Comparison between Colorimetric In Situ Hybridization, Histopathology, and Immunohistochemistry for the Diagnosis of New World Cutaneous Leishmaniasis in Human Skin Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Parasite Culture and Isoenzyme Characterization
2.3. Histopathology
2.4. Immunohistochemistry
2.5. Colorimetric In Situ Hybridization
2.6. Slide Reading
2.7. Statistical Analysis
2.8. Ethics Statement
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lainson, R. Espécies neotropicais de Leishmania: Uma breve revisão histórica sobre sua descoberta, ecologia e taxonomia. Rev. Panamazonica Saude. 2010, 1, 13–32. [Google Scholar] [CrossRef]
- Brazil Ministério da Saúde. Manual de Vigilância da Leishmaniose Tegumentar Americana, 2nd ed.; Ministério da Saúde: Brasília, Brazil, 2017.
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Pan American Health Organization. Leishmaniasis. Available online: https://www.paho.org/en/topics/leishmaniasis (accessed on 26 July 2022).
- Pena, H.P.; Belo, V.S.; Xavier-Junior, J.C.C.; Teixeira-Neto, R.G.; Melo, S.N.; Pereira, D.A.; Fontes, I.C.; Santos, I.M.; Lopes, V.V.; Tafuri, W.L.; et al. Accuracy of diagnostic tests for American tegumentary leishmaniasis: A systematic literature review with meta-analyses. Trop. Med. Int. Health 2020, 10, 168–1181. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.B.; Porto, C.; Da Motta, J.O.C.; Sampaio, R.N.R. Tratamento da leishmaniose tegumentar americana. An. Bras. Dermatol. 2007, 82, 111–124. [Google Scholar] [CrossRef] [Green Version]
- Da-Cruz, A.M.; Pirmez, C. Leishmaniose tegumentar americana. In Dinâmica das Doenças Infecciosas e Parasitárias, 2nd ed.; Coura, J.R., Ed.; Guanabara Koogan: Rio de Janeiro, Brazil, 2013; Volume 1, pp. 746–760. [Google Scholar]
- Pinheiro, A.B.S.; Kurizky, P.S.; Ferreira, M.F.; Mota, M.A.S.; Ribeiro, J.S.; Filho, E.Z.O.; Souza, C.A.; Barroso, D.H.; Sampaio, R.N.R.; Gomes, C.M. The accuracy of the Montenegro skin test for leishmaniasis in PCR-negative patients. Rev. Soc. Bras. Med. Trop. 2020, 53, e20190433. [Google Scholar] [CrossRef]
- Veasey, J.V.; Zampieri, R.A.; Lellis, R.F.; Freitas, T.H.P.; Winter, L.M.F. Identification of Leishmania species by high-resolution DNA dissociation in cases of American cutaneous leishmaniasis. An. Bras. Dermatol. 2020, 95, 459–468. [Google Scholar] [CrossRef]
- Quintella, L.P.; Cuzzi, T.; Madeira, M.D.F.; Okamoto, T.; Schubach, A.D.O. Immunoperoxidade technique using an anti-Leishmania (L.) chagasi hyperimmune serum in the diagnosis of culture-confirmed American tegumentary leishmaniasis. Rev. Inst. Med. Trop. Sao Paulo 2009, 51, 83–86. [Google Scholar] [CrossRef] [Green Version]
- Dinhopl, N.; Mostegl, M.M.; Richter, B.; Nedorost, N.; Maderner, A.; Fragner, K.; Weissenböck, H. In situ hybridization for the detection of Leishmania species in paraffin wax-embedded canine tissues using a digoxigenin-labelled oligonucleotide probe. Vet. Rec. 2011, 169, 525. [Google Scholar] [CrossRef] [Green Version]
- Frickmann, H.; Alnamar, Y.; Essig, A.; Clos, J.; Racz, P.; Barth, T.F.; Hagen, R.M.; Fischer, M.; Poppert, S. Rapid identification of Leishmania spp. in formalin-fixed, paraffin-embedded tissue samples by fluorescence in situ hybridization. Trop. Med. Int. Health 2012, 17, 1117–1126. [Google Scholar] [CrossRef]
- Menezes, R.C.; Figueiredo, F.B.; Wise, A.G.; Madeira, M.F.; Oliveira, R.V.; Schubach, T.M.; Kiupel, M.; Langohr, I.M. Sensitivity and specificity of in situ hybridization for the diagnosis of cutaneous infection by Leishmania infantum in dogs. J. Clin. Microbiol. 2013, 51, 206–211. [Google Scholar] [CrossRef]
- Segalés, J.; Ramos-Vara, J.A.; Duran, C.O.; Porter, A.; Domingo, M. Diagnosing infectious diseases using in situ hybridization. Swine Health Prod. 1999, 7, 125–128. [Google Scholar]
- Frickmann, H.; Zautner, A.E.; Moter, A.; Kikhney, J.; Hagen, R.M.; Stender, H.; Poppert, S. Fluorescence in situ hybridization (FISH) in the microbiological diagnostic routine laboratory: A review. Crit. Rev. Microbiol. 2017, 43, 263–293. [Google Scholar] [CrossRef] [PubMed]
- Cupolillo, E.; Grimaldi, G., Jr.; Momen, H. A general classification of New World Leishmania using numeral zymotaxomomy. Am. J. Trop. Med. Hyg. 1994, 50, 296–311. [Google Scholar] [CrossRef] [PubMed]
- Carson, F.L.; Hladick, C. Histotechnology: A Self-Instructional Text, 3rd ed.; ASCP Press: Chicago, IL, USA, 2009. [Google Scholar]
- Oliveira, V.C.; Junior, A.A.V.M.; Ferreira, L.C.; Calvet, T.M.Q.; Santos, S.A.; Figueiredo, F.B.; Campos, M.P.; Rodrigues, F.C.C.; Oliveira, R.V.C.O.; Lemos, E.R.S.; et al. Frequency of co-seropositivities for certain pathogens and their relationship with clinical and histopathological changes and parasite load in dogs infected with Leishmania infantum. PLoS ONE 2021, 16, e0247560. [Google Scholar] [CrossRef]
- Boechat, V.C.; Mendes Junior, A.A.V.; Madeira, M.F.; Ferreira, L.C.; Figueiredo, F.B.; Rodrigues, F.C.C.; Oliveira, V.C.; Oliveira, R.V.C.; Menezes, R.C. Occurrence of Leishmania infantum and associated histological alterations in the genital tract and mammary glands of naturally infected dogs. Parasitol Res. 2016, 115, 2371–2379. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 26 July 2022).
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [Green Version]
- Salinas, G.; Valderrama, L.; Palma, G.; Montes, G.; Saravia, N.G. Deteccion de amastigotas en leishmaniasis cutanea y mucocutanea por el metodo de imunoperoxidasa, usando anticuerpo policlonal: Sensibilidad y especificidad comparadas com metodos convencionales de diagnostico. Mem. Inst. Oswaldo Cruz. 1989, 84, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Sotto, M.N.; Yamashiro-Kanashiro, E.H.; Da Matta, V.L.; de Brito, T. Cutaneous leishmaniasis of the New World: Diagnostic immunopathology and antigen pathways in skin and mucosa. Acta Trop. 1989, 46, 121–130. [Google Scholar] [CrossRef]
- Kenner, J.R.; Aronson, N.E.; Bratthauer, G.L.; Turnicky, R.P.; Jackson, J.E.; Tang, D.B.; Sau, P. Immunohistochemistry to identify Leishmania parasites in fixed tissues. J. Cutan. Pathol. 1999, 26, 130–136. [Google Scholar] [CrossRef]
- Schubach, A.; Cuzzi-Maya, T.; Oliveira, A.V.; Sartori, A. Leishmanial antigens in the diagnosis of active lesions and ancient scars of American tegumentary leishmaniasis patients. Mem. Inst. Oswaldo Cruz. 2001, 96, 987–996. [Google Scholar] [CrossRef]
- Amato, V.S.; Duarte, M.I.; Nicodemo, A.C.; de Carvalho, L.V.; Pagliari, C.; da Matta, V.L.; de Oliveira, L.S.; de Castro, S.M.; Uip, D.E.; Amato, J.G.; et al. An evaluation of clinical, serologic, anatomopathologic and immunohistochemical findings for fifteen patients with mucosal leishmaniasis bebore and after treatment. Rev. Inst. Med. Trop. São Paulo 1998, 40, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.A.; Soares, R.P.; Almeida, G.G.; Souza, C.C.; Melo, M.N.; Pinto, S.A.; Quixabeira, V.B.; Pereira, L.I.; Dorta, M.L.; Ribeiro-Dias, F.; et al. Effectiveness of an immunohistochemical protocol for Leishmania detection in different clinical forms of American tegumentar leishmaniasis. Parasitol. Inter. 2017, 66, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.F.; Alves, C.F.; Figueiredo, M.M.; Souza, C.C.; Machado-Coelho, G.L.; Melo, M.N.; Tafuri, W.L.; Raso, P.; Soares, R.P.; Tafuri, W.L. American tegumentary leishmaniasis: Effectiveness of an immunohistochemical protocol for the detection of Leishmania in skin. PLoS ONE 2013, 8, e63343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, I.B.; Tortelly, R.; Quintella, L.P.; Quintella, L.P.; Madeira, M.F.; Miranda, L.H.M.; Figueiredo, F.B.; Oliveira, R.V.C.O.; Schubach, T.M.P. Higher sensitivity of immunohistochemistry for bona fide diagnosis of dog Leishmania (Viannia) braziliensis—Driven American tegumentary leishmaniasis: Description of an optimized immunohistochemistry method. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Vega-López, F. Diagnosis of cutaneous leishmaniasis. Curr. Opin. Infect. Dis. 2003, 16, 97–101. [Google Scholar] [CrossRef]
- Gomes, A.H.; Armelin, I.M.; Menon, S.Z.; Pereira-Chioccola, V.L. Leishmania (V.) braziliensis: Detection by PCR in biopsies from patients with cutaneous leishmaniasis. Exp. Parasitol. 2008, 119, 319–324. [Google Scholar] [CrossRef]
- Ameen, M. Cutaneous leishmaniasis: Advances in disease pathogenesis, diagnostics and therapeutics. Clin. Exp. Dermatol. 2010, 35, 699–705. [Google Scholar] [CrossRef]
- Andrade, R.V.; Massone, C.; Lucena, M.N.B.; Talhari, A.C.; Talhari, S.; Guerra, J.A.O.; Ferreira, L.C.L. The use of polymerase chain reaction to confirm diagnosis in skin biopsies consistent with American tegumentary leishmaniasis at histopathology: A study of 90 cases. An. Bras. Dermatol. 2011, 86, 892–896. [Google Scholar] [CrossRef] [Green Version]
- Satow, M.M.; Yamashiro-Kanashiro, E.H.; Rocha, M.C.; Oyafuso, L.K.; Soler, R.C.; Cotrim, P.C.; Lindodo, J.A.L. Applicability of kDNA PCR for routine diagnosis of American tegumentary leishmaniasis in a tertiary reference hospital. Rev. Inst. Med. Trop. Sao Paulo 2013, 55, 393–399. [Google Scholar] [CrossRef] [Green Version]
- Furtado, M.C.; Menezes, R.C.; Kiupel, M.; Madeira, M.F.; Oliveira, R.V.; Langohr, I.M.; Figueiredo, F.B. Comparative study of in situ hybridization, immunohistochemistry and parasite culture for the diagnosis of canine leishmaniosis. Parasit. Vectors. 2015, 8, 620. [Google Scholar] [CrossRef] [Green Version]
- Kaluarachchi, T.J.; Wickremasinghe, R.; Weerasekera, M.; Yasawardene, S.; McBain, A.J.; Yapa, B.; De Silva, H.; Menike, C.; Jayathilake, S.; Munasinghe, A.; et al. Diagnosing human cutaneous leishmaniasis using fluorescence in situ hybridization. Pathog. Glob. Health 2021, 115, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Maes, R.K.; Langohr, I.M.; Wise, A.G.; Smedley, R.C.; Thaiwong, T.; Kiupel, M. Beyond H&E: Integration of nucleic acid-based analyses into diagnostic pathology. Vet. Pathol. 2014, 51, 238–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guyard, A.; Boyez, A.; Pujals, A.; Robe, C.; Tran Van Nhieu, J.; Allory, Y.; Moroch, J.; Georges, O.; Fournet, J.C.; Zafrani, E.S.; et al. DNA degrades during storage in formalin-fixed and paraffin-embedded tissue blocks. Virchows Arch. 2017, 471, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Vara, J.A. Technical aspects of immunohistochemistry. Vet. Pathol. 2005, 42, 405–426. [Google Scholar] [CrossRef]
- Vasconcelos, T.; Machado, L.; Abrantes, T.; Menezes, R.; Madeira, M.; Ferreira, L.; Figueiredo, F. Avaliação da confiabilidade entre dois observadores em exame citopatológico e imunocitoquímico de aspirado de medula óssea no diagnóstico da leishmaniose visceral canina. Arq. Bras. Med. Vet. Zootec. 2016, 69, 821–824. [Google Scholar] [CrossRef]
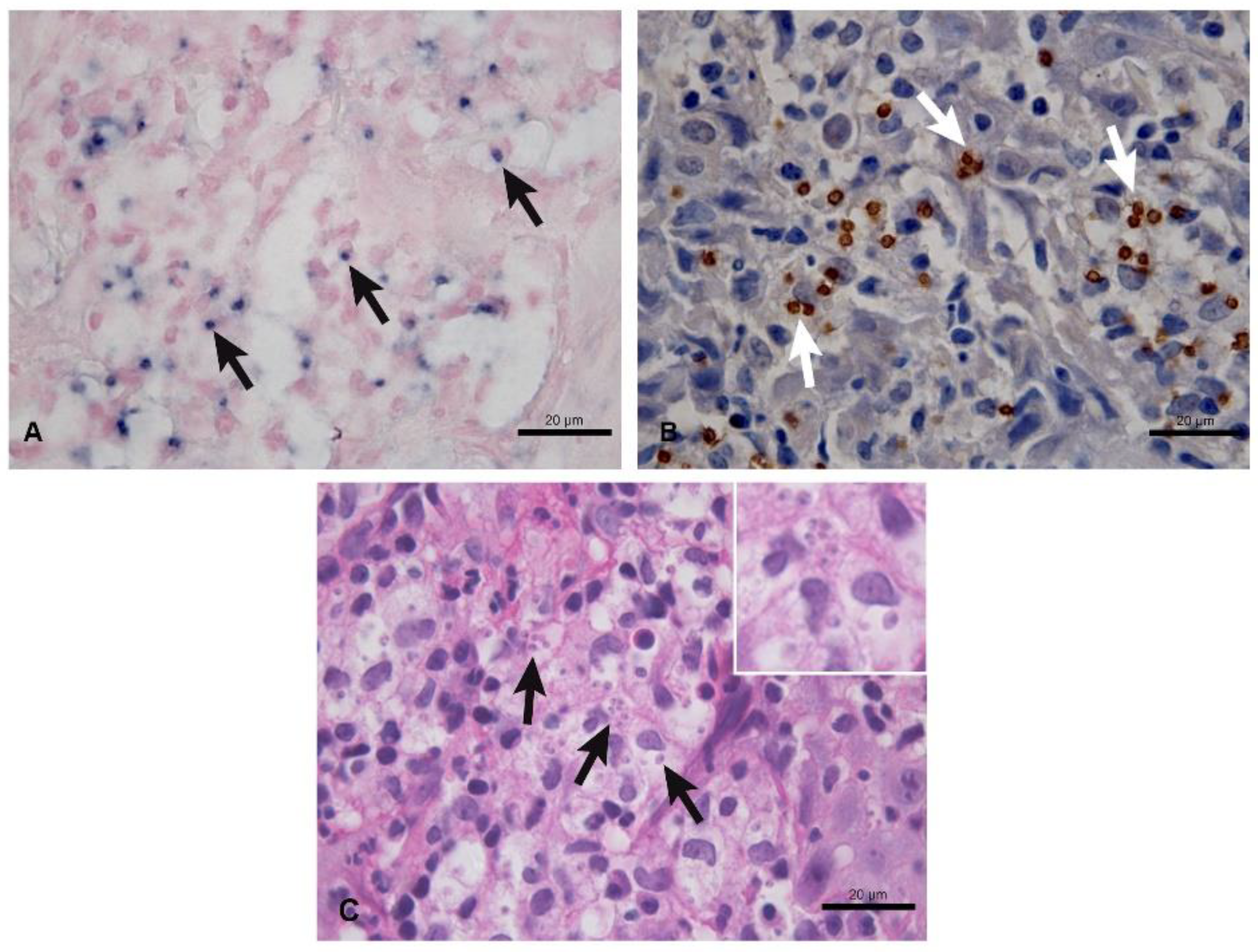
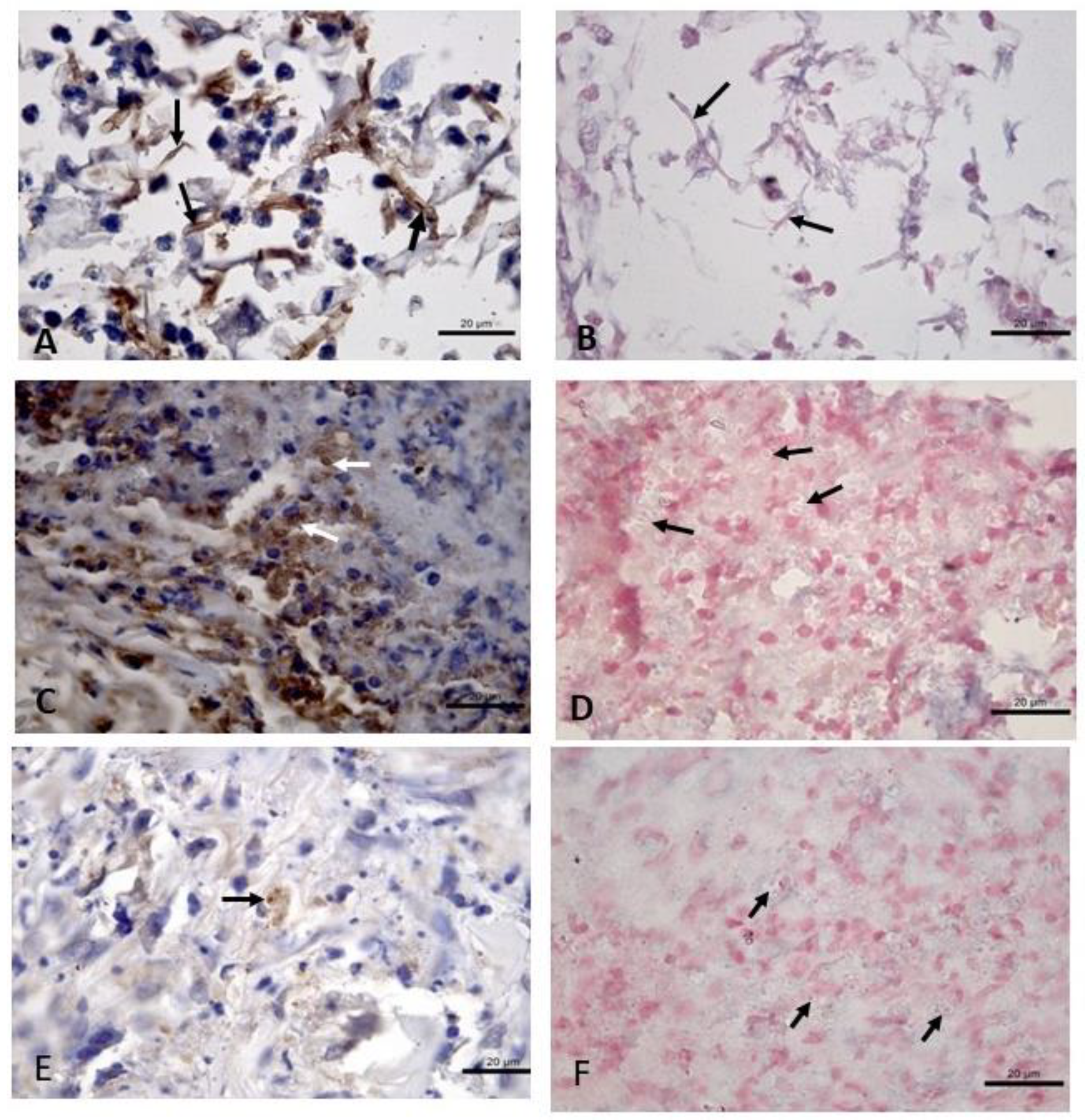
| Technique | Sensitivity (CI 95%) | Positive Samples | Negative Samples |
|---|---|---|---|
| IHC | 66.0% (51.2–78.8%) | 33 | 17 |
| CISH | 54.0% (39.3–68.2%) | 27 | 23 |
| HP | 50.0% (35.5–64.5%) | 25 | 25 |
| Time of Storage (Years) | N | Sensitivity (CI 95%) | ||
|---|---|---|---|---|
| IHC | CISH | HP | ||
| 14 to 17 | 20 | 70.0% (45.7–88.2%) | 40.0% (19.1–64.0%) | 50.0% (27.2–72.8%) |
| 12 to 13 | 30 | 63.3 % (43.9–80.0%) | 66.7% (47.2–82.7%) | 50.0% (31.3–68.7%) |
| Technique | IHC | HP | |||
|---|---|---|---|---|---|
| Positive n (%) | Negative n (%) | Positive n (%) | Negative n (%) | ||
| CISH | Positive | 24 (48%) | 3 (6%) | 19 (38%) | 8 (16%) |
| Negative | 9 (18%) | 14 (28%) | 6 (12%) | 17 (34%) | |
| HP | Positive | 23 (46%) | 10 (20%) | 25 (50%) | 0 |
| Negative | 2 (4%) | 15 (30%) | 0 | 25 (50%) | |
| Comparison of Techniques * | Cohen Kappa Index | Agreement a | Total Agreement |
|---|---|---|---|
| CISH vs. IHC | 0.51 (CI 0.27–0.74) | Moderate | 76% |
| CISH vs. HP | 0.44 (CI 0.19–0.69) | Moderate | 72% |
| IHC vs. HP | 0.52 (CI 0.3–0.74) | Moderate | 76% |
| Techniques * | Time of Storage of Paraffin Blocks | |||||
|---|---|---|---|---|---|---|
| 14 to 17 Years | 12 to 13 Years | |||||
| Cohen Kappa | Agreement a | Total Agreement | Cohen Kappa | Agreement a | Total Agreement | |
| CISH vs. IHC | 0.37 (CI 0.08–0.67) | fair | 65.0% | 0.63 (CI 0.34–0.92) | substantial | 83.3% |
| CISH vs. HP | 0.50 (CI 0.14–0.86) | moderate | 75.0% | 0.40 (CI 0.09–0.71) | moderate | 70.0% |
| IHC vs. HP | 0.60 (CI 0.28–0.92) | substantial | 80.0% | 0.47 (CI 0.16–0.77) | moderate | 73.3% |
| Technique | Observer 1 vs. 2 | Observer 1 vs. 3 | Observer 2 vs. 3 | |||
|---|---|---|---|---|---|---|
| Cohen Kappa | Agreement a | Cohen Kappa | Agreement a | Cohen Kappa | Agreement a | |
| CISH | 0.80 (IC 0.64–0.97) | almost perfect | 0.68 (CI 0.49–0.88) | substantial | 0.64 (CI 0.42–0.85) | substantial |
| IHC | 0.46 (IC 0.21–0.72) | moderate | 0.58 (CI 0.35–0.81) | moderate | 0.75 (CI 0.56–0.93) | substantial |
| HP | 0.76 (IC 0.58–0.94) | substantial | 0.72 (CI 0.53–0.91) | substantial | 0.88 (CI 0.75–1.00) | almost perfect |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, L.C.; Quintella, L.P.; Schubach, A.d.O.; Miranda, L.d.F.C.; Madeira, M.d.F.; Pimentel, M.I.F.; Vasconcellos, É.d.C.F.e.; Lyra, M.R.; Oliveira, R.d.V.C.d.; Menezes, R.C. Comparison between Colorimetric In Situ Hybridization, Histopathology, and Immunohistochemistry for the Diagnosis of New World Cutaneous Leishmaniasis in Human Skin Samples. Trop. Med. Infect. Dis. 2022, 7, 344. https://doi.org/10.3390/tropicalmed7110344
Ferreira LC, Quintella LP, Schubach AdO, Miranda LdFC, Madeira MdF, Pimentel MIF, Vasconcellos ÉdCFe, Lyra MR, Oliveira RdVCd, Menezes RC. Comparison between Colorimetric In Situ Hybridization, Histopathology, and Immunohistochemistry for the Diagnosis of New World Cutaneous Leishmaniasis in Human Skin Samples. Tropical Medicine and Infectious Disease. 2022; 7(11):344. https://doi.org/10.3390/tropicalmed7110344
Chicago/Turabian StyleFerreira, Luiz Cláudio, Leonardo Pereira Quintella, Armando de Oliveira Schubach, Luciana de Freitas Campos Miranda, Maria de Fátima Madeira, Maria Inês Fernandes Pimentel, Érica de Camargo Ferreira e Vasconcellos, Marcelo Rosandiski Lyra, Raquel de Vasconcellos Carvalhaes de Oliveira, and Rodrigo Caldas Menezes. 2022. "Comparison between Colorimetric In Situ Hybridization, Histopathology, and Immunohistochemistry for the Diagnosis of New World Cutaneous Leishmaniasis in Human Skin Samples" Tropical Medicine and Infectious Disease 7, no. 11: 344. https://doi.org/10.3390/tropicalmed7110344
APA StyleFerreira, L. C., Quintella, L. P., Schubach, A. d. O., Miranda, L. d. F. C., Madeira, M. d. F., Pimentel, M. I. F., Vasconcellos, É. d. C. F. e., Lyra, M. R., Oliveira, R. d. V. C. d., & Menezes, R. C. (2022). Comparison between Colorimetric In Situ Hybridization, Histopathology, and Immunohistochemistry for the Diagnosis of New World Cutaneous Leishmaniasis in Human Skin Samples. Tropical Medicine and Infectious Disease, 7(11), 344. https://doi.org/10.3390/tropicalmed7110344







