Antiviral Treatment against Monkeypox: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources and Search Strategy
2.4. Study Selection
2.5. Outcomes
2.6. Data Collection Process and Data Items
3. Results
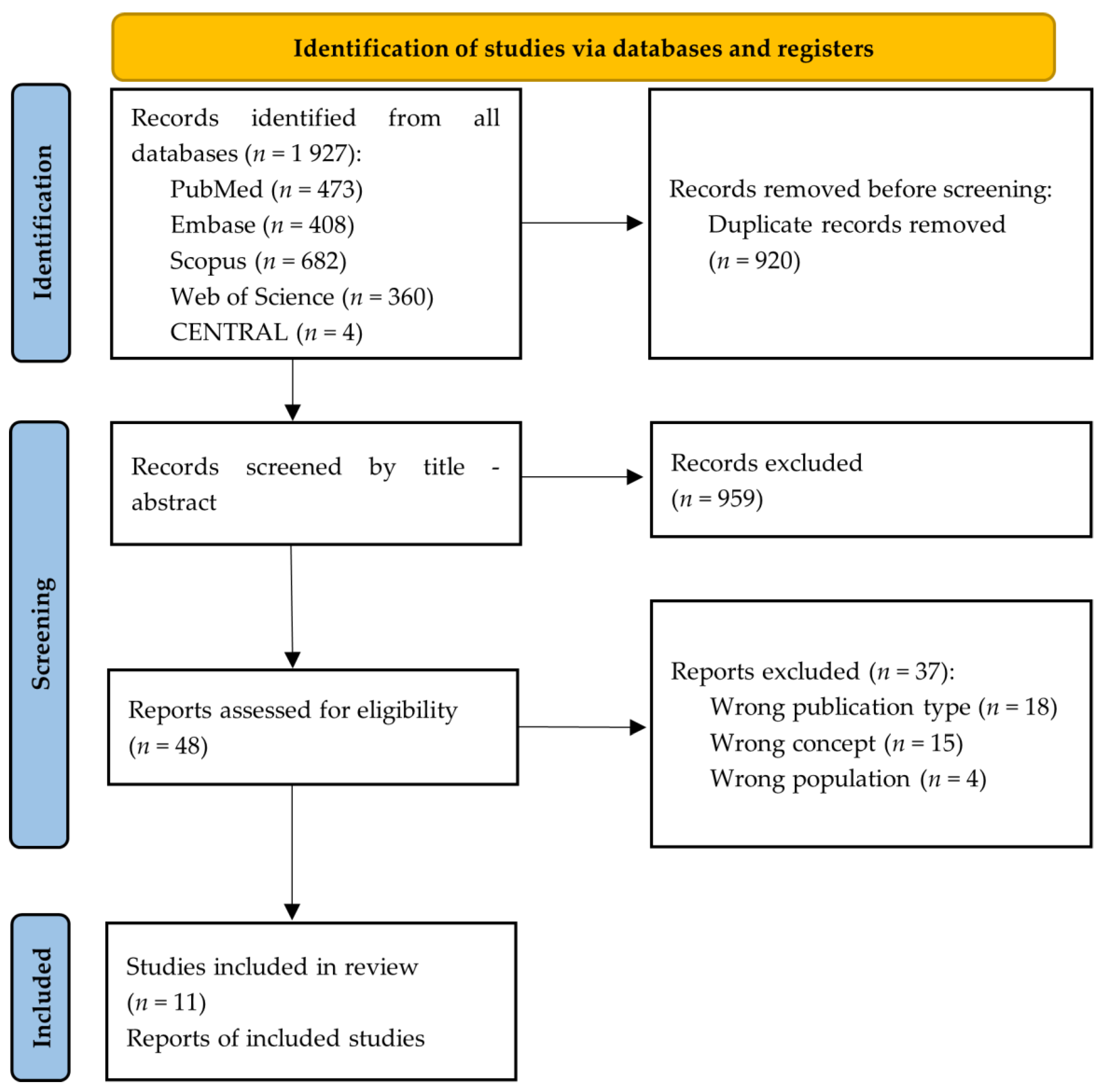
3.1. Study Selection
3.2. Study Characteristics
3.3. Demographical Characteristics and Diagnostic Method for Monkeypox
3.4. Clinical Manifestations, Localization of Skin Lesions, and Treatment
4. Discussion
Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Monkeypox Virus Infection in Humans across 16 Countries—April–June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef] [PubMed]
- 2022 Monkeypox Outbreak Global Map|Monkeypox|Poxvirus|CDC n.d. Available online: https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html (accessed on 9 September 2022).
- Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Is monkeypox another reemerging viral zoonosis with many animal hosts yet to be defined? Vet. Quart. 2022, 42, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Farahat, R.A.; Sah, R.; El-Sakka, A.A.; Benmelouka, A.Y.; Kundu, M.; Labieb, F.; Shaheen, R.S.; Abdelaal, A.; Kundu, M.; Labieb, F.; et al. Human monkeypox disease (MPX). Infez. Med. 2022, 30, 372–391. [Google Scholar] [CrossRef] [PubMed]
- Alakunle, E.; Moens, U.; Nchinda, G.; Okeke, M.I. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses 2020, 12, 1257. [Google Scholar] [CrossRef]
- Martín-Delgado, M.C.; Martín-Sánchez, F.J.; Martínez-Sellés, M.; Molero García, J.M.; Moreno Guillén, S.; Rodríguez-Artalejo, F.; Ruiz-Galiana, J.; Cantón, R.; De Lucas Ramos, P.; García-Botella, A.; et al. Monkeypox in humans: A new outbreak. Rev. Esp. Quimioter. 2022, martin06jul2022. [Google Scholar] [CrossRef]
- Ihekweazu, C.; Yinka-Ogunleye, A.; Lule, S.; Ibrahim, A. Importance of epidemiological research of monkeypox: Is incidence increasing? Expert Rev. Anti-Infect. Ther. 2020, 18, 389–392. [Google Scholar] [CrossRef]
- Lum, F.-M.; Torres-Ruesta, A.; Tay, M.Z.; Lin, R.T.P.; Lye, D.C.; Rénia, L.; Ng, L.F.P. Monkeypox: Disease epidemiology, host immunity and clinical interventions. Nat. Rev. Immunol. 2022, 22, 597–613. [Google Scholar] [CrossRef]
- León-Figueroa, D.A.; Bonilla-Aldana, D.K.; Pachar, M.; Romaní, L.; Saldaña-Cumpa, H.M.; Anchay-Zuloeta, C.; Diaz-Torres, M.; Franco-Paredes, C.; Suárez, J.A.; Ramirez, J.D.; et al. The never-ending global emergence of viral zoonoses after COVID-19? The rising concern of monkeypox in Europe, North America and beyond. Travel. Med. Infect. Dis. 2022, 49, 102362. [Google Scholar] [CrossRef]
- Rizk, J.G.; Lippi, G.; Henry, B.M.; Forthal, D.N.; Rizk, Y. Prevention and Treatment of Monkeypox. Drugs 2022, 82, 957–963. [Google Scholar] [CrossRef]
- Sookaromdee, P.; Wiwanitkit, V. Treatments for Monkeypox. Actas Dermosifiliogr. 2022. [Google Scholar] [CrossRef]
- León-Figueroa, D.A.; Barboza, J.J.; Garcia-Vasquez, E.A.; Bonilla-Aldana, D.K.; Diaz-Torres, M.; Saldaña-Cumpa, H.M.; Diaz-Murillo, M.T.; Cruz, O.C.-S.; Rodriguez-Morales, A.J. Epidemiological Situation of Monkeypox Transmission by Possible Sexual Contact: A Systematic Review. Trop. Med. Infect. Dis. 2022, 7, 267. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, A.; Reda, A.; Lashin, B.I.; Katamesh, B.E.; Brakat, A.M.; Al-Manaseer, B.M.; Kaur, S.; Asija, A.; Patel, N.K.; Basnyat, S.; et al. Preventing the Next Pandemic: Is Live Vaccine Efficacious against Monkeypox, or Is There a Need for Killed Virus and mRNA Vaccines? Vaccines 2022, 10, 1419. [Google Scholar] [CrossRef] [PubMed]
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The changing epidemiology of human monkeypox—A potential threat? A systematic review. PLoS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.D.J.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for conducting systematic scoping reviews. Int. J. Evid. Based Healthc. 2015, 13, 141–146. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Adler, H.; Gould, S.; Hine, P.; Snell, L.B.; Wong, W.; Houlihan, C.F.; Osborne, J.C.; Rampling, T.; Beadsworth, M.B.; Duncan, C.J.; et al. Clinical features and management of human monkeypox: A retrospective observational study in the UK. Lancet Infect. Dis. 2022, 22, 1153–1162. [Google Scholar] [CrossRef]
- Desai, A.N.; Thompson, G.R.; Neumeister, S.M.; Arutyunova, A.M.; Trigg, K.; Cohen, S.H. Compassionate Use of Tecovirimat for the Treatment of Monkeypox Infection. JAMA 2022, 328, 1348. [Google Scholar] [CrossRef]
- Matias, W.R.; Koshy, J.M.; Nagami, E.H.; Kovac, V.; Moeng, L.R.; Shenoy, E.S.; Hooper, D.C.; Madoff, L.C.; Barshak, M.B.; Johnson, A.J.; et al. Tecovirimat for the Treatment of Human Monkeypox: An Initial Series From Massachusetts, United States. Open Forum Infect. Dis. 2022, 9, ofac377. [Google Scholar] [CrossRef]
- Moschese, D.; Giacomelli, A.; Beltrami, M.; Pozza, G.; Mileto, D.; Reato, S.; Zacheo, M.; Corbellino, M.; Rizzardini, G.; Antinori, S. Hospitalisation for monkeypox in Milan, Italy. Travel Med. Infect. Dis. 2022, 49, 102417. [Google Scholar] [CrossRef]
- Rao, A.K.; Schulte, J.; Chen, T.-H.; Hughes, C.M.; Davidson, W.; Neff, J.M.; Markarian, M.; Delea, K.C.; Wada, S.; Liddell, A.; et al. Monkeypox in a Traveler Returning from Nigeria—Dallas, Texas, July 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Mailhe, M.; Beaumont, A.-L.; Thy, M.; le Pluart, D.; Perrineau, S.; Houhou-Fidouh, N.; Deconinck, L.; Bertin, C.; Ferré, V.M.; Cortier, M.; et al. Clinical characteristics of ambulatory and hospitalised patients with monkeypox virus infection: An observational cohort study. Clin. Microbiol. Infect. 2022. [Google Scholar] [CrossRef]
- Minhaj, F.S.; Ogale, Y.P.; Whitehill, F.; Schultz, J.; Foote, M.; Davidson, W.; Hughes, C.M.; Wilkins, K.; Bachmann, L.; Chatelain, R.; et al. Monkeypox Outbreak—Nine States, May 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Girometti, N.; Byrne, R.; Bracchi, M.; Heskin, J.; McOwan, A.; Tittle, V.; Gedela, K.; Scott, C.; Patel, S.; Gohil, J.; et al. Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, UK: An observational analysis. Lancet Infect. Dis. 2022, 22, 1321–1328. [Google Scholar] [CrossRef]
- Tarín-Vicente, E.J.; Alemany, A.; Agud-Dios, M.; Ubals, M.; Suñer, C.; Antón, A.; Arando, M.; Arroyo-Andrés, J.; Calderón-Lozano, L.; Casañ, C.; et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: A prospective observational cohort study. Lancet 2022, 400, 661–669. [Google Scholar] [CrossRef]
- Patel, A.; Bilinska, J.; Tam, J.C.H.; da Silva Fontoura, D.; Mason, C.Y.; Daunt, A.; Snell, L.B.; Murphy, J.; Potter, J.; Tuudah, C.; et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: Descriptive case series. BMJ 2022, 378, e072410. [Google Scholar] [CrossRef]
- Treatment Information for Healthcare Professionals|Monkeypox|Poxvirus|CDC n.d. Available online: https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html (accessed on 13 September 2022).
- Multi-Country Outbreak of Monkeypox—External Situation Report 4, Published 24 August 2022—World|ReliefWeb n.d. Available online: https://reliefweb.int/report/world/multi-country-outbreak-monkeypox-external-situation-report-4-published-24-august-2022 (accessed on 25 August 2022).
- Grosenbach, D.W.; Honeychurch, K.; Rose, E.A.; Chinsangaram, J.; Frimm, A.; Maiti, B.; Lovejoy, C.; Meara, I.; Long, P.; Hruby, D.E. Oral Tecovirimat for the Treatment of Smallpox. N. Engl. J. Med. 2018, 379, 44. [Google Scholar] [CrossRef]
- Siegrist, E.A.; Sassine, J. Antivirals with Activity Against Monkeypox: A Clinically Oriented Review. Clin. Infect. Dis. 2022. [Google Scholar] [CrossRef]
- Li, D.; Liu, Y.; Li, K.; Zhang, L. Targeting F13 from monkeypox virus and variola virus by tecovirimat: Molecular simulation analysis. J. Infect. 2022, 85, e99–e101. [Google Scholar] [CrossRef]
- Lam, H.Y.I.; Guan, J.S.; Mu, Y. In Silico Repurposed Drugs against Monkeypox Virus. Molecules 2022, 27, 5277. [Google Scholar] [CrossRef]
- Jordan, R.; Chinsangaram, J.; Bolken, T.C.; Tyavanagimatt, S.R.; Tien, D.; Jones, K.F.; Frimm, A.; Corrado, M.L.; Pickens, M.; Landis, P.; et al. Safety and pharmacokinetics of the antiorthopoxvirus compound ST-246 following repeat oral dosing in healthy adult subjects. Antimicrob. Agents Chemother. 2010, 54, 2560–2566. [Google Scholar] [CrossRef] [PubMed]
- Chinsangaram, J.; Honeychurch, K.M.; Tyavanagimatt, S.R.; Leeds, J.M.; Bolken, T.C.; Jones, K.F.; Jordan, R.; Marbury, T.; Ruckle, J.; Mee-Lee, D.; et al. Safety and pharmacokinetics of the anti-orthopoxvirus compound ST-246 following a single daily oral dose for 14 days in human volunteers. Antimicrob. Agents Chemother. 2012, 56, 4900–4905. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.K.; Olson, V.A.; Karem, K.L.; Jordan, R.; Hruby, D.E.; Damon, I.K. In vitro efficacy of ST246 against smallpox and monkeypox. Antimicrob. Agents Chemother. 2009, 53, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Meadows, K.P.; Tyring, S.K.; Pavia, A.T.; Rallis, T.M. Resolution of Recalcitrant Molluscum Contagiosum Virus Lesions in Human Immunodeficiency Virus-Infected Patients Treated with Cidofovir. Arch. Dermatol. 1997, 133, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Kurth, A.; Hessler, F.; Kramp, H.; Gokel, M.; Hoffmann, R.; Kuczka, A.; Nitsche, A. Cowpox virus infection in pet rat owners: Not always immediately recognized. Dtsch. Arztebl. Int. 2009, 106, 329–334. [Google Scholar] [CrossRef]
- Graef, S.; Kurth, A.; Auw-Haedrich, C.; Plange, N.; Kern, W.V.; Nitsche, A.; Reinhard, T. Clinicopathological findings in persistent corneal cowpox infection. JAMA Ophthalmol. 2013, 131, 1089–1091. [Google Scholar] [CrossRef]
- Baker, R.O.; Bray, M.; Huggins, J.W. Potential antiviral therapeutics for smallpox, monkeypox and other orthopoxvirus infections. Antivir. Res. 2003, 57, 13–23. [Google Scholar] [CrossRef]
- Smee, D.F. Progress in the discovery of compounds inhibiting orthopoxviruses in animal models. Antivir. Chem. Chemother. 2008, 19, 115–124. [Google Scholar] [CrossRef]
- Hostetler, K.Y. Alkoxyalkyl prodrugs of acyclic nucleoside phosphonates enhance oral antiviral activity and reduce toxicity: Current state of the art. Antivir. Res. 2009, 82, A84–A98. [Google Scholar] [CrossRef]
- Tippin, T.K.; Morrison, M.E.; Brundage, T.M.; Momméja-Marin, H. Brincidofovir Is Not a Substrate for the Human Organic Anion Transporter 1, A Mechanistic Explanation for the Lack of Nephrotoxicity Observed in Clinical Studies. Ther. Drug Monit. 2016, 38, 777. [Google Scholar] [CrossRef]
- Voigt, S.; Hofmann, J.; Edelmann, A.; Sauerbrei, A.; Kühl, J.S. Brincidofovir clearance of acyclovir-resistant herpes simplex virus-1 and adenovirus infection after stem cell transplantation. Transpl. Infect. Dis. 2016, 18, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Chittick, G.; Morrison, M.; Brundage, T.; Nichols, W.G. Short-term clinical safety profile of brincidofovir: A favorable benefit-risk proposition in the treatment of smallpox. Antivir. Res. 2017, 143, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Singhal, T.; Kabra, S.K.; Lodha, R. Monkeypox: A Review. Indian J. Pediatr. 2022, 89, 955–960. [Google Scholar] [CrossRef] [PubMed]
| Base | Search Strategy |
|---|---|
| PUBMED | #1 “Monkeypox” [MH] OR “Monkeypox virus” [MH] OR “Monkeypox” [TIAB] OR “Monkey Pox” [TIAB] OR “Monkeypoxvirus*” [TIAB] #2 “Therapeutics” [MH] OR “Therapeutic Uses” [MH] OR “Therap*” [TIAB] OR “Treatment*” [TIAB] OR “Pharmaco*” [TIAB] OR “Antiviral*” [TIAB] OR “Management*” [TIAB] OR “Drug*” [TIAB] OR “Agent*” [TIAB] #3 = #1 AND #2 |
| SCOPUS | #1 TITLE-ABS-KEY (“Monkeypox” OR “Monkeypox virus” OR “Monkey Pox” OR “Monkeypoxvirus*”) #2 TITLE-ABS-KEY (“Therap*” OR “Treatment*” OR “Pharmaco*” OR “Antiviral*” OR “Management*” OR “Drug*” OR “Agent*”) #3 = #1 AND #2 |
| WEB OF SCIENCE | #1 ALL = (“Monkeypox” OR “Monkeypox virus” OR “Monkey Pox” OR “Monkeypoxvirus*”) #2 ALL = (“Therap*” OR “Treatment*” OR “Pharmaco*” OR “Antiviral*” OR “Management*” OR “Drug*” OR “Agent*”) #3 = #1 AND #2 |
| EMBASE | #1 ‘monkeypox’/exp OR ‘monkeypox’ #2 ‘therapy’ #3 = #1 AND #2 |
| CENTRAL | #1 “Monkeypox” OR “Monkeypox virus” OR “Monkey Pox” OR “Monkeypoxvirus*” #2 “Therap*” OR “Treatment*” OR “Pharmaco*” OR “Antiviral*” OR “Management*” OR “Drug*” #3 = #1 AND #2 |
| Authors | Year | Design | Country | Number of Patients (n) | Age (Years) | Sex (M/F) | Sexual Behavior | Previous STIs | HIV Status | Diagnostic Method for Monkeypox | Patients Who Received Antiviral Treatmen (n) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adler H, et al. [18] | 2022 | Case series | United Kingdom | 1 | Range (30–40) | M | NR | None | Negative | RT-PCR | n = 4 |
| 2 | Range (30–40) | M | NR | None | Negative | RT-PCR | |||||
| 3 | Range (30–40) | F | NR | None | Negative | RT-PCR | |||||
| 4 | Range (41–50) | M | NR | None | Negative | RT-PCR | |||||
| 5 | Range (30–40) | M | NR | None | Negative | RT-PCR | |||||
| 6 | Range (<2) | F | NR | None | Negative | RT-PCR | |||||
| 7 | Range (30–40) | F | NR | None | Negative | RT-PCR | |||||
| Desai AN, et al. [19] | 2022 | Case series | United States | 25 | Median = 40.7 (26–76) | M (n = 25) | MSM (n = 25) | NR | Positive (n = 9) | RT-PCR | n = 25 |
| Matias WR, et al. [20] | 2022 | Case series | United States | 1 | 20 | M | MSM | Gonococcal urethritis | Negative | RT-PCR | n = 3 |
| 2 | 20 | M | MSM | HIV | Positive | RT-PCR | |||||
| 3 | 40 | M | MSM | None | Negative | RT-PCR | |||||
| Thornhill JP, et al. [1] | 2022 | Case series | 16 countries | 528 | Median: 38 (18–68) | M (n = 527) F (n = 0) Trans (n = 1) | Heterose-xual (n = 9) Homose-xual (n = 509) Bisexual (n = 10) | STI (n = 377) Gonorrhea (n = 32/377), Chlamydia (n = 20/377), Syphilis (n = 33/377), Herpes simplex (n = 3/377), Lympho-granuloma venereum (n = 2/377), Chlamydia and gonor-rhea (n = 5/377), Other or not stated (n = 14/377) | Positive (n = 218) | RT-PCR | n = 21 |
| Moschese D, et al. [21] | 2022 | Case series | Italy | 1 | 26 | M | NR | NR | Negative | RT-PCR | n = 1 |
| 2 | 35 | M | NR | NR | Negative | RT-PCR | |||||
| 3 | 34 | M | NR | NR | Positive | RT-PCR | |||||
| 4 | 37 | M | NR | NR | Positive | RT-PCR | |||||
| Rao AK, et al. [22] | 2022 | Case report | United States | 1 | NR | M | Hetero-sexual | None | None | RT-PCR | n = 1 |
| Mailhe M, et al. [23] | 2022 | Cohort study | France | 264 | Median: 35 (30–41) | M (n = 262) F (n = 1) Trans (n = 1) | MSM (n = 245) | STI (n = 209) | Positive (n = 73) | RT-PCR | n = 1 |
| Minhaj, F.S. et al. [24] | 2022 | Case reports | United States | 17 | Median: 40 (28–61) | M (n = 17) | GBMSM (n = 17) | NR | NR | RT-PCR | n = 1 |
| Girometti, N. et al. [25] | 2022 | Cohort study | United Kingdom | 54 | Median: 41 (34–45) | M (n = 54) | MSM (n = 54) | HIV (n = 13), Syphilis (n = 14), Herpes simplex (n = 24) and Gonorrhea (n = 13) | Positive (n = 13) | RT-PCR | n = 1 |
| Tarín-Vicente, E.J. et al. [26] | 2022 | Cohort study | Spain | 181 | Median: 37 (31–42) | M (n = 175) F (n = 6) | MSM (n = 166) MSW (n = 15) | HIV (n = 72), Syphilis (n = 13), Chlamydia (n = 10) | Positive (n = 72) | RT-PCR | n = 6 |
| Patel, A. et al. [27] | 2022 | Case report | United Kingdom | 197 | Median: 38 (32–42) | M (n = 197) | MSM (n = 197) | HIV (n = 70), Gonorrhea (n = 43/161), Chlamydia (n = 13/161), Syphilis (n = 6/163), Herpes simplex (n = 11/157) | Positive (n = 70) | RT-PCR | n = 1 |
| Authors | Number of Patients (n) | Clinical Manifestations | Localization of Skin Lesions | Antiviral Treatment | Route of Administration | Associated Adverse Effects | Outcome |
|---|---|---|---|---|---|---|---|
| Adler H, et al. [18] | 1 | Skin lesions, lymphadenopathy, fever, and night sweats | Face, scalp, trunk, limbs, palms, glans penis, and scrotum | Brincidofovir 200 mg (one dose) | Oral | Transaminitis | Full recovery |
| 2 | Skin lesions, lymphadenopathy, fever, and groin swelling | Face, trunk, limbs, palms, soles, and scrotum | Brincidofovir 200 mg (two doses) | Oral | Transaminitis | Full recovery | |
| 3 | Skin lesions and coryzal illness | Face, trunk, hands (including nail bed), and labia majora | Brincidofovir 200 mg (two doses) | Oral | Transaminitis, nausea, and abdominal discomfort | Full recovery | |
| 4 | Skin lesions, lymphadenopathy, fever, and headache | Face, scalp, trunk, limbs, penile shaft, palms, and soles | None | None | None | Full recovery | |
| 5 | Skin lesions and lymphadenopathy | Face, trunk, limbs, palms, and penile shaft | None | None | None | Full recovery | |
| 6 | Skin lesions and lymphadenopathy | Face, trunk, arms, and legs | None | None | None | Full recovery | |
| 7 | Skin lesions | Face, trunk, arms, and hands | Tecovirimat 600 mg twice daily for 2 weeks | Oral | None | Full recovery | |
| Desai AN, et al. [19] | 25 | Skin lesions (n = 25), fever (n = 19), lymphadenopathy (n = 13), headache (n = 8), fatigue (n = 7), sore throat (n = 5), chills (n = 5), back pain (n = 3), myalgia (n = 2), nausea (n = 1), and diarrhea (n = 1). | Genital and/or perianal (n = 23), chest (n = 9), arms (n = 13), back (n = 8), face (n = 7), and legs (n = 6). | Tecovirimat every 8 or 12 h for 2 weeks (n = 25) | Oral | Fatigue (n = 7), headache (n = 5), nausea (n = 4), itching (n = 2), and diarrhea (n = 2) | Full recovery |
| Matias WR, et al. [20] | 1 | Skin lesions, lymphadenopathy, fever, chills, and general malaise. | Penis, pubis, and arm | Tecovirimat 600 mg twice daily for 2 weeks | Oral | Transaminitis, headache | Full recovery |
| 2 | Skin lesions, lymphadenopathy, fever, chills, myalgias, left tonsillar pain, and odynophagia | Forearms and hands | Tecovirimat 600 mg twice daily for 2 weeks | Oral | Liquid stools | Full recovery | |
| 3 | Skin lesions, lymphadenopathy, malaise, and subjective fevers | Penis, chest, and arm | Tecovirimat 600 mg twice daily for 2 weeks | Oral | None | Full recovery | |
| Thornhill JP, et al. [1] | 528 | Rash or skin lesions (n = 500), fever (n = 330), lymphadenopathy (n = 295), lethargy or exhaustion (n = 216), myalgia (n = 165), headache (n = 145), pharyngitis (n = 113), low mood (n = 54), and proctitis or anorectal pain (n = 75). | Anogenital area (n = 383), trunk or limbs (n = 292), face (n = 134), palms or soles (n = 51), and mucosal lesions (n = 217). | Cidofovir (n = 12), tecovirimat (n = 8), vaccinia immune globulin (n = 1) | Oral and parenteral | NR | Full recovery |
| Moschese D, et al. [21] | 1 | Skin lesions, fever, chills, sweats, and lymphadenopathy | Nose, limb | Cidofovir 5 mg/kg day 1 and 7 | Intravenous | None | Full recovery |
| 2 | Skin lesions, fever, and lymphadenopathy | Head, limbs, and trunk | None | NR | None | Full recovery | |
| 3 | Skin lesions, fever, and lymphadenopathy | Perianal, foot, face, and arm | None | NR | None | Full recovery | |
| 4 | Skin lesions, fever, headache, and lymphadenopathy | Inguinal, penis, scrotum, and face | None | NR | None | Full recovery | |
| Rao AK, et al. [22] | 1 | Purulent rash, diarrhea, vomiting, cough, subjective fever, and fatigue | NR | Tecovirimat | Oral | None | Full recovery |
| Mailhe M, et al. [23] | 264 | Skin lesions (n = 264), lymphadenopathy (n = 174), fever (n = 171), pharyngitis (n = 51), angina (n = 41), respiratory signs (n = 31), and headaches (n = 89) | Genital area (n = 135), limbs (n = 121), trunk (n = 105), perianal (n = 100), face (n = 88), and palmoplantar area (n = 36) | Two doses of Cidofovir 5 mg/kg. (n = 1) | Intravenous | None | Full recovery |
| Minhaj, F.S. et al. [24] | 17 | Skin lesions (n = 17), fatigue or malaise (n = 13), chills (n = 12), lymphadenopathy (n = 9), headache (n = 8), fever (n = 7), body aches (n = 6), sore throat or cough (n = 5), and sweat (n = 4). | Arm (n = 9), trunk (n = 9), legs (n = 8), face (n = 7), hands (n = 6), perianal (n = 6), oral (n = 5), neck (n = 5), genital (penis or vagina) (n = 4), and feet (n = 4). | Tecovirimat (n = 1) | Oral | None | Full recovery |
| Girometti, N. et al. [25] | 54 | Skin lesions (n = 54), Fatigue (n = 36), fever (n = 31), lymphadenopathy (n = 30), myalgia (n = 16), and sore throat (n = 11) | Genital (n = 33), perianal (n = 24), upper and lower extremities (n = 27), facial (n = 11), oropharyngeal (n = 4), and torso (n = 14) | Tecovirimat (n = 1). | Oral | None | Full recovery |
| Tarín-Vicente, E.J. et al. [26] | 181 | Skin lesions (n = 181), lymphadenopathy (n = 153), Influenza-like illness (n = 147), fever (n = 131), headache (n = 96), and sore throat (n = 66) | Genital (n = 100), perianal area (n = 66), oral ulcer (n = 45), perioral (n = 51), hands and feet (n = 108), trunk and extremities (n = 104) | Cidofovir (n = 6) | Cutaneous | None | Full recovery |
| Patel, A. et al. [27] | 197 | Mucocutaneous manifestations (n = 197), fever (n = 122), lymphadenopathy (n = 114), headache (n = 49), fatigue/lethargy (n = 46), myalgia (n = 62), arthralgia (n = 21), back pain (n = 21), and rectal pain or pain on defecation (n = 71) | Face (n = 71), trunk (n = 70), arms/legs (n = 74), hands/feet (n = 56), genitals (n = 111), anus or perianal area (n = 82), and oropharyngeal (n = 27) | Tecovirimat 600 mg twice daily for 14 days (n = 1). | Oral | None | Full recovery |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Saavedra, B.; León-Figueroa, D.A.; Montes-Madariaga, E.S.; Ricardo-Martínez, A.; Alva, N.; Cabanillas-Ramirez, C.; Barboza, J.J.; Siddiq, A.; Coaguila Cusicanqui, L.A.; Bonilla-Aldana, D.K.; et al. Antiviral Treatment against Monkeypox: A Scoping Review. Trop. Med. Infect. Dis. 2022, 7, 369. https://doi.org/10.3390/tropicalmed7110369
Ortiz-Saavedra B, León-Figueroa DA, Montes-Madariaga ES, Ricardo-Martínez A, Alva N, Cabanillas-Ramirez C, Barboza JJ, Siddiq A, Coaguila Cusicanqui LA, Bonilla-Aldana DK, et al. Antiviral Treatment against Monkeypox: A Scoping Review. Tropical Medicine and Infectious Disease. 2022; 7(11):369. https://doi.org/10.3390/tropicalmed7110369
Chicago/Turabian StyleOrtiz-Saavedra, Brando, Darwin A. León-Figueroa, Elizbet S. Montes-Madariaga, Alex Ricardo-Martínez, Niza Alva, Cielo Cabanillas-Ramirez, Joshuan J. Barboza, Abdelmonem Siddiq, Luis A. Coaguila Cusicanqui, D. Katterine Bonilla-Aldana, and et al. 2022. "Antiviral Treatment against Monkeypox: A Scoping Review" Tropical Medicine and Infectious Disease 7, no. 11: 369. https://doi.org/10.3390/tropicalmed7110369
APA StyleOrtiz-Saavedra, B., León-Figueroa, D. A., Montes-Madariaga, E. S., Ricardo-Martínez, A., Alva, N., Cabanillas-Ramirez, C., Barboza, J. J., Siddiq, A., Coaguila Cusicanqui, L. A., Bonilla-Aldana, D. K., & Rodriguez-Morales, A. J. (2022). Antiviral Treatment against Monkeypox: A Scoping Review. Tropical Medicine and Infectious Disease, 7(11), 369. https://doi.org/10.3390/tropicalmed7110369










