Monkeypox and HIV in the Canary Islands: A Different Pattern in a Mobile Population
Abstract
:1. Introduction
2. Materials and Methods
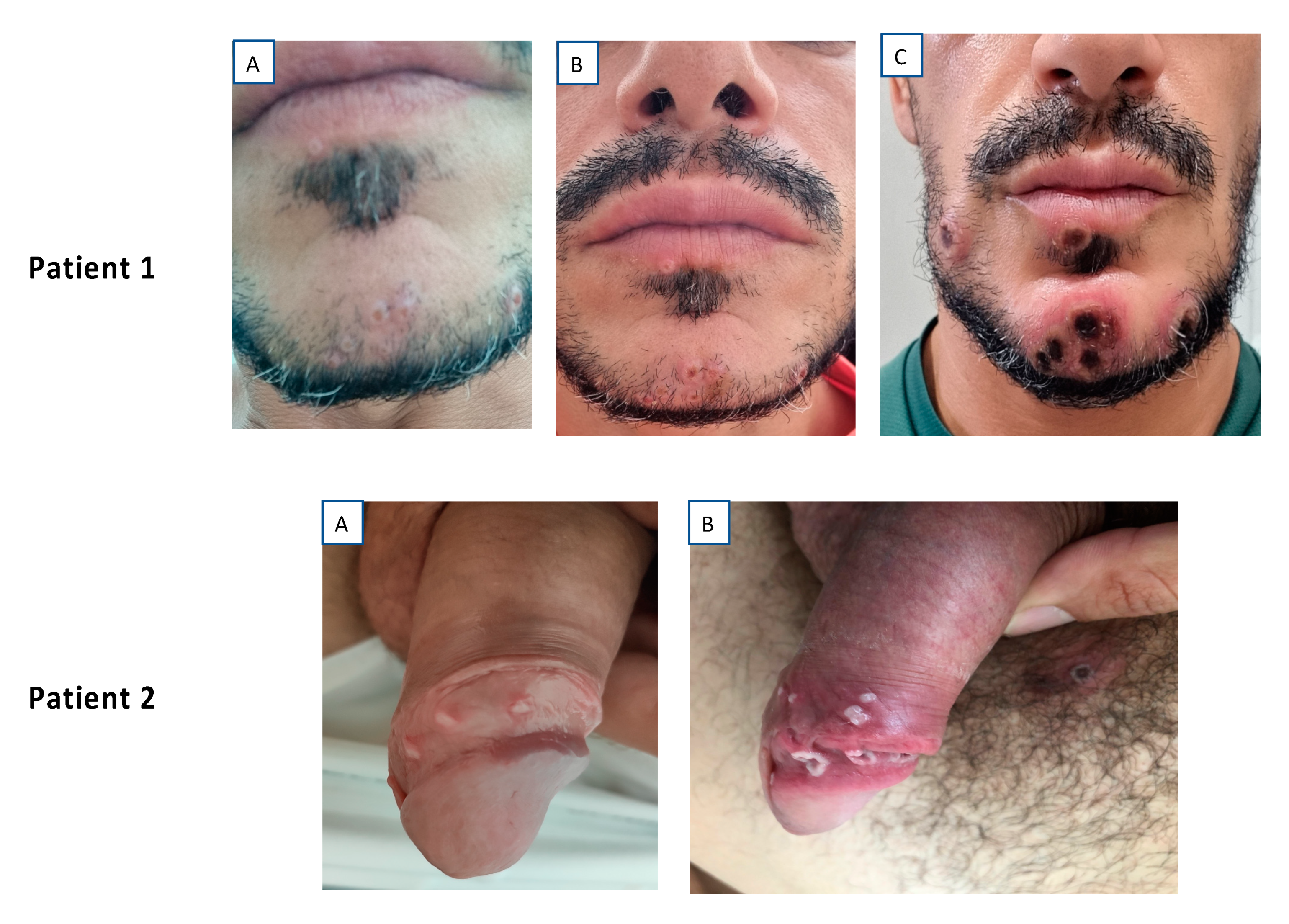
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.I.O.; de Oliveira, J.S.; Kroon, E.G.; Trindade, G.S.; Drumond, B.P. Here, There, and Everywhere: The Wide Host Range and Geographic Distribution of Zoonotic Orthopoxviruses. Viruses 2020, 1, 43. [Google Scholar] [CrossRef] [PubMed]
- Harris, E. What to Know About Monkeypox. JAMA 2022, 327, 2278–2279. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Monkeypox outbreak questions intensify as cases soar. Science 2022, 376, 902–903. [Google Scholar] [CrossRef]
- Velavan, T.P.; Meyer, C.G. Monkeypox 2022 outbreak: An update. Trop Med. Int. Health 2022, 27, 604–605. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control/WHO Regional Office for Europe. Monkeypox, Joint Epidemiological Overview. Available online: https://www.ecdc.europa.eu/en/news-events/monkeypox-situation-update (accessed on 19 August 2022).
- Kupferschmidt, K. Why monkeypox is mostly hitting men who have sex with men. Science 2022, 376, 1364–1365. [Google Scholar] [CrossRef]
- Orviz, E.; Negredo, A.; Ayerdi, O.; Vázquez, A.; Muñoz-Gomez, A.; Monzón, S.; Clavo, P.; Zaballos, A.; Vera, M.; Sánchez, P.; et al. Monkeypox outbreak in Madrid (Spain): Clinical and virological aspects. J. Infect. 2022, 85, 412–417. [Google Scholar] [CrossRef]
- Girometti, N.; Byrne, R.; Bracchi, M.; Heskin, J.; McOwan, A.; Tittle, V.; Gedel, K.; Scott, C.; Patel, S.; Gohil, J.; et al. Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, UK: An observational analysis. Lancet Infect. Dis. 2022, 22, 1321–1328. [Google Scholar] [CrossRef]
- Tarín-Vicente, E.J.; Alemany, A.; Agud-Dios, M.; Ubals, M.; Suñer, C.; Antón, A.; Arando, M.; Arroyo-Andrés, J.; Calderón-Lozano, L.; Casañ, C.; et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: A prospective observational cohort study. Lancet 2022, 10353, 661–669. [Google Scholar] [CrossRef]
- Català, A.; Clavo Escribano, P.; Riera, J.; Martín-Ezquerra, G.; Fernandez-Gonzalez, P.; Revelles Peñas, L.; Simon-Gozalbo, A.; Rodríguez-Cuadrado, F.J.; Castells, V.G.; de la Torre Gomar, F.J.; et al. Monkeypox outbreak in Spain: Clinical and epidemiological findings in a prospective cross-sectional study of 185 cases. Br. J. Dermatol. 2022. [Google Scholar] [CrossRef]
- Patel, A.; Bilinska, J.; Tam, J.C.H.; Da Silva Fontoura, D.; Mason, C.Y.; Daunt, A. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: Descriptive case series. BMJ 2022, 378, e072410. [Google Scholar] [CrossRef] [PubMed]
- Iñigo Martínez, J.; Gil Montalbán, E.; Jiménez Bueno, S.; Martín Martínez, F.; Nieto Juliá, A.; Sánchez Díaz, J.; Marín, N.G.; Deorador, E.C.; Forte, A.N.; García, M.A.; et al. Monkeypox outbreak predominantly affecting men who have sex with men, Madrid, Spain, 26 April to 16 June 2022. Euro Surveill. 2022, 27, 2200471. [Google Scholar] [CrossRef] [PubMed]
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Monkeypox Virus Infection in Humans across 16 Countries-April-June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Maronese, C.A.; Beretta, A.; Avallone, G.; Boggio, F.L.; Marletta, D.A.; Murgia, G.; Cusini, M.; Gori, A.; Carrera, G.C.; Di Benedetto, A.; et al. Clinical, dermoscopic and histopathological findings in localized human monkeypox: A case from northern Italy. Br. J. Dermatol. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.sanidad.gob.es/en/profesionales/saludPublica/ccayes/alertasActual/alertaMonkeypox/docs/Informe_de_situacion_MPX_20220816.pdf (accessed on 19 August 2022).
- Ortiz-Martínez, Y.; Zambrano-Sánchez, G.; Rodríguez-Morales, A.J. Monkeypox and HIV/AIDS: When the outbreak faces the epidemic. Int. J. STD AIDS 2022, 33, 949–950. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, J.B.; Borio, L.L.; Gostin, L.O. The WHO Declaration of Monkeypox as a Global Public Health Emergency. JAMA 2022, 328, 615–617. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON396 (accessed on 10 August 2022).
- Miura, F.; van Ewijk, C.E.; Backer, J.A.; Xiridou, M.; Franz, E.; Op de Coul, E.; Brandwagt, D.; van Cleef, B.; van Rijckevorsel, G.; Swaan, C.; et al. Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Euro Surveill. 2022, 27, 2200448. [Google Scholar] [CrossRef]
- Heskin, J.; Belfield, A.; Milne, C.; Brown, N.; Walters, Y.; Scott, C.; Bracchi, M.; Moore, L.S.P.; Mughal, N.; Rampling, T.; et al. Transmission of monkeypox virus through sexual contact-A novel route of infection. J. Infect. 2022, 85, 334–363. [Google Scholar] [CrossRef]
- Peiró-Mestres, A.; Fuertes, I.; Camprubí-Ferrer, D.; Marcos, M.Á.; Vilella, A.; Navarro, M.; Rodriguez-Elena, L.; Riera, J.; Català, A.; Martínez, M.J.; et al. Frequent detection of monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022. Euro Surveill. 2022, 27, 2200503. [Google Scholar] [CrossRef]
- Quattri, E.; Avallone, G.; Maronese, C.A.; Cusini, M.; Carrera, C.G.; Marzano, A.V. Unilesional monkeypox: A report of two cases from Italy. Travel Med. Infect. Dis. 2022, 49, 102424. [Google Scholar] [CrossRef]
- Luna, N.; Ramírez, A.L.; Muñoz, M.; Ballesteros, N.; Patiño, L.H.; Castañeda, S.A.; Bonilla-Aldan, D.K.; Paniz, A.; Juan, M.; Ramírez, D.; et al. Phylogenomic analysis of the monkeypox virus (MPXV) 2022 outbreak: Emergence of a novel viral lineage? Travel Med. Infect. Dis. 2022, 49, 102402. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, D.; McClung, R.P.; Mena, L.; Mermin, J.; Centers for Disease Control and Prevention’s Monkeypox Response Team. Monkeypox: Avoiding the Mistakes of Past Infectious Disease Epidemics. Ann. Intern. Med. 2022, 175, 1177–1178. [Google Scholar] [CrossRef] [PubMed]
- Karbalaei, M.; Keikha, M. Human monkeypox coinfections; lessons from available cases. Int. J. Surg. 2022, 104, 106734. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.M.; Liu, L.; Davidson, W.B.; Radford, K.W.; Wilkins, K.; Monroe, B.; Metcalfe, M.G.; Likafi, T.; Lushima, R.S.; Kabamba, J.; et al. A Tale of Two Viruses: Coinfections of Monkeypox and Varicella Zoster Virus in the Democratic Republic of Congo. Am. J. Trop. Med. Hyg. 2020, 104, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Bížová, B.; Veselý, D.; Trojánek, M.; Rob, F. Coinfection of syphilis and monkeypox in HIV positive man in Prague, Czech Republic. Travel Med. Infect. Dis. 2022, 49, 102368. [Google Scholar] [CrossRef]
- Davido, B.; D’Anglejan, E.; Jourdan, J.; Robinault, A.; Davido, G. Monkeypox 2022 outbreak: Cases with exclusive genital lesions. J. Travel Med. 2022, 6, taac077. [Google Scholar] [CrossRef]
- Ramoni, S.; Maronese, C.A.; Morini, N.; Avallone, G.; Quattri, E.; Carrera, C.G.; Boggio, F.L.; Marzano, A.V. Syphilis and monkeypox co-infection: Coincidence, synergy or asymptomatic carriage? Travel Med. Infect. Dis. 2022, in press. [Google Scholar] [CrossRef]
- de Sousa, D.; Patrocínio, J.; Frade, J.; Correia, C.; Borges-Costa, J.; Filipe, P. Human monkeypox coinfection with acute HIV: An exuberant presentation. Int. J. STD AIDS 2022, 33, 936. [Google Scholar] [CrossRef]
- Ogoina, D. Sexual behaviours and clinical course of human monkeypox in Spain. Lancet 2022, 10353, 636–637. [Google Scholar] [CrossRef]



| Total (n = 42) | HIV (n = 27) | Non HIV (n = 15) | p | |
|---|---|---|---|---|
| Age (median; IQR) | 40; 16 (35–51) | 45; 16 (36–52) | 37: 18 (32–50) | NS |
| Origin (n; %) | NS | |||
| Spanish | 18 (43%) | 10/18 | 8/18 | |
| Foreign | 24 (57%) | 17/24 | 7/24 | |
| Europe | 14 | 9 | 5 | |
| Germany | 3 | 3 | 0 | |
| France | 1 | 0 | 1 | |
| United Kingdom | 1 | 0 | 1 | |
| Italy | 3 | 3 | 0 | |
| Poland | 3 | 1 | 2 | |
| Portugal | 1 | 1 | 0 | |
| Russia | 1 | 0 | 1 | |
| Switzerland | 1 | 1 | 0 | |
| Latin America | 9 | 8 | 1 | |
| Argentina | 1 | 1 | 0 | |
| Brazil | 1 | 1 | 0 | |
| Colombia | 1 | 1 | 0 | |
| Cuba | 3 | 3 | 0 | |
| Honduras | 1 | 1 | 0 | |
| Peru | 1 | 1 | 0 | |
| Dominican Republic | 1 | 0 | 1 | |
| Africa | 1 | 0 | 1 | |
| Morocco | 1 | 0 | 1 | |
| Fever (n; %) | 15 (36%) | 8/15 | 7/15 | NS |
| Lymphadenopathies (n; %) | 17 (40%) | 10/17 | 7/17 | NS |
| Cervical | 7 (17%) | 4/7 | 3/7 | |
| Inguinal | 10 (24%) | 6/7 | 4/7 | |
| Skin and mucosal lessions | 42 | |||
| Head and neck (n; %) | 25 (60%) | 17/25 | 8/25 | 0.01 |
| Perioral | 15 | 12/15 | 3/15 | |
| Other | 10 | 3/10 | 7/10 | |
| Trunk (n; %) | 20 (48%) | 13/20 | 7/25 | NS |
| Abdomen/Buttocks (n; %) | 19 (45%) | 12/19 | 7/19 | NS |
| Genital lesions (n; %) | 22 (52%) | 15/21 | 7/21 | NS |
| Perianal (n; %) | 6 (14%) | 3/6 | 3/6 | NS |
| Limbs (n; %) | 30 (71%) | 17/29 | 13/19 | NS |
| Arms | 14 | 7/14 | 7/14 | |
| Legs | 3 | 3/3 | 0/3 | |
| Both | 13 | 7/13 | 6/13 | |
| Pharyngitis (n; %) | 7 (17%) | 7/7 | 0/7 | 0.03 |
| Proctitis (n; %) | 5 (12%) | 3/5 | 2/5 | NS |
| Penis and or scrotal edema (n; %) | 5 (12%) | 3/5 | 2/5 | NS |
| Local complications (n; %) | 2 (5%) | 1/2 Severe dysphagia | 1/2 Urinary retention | NS |
| Other infectious diseases (n; %) | 11/24 * (50%) | 10/11 | 1/11 | 0.03 |
| Chlamydia trachomatis | 1 | 0 | ||
| Haemophilus parainfluenzae | 1 | 0 | ||
| Herpesvirus type 2 | 1 | 0 | ||
| MS Staphylococcus aureus | 1 | 0 | ||
| Mycoplasma genitalium | 2 | 0 | ||
| Mycoplasma hominis | 1 | 1 | ||
| Pantoea septica | 1 | 0 | ||
| Streptococcus dysgalactiae (1) | 1 | 0 | ||
| Ureaplasma urealyticum (4) | 4 | 0 |
| Present Case Series | Orviz (8) | Girometti (9) | Tarín (10) | Catalá (11) | Patel (12) | Iñigo (13) | Thornhill (14) | p * | |
|---|---|---|---|---|---|---|---|---|---|
| Country | Spain (Canary Islands) | Spain (Madrid) | United Kingdom (London) | Spain (Multicentric) | Spain (Multicentric) | United Kingdom (London) | Spain (Madrid) | International (Multicentric) | - |
| Setting | Infectious & Tropical Service | Reference Sexual Transmitted Infections | Reference Sexual Transmitted Infections | Clinical and University Centers | Dermatology Services | High Consequence Infectious Diseases | Public Health Directorate | - | - |
| Patients | 42 | 48 | 54 | 181 | 185 | 197 | 508 | 528 | - |
| Median age (years) | 40 | 35 | 41 | 37 | 38 | 38 | 35 | 38 | NS |
| MSM (%) | 90 | 87 | 100 | 92 | 100 | 99 | 99 | 98 | NS |
| Fever (%) | 36 | 52 | 57 | 72 | 54 | 62 | 64 | 62 | <0.01 |
| Lymphadenopathies (%) | 40 | 39 | 55 | 85 | 56 | 58 | 61 | 56 | <0.01 |
| Skin and mucosal lessions | 100 | - | 100 | 100 | 100 | 100 | 98 | 95 | NS |
| Genital lesions (%) | 52 | 54 | 61 | 55 | 53 | 56 | 72 | 73 | NS |
| Perianal (%) | 14 | 35 | 44 | 36 | 34 | 41 | - | <0.01 | |
| Pharyngitis (%) | 17 | - | 7 | 10 | - | 4.6 | 28 | 21 | <0.01 |
| Proctitis (%) | 12 | 27 | - | 25 | 22 | 17 | 16 | 14 | NS |
| Penile and/or scrotal edema | 12 | - | - | 8 | - | 15 | - | - | NS |
| Local complications (%) ** | 5 | 2 | 9 | 2 | 2 | 10 | 4 | 13 | <0.01 |
| HIV (%) | 64 | 39 | 13 | 40 | 42 | 35.9 | 44 | 41 | <0.01 |
| Other infectious diseases (%) | 50 *** | 25 | - | 17 | 76 | 32 | - | 29 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betancort-Plata, C.; Lopez-Delgado, L.; Jaén-Sanchez, N.; Tosco-Nuñez, T.; Suarez-Hormiga, L.; Lavilla-Salgado, C.; Pisos-Álamo, E.; Hernández-Betancor, A.; Hernández-Cabrera, M.; Carranza-Rodríguez, C.; et al. Monkeypox and HIV in the Canary Islands: A Different Pattern in a Mobile Population. Trop. Med. Infect. Dis. 2022, 7, 318. https://doi.org/10.3390/tropicalmed7100318
Betancort-Plata C, Lopez-Delgado L, Jaén-Sanchez N, Tosco-Nuñez T, Suarez-Hormiga L, Lavilla-Salgado C, Pisos-Álamo E, Hernández-Betancor A, Hernández-Cabrera M, Carranza-Rodríguez C, et al. Monkeypox and HIV in the Canary Islands: A Different Pattern in a Mobile Population. Tropical Medicine and Infectious Disease. 2022; 7(10):318. https://doi.org/10.3390/tropicalmed7100318
Chicago/Turabian StyleBetancort-Plata, Christian, Laura Lopez-Delgado, Nieves Jaén-Sanchez, Tomás Tosco-Nuñez, Laura Suarez-Hormiga, Carmen Lavilla-Salgado, Elena Pisos-Álamo, Araceli Hernández-Betancor, Michele Hernández-Cabrera, Cristina Carranza-Rodríguez, and et al. 2022. "Monkeypox and HIV in the Canary Islands: A Different Pattern in a Mobile Population" Tropical Medicine and Infectious Disease 7, no. 10: 318. https://doi.org/10.3390/tropicalmed7100318
APA StyleBetancort-Plata, C., Lopez-Delgado, L., Jaén-Sanchez, N., Tosco-Nuñez, T., Suarez-Hormiga, L., Lavilla-Salgado, C., Pisos-Álamo, E., Hernández-Betancor, A., Hernández-Cabrera, M., Carranza-Rodríguez, C., Briega-Molina, M., & Pérez-Arellano, J.-L. (2022). Monkeypox and HIV in the Canary Islands: A Different Pattern in a Mobile Population. Tropical Medicine and Infectious Disease, 7(10), 318. https://doi.org/10.3390/tropicalmed7100318






