis and Opisthorchis-associated cholangiocarcinoma in Thailand and Laos. Acta Trop. 2011, 120, S158–S168. [Google Scholar] [CrossRef]
- Kaewpitoon, N.; Kootanavanichpong, N.; Kompor, P.; Chavenkun, W.; Kujapun, J.; Norkaew, J.; Ponphimai, S.; Matrakool, L.; Tongtawee, T.; Panpimanmas, S.; et al. Review and current status of Opisthorchis viverrini infection at the community level in Thailand. Asian Pac. J. Cancer Prev. 2015, 16, 6825–6830. [Google Scholar] [CrossRef]
- Sripa, B.; Brindley, P.J.; Mulvenna, J.; Laha, T.; Smout, M.J.; Mairiang, E.; Bethony, J.M.; Loukas, A. The tumorigenic liver fluke Opisthorchis viverrini–multiple pathways to cancer. Trends Parasitol. 2012, 28, 395–407. [Google Scholar] [CrossRef]
- Sripa, B.; Kaewkes, S.; Sithithaworn, P.; Mairiang, E.; Laha, T.; Smout, M.; Pairojkul, C.; Bhudhisawasdi, V.; Tesana, S.; Thinkamrop, B.; et al. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007, 4, e201. [Google Scholar] [CrossRef]
- Mairiang, E.; Elkins, D.B.; Mairiang, P.; Chaiyakum, J.; Chamadol, N.; Loapaiboon, V.; Posri, S.; Sithithaworn, P.; Haswell-elkins, M. Relationship between intensity of Opisthorchis viverrini infection and hepatobiliary disease detected by ultrasonography. J. Gastroenterol. Hepatol. 1992, 7, 17–21. [Google Scholar] [CrossRef]
- Haswell, M.R.; Satarug, S.; Elkins, D.B. Opisthorchis viverrini infection in northeast Thailand and its relationship to cholangiocarcinoma. J. Gastroenterol. Hepatol. 1992, 7, 538–548. [Google Scholar] [CrossRef]
- Pengput, A.; Schwartz, D.G. Risk factors for Opisthorchis viverrini infection: A systematic review. J. Infect. Public Health 2020, 13, 1265–1273. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Hasegawa, S.; Ikai, I.; Fujii, H.; Hatano, E.; Shimahara, Y. Surgical resection of hilar cholangiocarcinoma: Analysis of survival and postoperative complications. World J. Surg. 2007, 31, 1258–1265. [Google Scholar] [CrossRef]
- Kamsa-Ard, S.; Luvira, V.; Suwanrungruang, K.; Kamsa-Ard, S.; Luvira, V.; Santong, C.; Srisuk, T.; Pugkhem, A.; Bhudhisawasdi, V.; Pairojkul, C. Cholangiocarcinoma trends, incidence, and relative survival in Khon Kaen, Thailand from 1989 through 2013: A population-based cancer registry study. J. Epidemiol. 2019, 29, 197–204. [Google Scholar] [CrossRef]
- Bureau of Epidemiology, Ministry of Public Health, Thailand. Annual Epidemiological Surveillance Report. 2009; pp. 128–129. Available online: https://apps-doe.moph.go.th/boeeng/annual.php (accessed on 24 July 2022).
- Jongsuksuntigul, P.; Imsomboon, T. The impact of a decade long opisthorchiasis control program in northeastern Thailand. Southeast Asian J. Trop. Med. Public Health 1997, 28, 551–557. [Google Scholar]
- CASCAP Fluke Free Thailand. 2017. Available online: https://cloud.cascap.in.th/report/report-flukefree (accessed on 12 July 2022).
- Srithai, C.; Chuangchaiya, S.; Jaichuang, S.; Idris, Z.M. Prevalence of Opisthorchis viverrini and its associated risk factors in the Phon Sawan District of Nakhon Phanom province, Thailand. Iran. J. Parasitol. 2021, 16, 474. [Google Scholar]
- Charoensuk, L.; Ribas, A.; Chedtabud, K.; Prakobwong, S. Infection rate of Opisthorchis viverrini metacercariae in cyprinoid fish from the markets and its association to human opisthorchiasis in the local community in the Northeast Thailand. Acta Trop. 2022, 225, 106216. [Google Scholar] [CrossRef]
- Sripa, B.; Tangkawattana, S.; Laha, T.; Kaewkes, S.; Mallory, F.F.; Smith, J.F.; Wilcox, B.A. Toward integrated opisthorchiasis control in northeast Thailand: The Lawa project. Acta Trop. 2015, 141, 361–367. [Google Scholar] [CrossRef]
- Rangsin, R.; Mungthin, M.; Taamasri, P.; Mongklon, S.; Aimpun, P.; Naaglor, T.; Leelayoova, S. Incidence and risk factors of Opisthorchis viverrini infections in a rural community in Thailand. Am. J. Trop. Med. Hyg. 2009, 81, 152–155. [Google Scholar] [CrossRef]
- Aunpromma, S.; Tangkawattana, P.; Papirom, P.; Kanjampa, P.; Tesana, S.; Sripa, B.; Tangkawattana, S. High prevalence of Opisthorchis viverrini infection in reservoir hosts in four districts of Khon Kaen Province, an opisthorchiasis endemic area of Thailand. Parasitol. Int. 2012, 61, 60–64. [Google Scholar]
- Sriamporn, S.; Pisani, P.; Pipitgool, V.; Suwanrungruang, K.; Kamsa-Ard, S.; Parkin, D.M. Prevalence of Opisthorchis viverrini infection and incidence of cholangiocarcinoma in Khon Kaen, Northeast Thailand. Trop. Med. Int. Health 2004, 9, 588–594. [Google Scholar] [CrossRef]
- Thaewnongiew, K.; Singthong, S.; Kutchamart, S.; Tangsawad, S.; Promthet, S.; Sailugkum, S.; Wongba, N. Prevalence and risk factors for Opisthorchis viverrini infections in upper Northeast Thailand. Asian Pac. J. Cancer Prev. 2014, 15, 6609–6612. [Google Scholar] [CrossRef]
- Saenna, P.; Hurst, C.; Echaubard, P.; Wilcox, B.A.; Sripa, B. Fish sharing as a risk factor for Opisthorchis viverrini infection: Evidence from two villages in north-eastern Thailand. Infect. Dis. Poverty 2017, 6, 66. [Google Scholar] [CrossRef]
- Suwannahitatorn, P.; Webster, J.; Riley, S.; Mungthin, M.; Donnelly, C.A. Uncooked fish consumption among those at risk of Opisthorchis viverrini infection in central Thailand. PLoS ONE 2019, 14, e0211540. [Google Scholar] [CrossRef]
- Yeoh, K.W.; Promthet, S.; Sithithaworn, P.; Kamsaard, S.; Parkin, D.M. Re-examination of Opisthorchis viverrini infection in northeast Thailand. Asian Pac. J. Cancer Prev. 2015, 16, 3413–3418. [Google Scholar]
- Saengsawang, P.; Promthet, S.; Bradshaw, P. Infection with Opisthorchis viverrini and use of praziquantel among a working-age population in northeast Thailand. Asian Pac. J. Cancer Prev. 2013, 14, 2963–2966. [Google Scholar] [CrossRef]
- Thinkhamrop, K.; Khuntikeo, N.; Sithithaworn, P.; Thinkhamrop, W.; Wangdi, K.; Kelly, M.J.; Suwannatrai, A.T.; Gray, D.J. Repeated praziquantel treatment and Opisthorchis viverrini infection: A population-based cross-sectional study in northeast Thailand. Infect. Dis. Poverty. 2019, 8, 34–42. [Google Scholar]
- Chudthaisong, N.; Promthet, S.; Bradshaw, P. Risk factors for Opisthorchis viverrini infection in Nong Khai province, Thailand. Asian Pac. J. Cancer Prev. 2015, 16, 4593–4596. [Google Scholar] [CrossRef]
- Forrer, A.; Sayasone, S.; Vounatsou, P.; Vonghachack, Y.; Bouakhasith, D.; Vogt, S.; Glaser, R.; Utzinger, J.; Akkhavong, K.; Odermatt, P. Spatial distribution of, and risk factors for, Opisthorchis viverrini infection in southern Lao PDR. PLoS Negl. Trop. Dis. 2012, 6, e1481. [Google Scholar] [CrossRef]
- Prakobwong, S.; Suwannatrai, A.; Sancomerang, A.; Chaipibool, S.; Siriwechtumrong, N. A large scale study of the epidemiology and risk factors for the carcinogenic liver fluke Opisthorchis viverrini in Udon Thani Province, Thailand. Asian Pac. J. Cancer Prev. 2017, 18, 2853. [Google Scholar]
- Kim, C.S.; Smith, J.F.; Suwannatrai, A.; Echaubard, P.; Wilcox, B.; Kaewkes, S.; Sithithaworn, P.; Sripa, B. Role of socio-cultural and economic factors in cyprinid fish distribution networks and consumption in Lawa Lake region, Northeast Thailand: Novel perspectives on Opisthorchis viverrini transmission dynamics. Acta Trop. 2017, 170, 85–94. [Google Scholar] [CrossRef]
- Wilcox, B.A.; Echaubard, P. Balancing biomedical and ecological perspectives in research framing of liver fluke and cholangiocarcinoma in NE Thailand. Parasitol. Int. 2017, 66, 372–377. [Google Scholar] [CrossRef]
- Sripa, B. Concerted action is needed to tackle liver fluke infections in Asia. PLoS Negl. Trop. Dis. 2008, 2, e232. [Google Scholar] [CrossRef]
- Saiyachak, K.; Tongsotsang, S.; Saenrueang, T.; Moore, M.A.; Promthet, S. Prevalence and factors associated with Opisthorchis viverrini infection in Khammouane province, Lao PDR. Asian Pac. J. Cancer Prev. 2016, 17, 1589–1593. [Google Scholar] [CrossRef][Green Version]
- Nakbun, S.; Thongkrajai, P.; Nithikathkul, C. Risk factors for infection in Nakhon Phanom, Thailand, where the infection is highly endemic. Asian Biomed. 2018, 12, 45–51. [Google Scholar] [CrossRef]
- Pumidonming, W.; Katahira, H.; Igarashi, M.; Salman, D.; Abdelbaset, A.E.; Sangkaeo, K. Potential risk of a liver fluke Opisthorchis viverrini infection brought by immigrants from prevalent areas: A case study in the lower Northern Thailand. Acta Trop. 2018, 178, 213–218. [Google Scholar] [CrossRef]
- Reiche, E.M.V.; Nunes, S.O.V.; Morimoto, H.K. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004, 5, 617–625. [Google Scholar] [CrossRef]
- Sakondhavat, A. Understanding Poverty Dynamics Using a Mixed-Method Study: Evidence from the Rural Village in the Northeast and Central Regions of Thailand. Ph.D. Thesis, University of Sussex, Falmer, UK, 2013. [Google Scholar]
- Kaewboonchoo, O.; Kongtip, P.; Woskie, S. Occupational health and safety for agricultural workers in Thailand: Gaps and recommendations, with a focus on pesticide use. New Solut. 2015, 25, 102–120. [Google Scholar] [CrossRef]
| Variable | Controls (n = 160) | Cases (n = 160) | Total | Univariate OR | 95%CI | p-Value |
|---|---|---|---|---|---|---|
| Number (%) | Number (%) | 320 | ||||
| Gender | ||||||
| Male | 84 (52.50) | 66 (41.25) | 150 (46.90) | 1 | ||
| Female | 76 (47.50) | 94 (58.75) | 170 (53.10) | 1.57 * | 0.99–2.51 | 0.044 |
| Age (years) | ||||||
| 55+ | 108 (67.50) | 99 (61.87) | 207 (64.70) | 1 | ||
| ≤55 | 52 (32.50) | 61 (38.13) | 113 (35.30) | 1.28 | 0.79–2.08 | 0.294 |
| Status | ||||||
| Widowed/ | ||||||
| divorced/ | ||||||
| separated | 18 (11.25) | 7 (4.37) | 25 (7.80) | 1 | ||
| Married | 142 (88.75) | 153 (95.62) | 295 (92.20) | 2.77 * | 1.06–8.06 | 0.022 |
| Education | ||||||
| Secondary school/upper | ||||||
| 16 (10.00) | 13 (8.10) | 29 (9.10) | 1 | |||
| Primary school/lower | ||||||
| 144 (90.00) | 147 (91.90) | 291 (90.90) | 1.26 | 0.55–2.95 | 0.560 | |
| Occupation | ||||||
| Other | 17 (10.60) | 9 (5.60) | 26 (8.10) | 1 | ||
| Agriculture | 143 (89.40) | 151 (94.40) | 294 (91.90) | 2.00 | 0.81–5.24 | 0.102 |
| Family income per month (THB) | ||||||
| <5000 | 39 (24.40) | 28 (17.50) | 67 (20.90) | 1 | ||
| 5001–10,000 | 91 (56.90) | 97 (60.60) | 188 (58.80) | 0.67 | 0.37–1.23 | 0.17 |
| 10,001–15,000 | 21 (13.10) | 29 (18.10) | 50 (15.60) | 0.52 | 0.23–1.16 | 0.08 |
| >15,000 | 9 (5.60) | 6 (3.80) | 15 (4.70) | 1.08 | 0.30–4.12 | 0.89 |
| Variable | Controls (n = 160) | Cases (n = 160) | Total | Univariate OR | 95%CI | p-Value |
|---|---|---|---|---|---|---|
| Number (%) | Number (%) | 320 | ||||
| Habit of eating raw fish | ||||||
| Several times | 30 (18.75) | 95 (59.38) | 125 (39.06) | 1 | ||
| Sometimes | 130 (81.25) | 65 (40.62) | 195 (60.94) | 6.33 *** | 3.71–10.90 | <0.0001 |
| History of OV examination | ||||||
| Never | 138 (86.25) | 66 (41.25) | 204 (63.75) | 1 | ||
| 1st time | 22 (13.75) | 94 (58.75) | 116 (36.25) | 8.93 *** | 5.15–16.21 | <0.0001 |
| History of OV infection | ||||||
| Never | 160 (100.00) | 71 (44.38) | 231 (72.20) | 1 | ||
| Ever | 0 (0) | 89 (55.62) | 89 (27.80) | 201.25 *** | 33.32–8082.76 | <0.0001 |
| History of praziquantel administration | ||||||
| Never use | 160 (100.00) | 71 (44.37) | 231 (72.20) | 1 | ||
| Have used | 0 (0) | 89 (55.63) | 89 (27.80) | 201.25 *** | 33.32–8082.76 | <0.0001 |
| Relative with CCA | ||||||
| None | 129 (80.62) | 138 (86.25) | 267 (83.43) | 1 | ||
| Have relative | 31 (19.38) | 22 (13.75) | 53 (16.57) | 1.07 | 0.36–1.20 | 0.175 |
| Alcohol consumption | ||||||
| No | 84 (52.50) | 89 (55.62) | 173 (54.06) | 1 | ||
| Yes | 76 (47.50) | 71 (44.38) | 147 (45.94) | 0.88 | 0.56–1.36 | 0.575 |
| Smoking | ||||||
| No | 113 (70.63) | 121 (75.63) | 234 (71.13) | 1 | ||
| Yes | 47 (29.37) | 39 (24.37) | 86 (26.87) | 0.77 | 0.47–1.27 | 0.314 |
| Defecation in latrine | ||||||
| No | 0 (0) | 0 (0) | 0 (0) | 1 | ||
| Yes | 160 (100.00) | 160 (100.00) | 320 (100.00) | - | - | - |
| Agriculture and pesticide used | ||||||
| Never used | 159 (99.38) | 155 (96.88) | 314 (98.13) | 1 | ||
| Have used | 1 (0.62) | 5 (3.12) | 6 (1.87) | 5.12 | 0.59–44.40 | 0.138 |
| House near wetlands | ||||||
| No | 110 (68.75) | 111 (69.37) | 221 (69.07) | 1 | ||
| Yes | 50 (31.25) | 49 (30.63) | 99 (30.93) | 0.97 | 0.61–1.56 | 0.904 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perakanya, P.; Ungcharoen, R.; Worrabannakorn, S.; Ongarj, P.; Artchayasawat, A.; Boonmars, T.; Boueroy, P. Prevalence and Risk Factors of Opisthorchis viverrini Infection in Sakon Nakhon Province, Thailand. Trop. Med. Infect. Dis. 2022, 7, 313. https://doi.org/10.3390/tropicalmed7100313
Perakanya P, Ungcharoen R, Worrabannakorn S, Ongarj P, Artchayasawat A, Boonmars T, Boueroy P. Prevalence and Risk Factors of Opisthorchis viverrini Infection in Sakon Nakhon Province, Thailand. Tropical Medicine and Infectious Disease. 2022; 7(10):313. https://doi.org/10.3390/tropicalmed7100313
Chicago/Turabian StylePerakanya, Pariyakorn, Ratchadaporn Ungcharoen, Sutthiporn Worrabannakorn, Passakorn Ongarj, Atchara Artchayasawat, Thidarut Boonmars, and Parichart Boueroy. 2022. "Prevalence and Risk Factors of Opisthorchis viverrini Infection in Sakon Nakhon Province, Thailand" Tropical Medicine and Infectious Disease 7, no. 10: 313. https://doi.org/10.3390/tropicalmed7100313
APA StylePerakanya, P., Ungcharoen, R., Worrabannakorn, S., Ongarj, P., Artchayasawat, A., Boonmars, T., & Boueroy, P. (2022). Prevalence and Risk Factors of Opisthorchis viverrini Infection in Sakon Nakhon Province, Thailand. Tropical Medicine and Infectious Disease, 7(10), 313. https://doi.org/10.3390/tropicalmed7100313






