Potential Drivers for the Re-Emergence of Canine Leptospirosis in the United States and Canada
Abstract
:1. Introduction
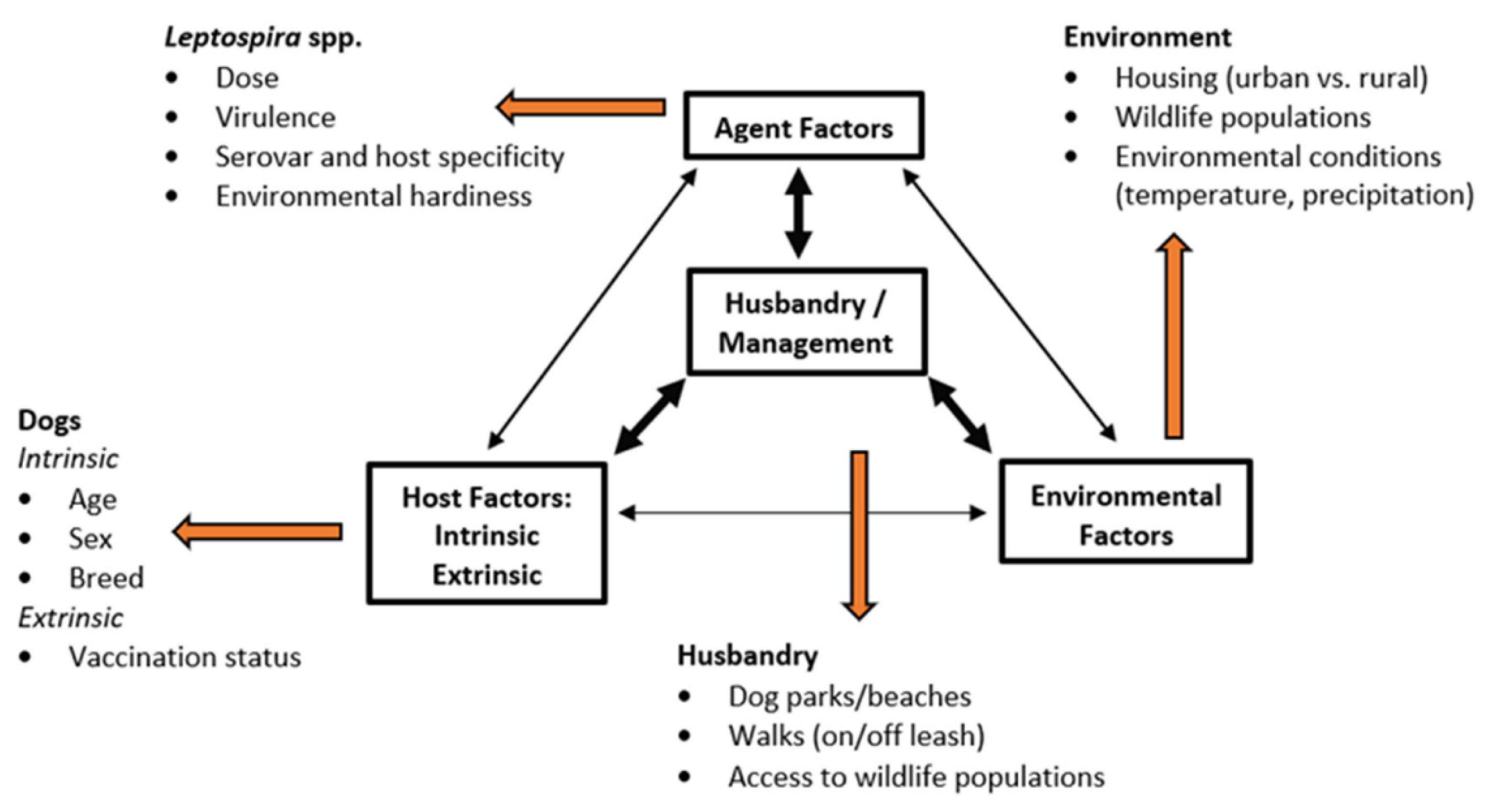
2. Epidemiologic Triad
3. Agent
4. Environment
4.1. Climate Change
4.2. Urbanization
4.3. Canine Importation, Feral Animals, and Wildlife Trade
5. Host
5.1. The Evolving Role and Lifestyle of the Dog
5.2. Role of Vaccination
5.3. Actions by Dog Owners
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sumi, A.; Telan, E.F.O.; Chagan-Yasutan, H.; Piolo, M.B.; Hattori, T.; Kobayashi, N. Effect of temperature, relative humidity and rainfall on dengue fever and leptospirosis infections in Manila, the Philippines. Epidemiol. Infect. 2017, 145, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Haake, D.A.; Levett, P.N. Leptospirosis in humans. Curr. Top. Microbiol. Immunol. 2015, 387, 65–97. [Google Scholar] [CrossRef] [Green Version]
- Randall, R. Canine leptospirosis. J. Am. Vet. Med. Assoc. 1948, 112, 136–139. [Google Scholar] [PubMed]
- Chernesky, S.J. A serological survey for canine leptospirosis in British Columbia. Can. J. Comp. Med. Rev. Can. Med. Comp. 1970, 34, 102–104. [Google Scholar]
- Ward, M.P.; Glickman, L.T.; Guptill, L.E. Prevalence of and risk factors for leptospirosis among dogs in the United States and Canada: 677 cases (1970–1998). J. Am. Vet. Med. Assoc. 2002, 220, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Bolin, C.A. Diagnosis of leptospirosis: A reemerging disease of companion animals. Semin. Vet. Med. Surg. (Small Anim.) 1996, 11, 166–171. [Google Scholar] [CrossRef]
- Sykes, J.E.; Hartmann, K.; Lunn, K.F.; Moore, G.E.; Stoddard, R.A.; Goldstein, R.E. 2010 ACVIM Small Animal Consensus Statement on Leptospirosis: Diagnosis, Epidemiology, Treatment, and Prevention. J. Vet. Intern. Med. 2011, 25, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautam, R.; Wu, C.-C.; Guptill, L.F.; Potter, A.; Moore, G.E. Detection of antibodies against Leptospira serovars via microscopic agglutination tests in dogs in the United States, 2000–2007. J. Am. Vet. Med. Assoc. 2010, 237, 293–298. [Google Scholar] [CrossRef]
- Moore, G.E.; Guptill, L.F.; Glickman, N.W.; Caldanaro, R.J.; Aucoin, D.; Glickman, L.T. Canine Leptospirosis, United States, 2002–2004. Emerg. Infect. Dis. 2006, 12, 501–503. [Google Scholar] [CrossRef]
- Balboni, A.; Mazzotta, E.; Boniotti, M.B.; Bertasio, C.; Bellinati, L.; Lucchese, L.; Battilani, M.; Ceglie, L.; Marchione, S.; Esposito, G.; et al. Outbreak of Leptospira borgpetersenii serogroup Sejroe infection in kennel: The role of dogs as sentinel in specific environments. Int. J. Environ. Res. Public. Health 2022, 19, 3906. [Google Scholar] [CrossRef]
- Hookey, J.V.; Bryden, J.; Gatehouse, L. The use of 16S rDNA sequence analysis to investigate the phylogeny of Leptospiraceae and related spirochaetes. J. Gen. Microbiol. 1993, 139, 2585–2590. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.T.; Schiettekatte, O.; Goarant, C.; Neela, V.K.; Bernet, E.; Thibeaux, R.; Ismail, N.; Mohd Khalid, M.K.N.; Amran, F.; Masuzawa, T.; et al. Revisiting the taxonomy and evolution of pathogenicity of the genus Leptospira through the prism of genomics. PLoS Negl. Trop. Dis. 2019, 13, e0007270. [Google Scholar] [CrossRef] [Green Version]
- Levett, P.N. Leptospirosis. Clin. Microbiol. Rev. 2001, 14, 296–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sykes, J.E.; Gamage, C.D.; Haake, D.A.; Nally, J.E. Understanding leptospirosis: Application of state-of-the-art molecular typing tools with a One Health lens. Am. J. Vet. Res. 2022, 83, ajvr.22.06.0104. [Google Scholar] [CrossRef] [PubMed]
- Sykes, J.E.; Reagan, K.L.; Nally, J.E.; Galloway, R.L.; Haake, D.A. Role of diagnostics in epidemiology, management, surveillance, and control of leptospirosis. Pathogens 2022, 11, 395. [Google Scholar] [CrossRef]
- Di Azevedo, M.I.N.; Santanna, R.; Carvalho-Costa, F.A.; Lilenbaum, W. The same strain leading to different clinical outcomes: The enigma behind the canine leptospirosis. Microb. Pathog. 2022, 165, 105500. [Google Scholar] [CrossRef]
- Adler, B.; Faine, S. Host immunological mechanisms in the resistance of mice to leptospiral infections. Infect. Immun. 1977, 17, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Van de Maele, I.; Claus, A.; Haesebrouck, F.; Daminet, S. Leptospirosis in dogs: A review with emphasis on clinical aspects. Vet. Rec. 2008, 163, 409–413. [Google Scholar] [CrossRef]
- Athapattu, T.; Fernando, R.; Abayawansha, R.; Fernando, P.; Fuward, M.; Samarakoon, N.; Koizumi, N.; Gamage, C. Carrier status of Leptospira spp. in healthy companion dogs in Sri Lanka. Vector Borne Zoonotic Dis. 2022, 22, 93–100. [Google Scholar] [CrossRef]
- Sant’Anna da Costa, R.; Di, N.; Azevedo, M.I.; Dos Santos Baptista Borges, A.L.; Carvalho-Costa, F.A.; Martins, G.; Lilenbaum, W. Persistent high Leptospiral shedding by asymptomatic dogs in endemic areas triggers a serious public health concern. Anim. Open Access J. 2021, 11, 937. [Google Scholar] [CrossRef]
- Bertasio, C.; Boniotti, M.B.; Lucchese, L.; Ceglie, L.; Bellinati, L.; Mazzucato, M.; Furlanello, T.; D’Incau, M.; Natale, A. Detection of new Leptospira genotypes infecting symptomatic dogs: Is a new vaccine formulation needed? Pathogens 2020, 9, 484. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Guptill, L.F.; Wu, C.C.; Potter, A.; Moore, G.E. Spatial and spatio-temporal clustering of overall and serovar-specific Leptospira microscopic agglutination test (MAT) seropositivity among dogs in the United States from 2000 through 2007. Prev. Vet. Med. 2010, 96, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Stull, J.W.; Evason, M.D.; Weese, J.S.; Wittum, T.E.; Szlosek, D.; Arruda, A.G. Investigation of spatio-temporal clusters of positive leptospirosis polymerase chain reaction test results in dogs in the United States, 2009 to 2016. J. Vet. Intern. Med. 2021, 35, 1355–1360. [Google Scholar] [CrossRef]
- Stull, J.W.; Evason, M.; Weese, J.S.; Yu, J.; Szlosek, D.; Smith, A.M. Canine leptospirosis in Canada, test-positive proportion and risk factors (2009 to 2018): A cross-sectional study. PLoS ONE 2022, 17, e0270313. [Google Scholar] [CrossRef] [PubMed]
- Iverson, S.A.; Levy, C.; Yaglom, H.D.; Venkat, H.L.; Artus, A.; Galloway, R.; Guagliardo, S.A.J.; Reynolds, L.; Kretschmer, M.J.; LaFerla Jenni, M.E.; et al. Clinical, diagnostic, and epidemiological features of a community-wide outbreak of canine leptospirosis in a low-prevalence region (Maricopa County, Arizona). J. Am. Vet. Med. Assoc. 2021, 258, 616–629. [Google Scholar] [CrossRef]
- Grimm, K.; Rivera, N.A.; Fredebaugh-Siller, S.; Weng, H.-Y.; Warner, R.E.; Maddox, C.W.; Mateus-Pinilla, N.E. Evidence of Leptospira serovars in wildlife and Leptospira DNA in water sources in a natural area in east-central Illinios, USA. J. Wildl. Dis. 2020, 56, 316–327. [Google Scholar] [CrossRef]
- Wuebbles, D.J.; Fahey, D.W.; Hibbard, K.A.; Dokken, D.J.; Stewart, B.C.; Maycock, T.K. Climate Science Special Report: Fourth National Climate Assessment; U.S. Global Change Research Program: Washington, DC, USA, 2017; Volume I. Available online: https://science2017.globalchange.gov/ (accessed on 27 September 2022).
- Ebi, K.L.; Hess, J.J. Health risks due to climate change: Inequity in causes and consequences. Health Aff. Proj. Hope 2020, 39, 2056–2062. [Google Scholar] [CrossRef]
- Gallo, T.; Fidino, M.; Gerber, B.; Ahlers, A.A.; Angstmann, J.L.; Amaya, M.; Concilio, A.L.; Drake, D.; Gay, D.; Lehrer, E.W.; et al. Mammals adjust diel activity across gradients of urbanization. eLife 2022, 11, e74756. [Google Scholar] [CrossRef]
- Ward, M.P. Seasonality of canine leptospirosis in the United States and Canada and its association with rainfall. Prev. Vet. Med. 2002, 56, 203–213. [Google Scholar] [CrossRef]
- Amilasan, A.T.; Ujiie, M.; Suzuki, M.; Salva, E.; Belo, M.C.P.; Koizumi, N.; Yoshimatsu, K.; Schmidt, W.-P.; Marte, S.; Dimaano, E.M.; et al. Outbreak of leptospirosis after flood, the Philippines, 2009. Emerg. Infect. Dis. 2012, 18, 91–94. [Google Scholar] [CrossRef] [Green Version]
- Leaning, J.; Guha-Sapir, D. Natural Disasters, Armed Conflict, and Public Health. N. Engl. J. Med. 2013, 369, 1836–1842. [Google Scholar] [CrossRef] [PubMed]
- Patz, J.A.; Kovats, R.S. Hotspots in climate change and human health. BMJ 2002, 325, 1094–1098. [Google Scholar] [CrossRef] [Green Version]
- Trevejo, R.T.; Rigau-Pérez, J.G.; Ashford, D.A.; McClure, E.M.; Jarquín-González, C.; Amador, J.J.; de los Reyes, J.O.; Gonzalez, A.; Zaki, S.R.; Shieh, W.-J.; et al. Epidemic leptospirosis associated with pulmonary hemorrhage—Nicaragua, 1995. J. Infect. Dis. 1998, 178, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, R.K.; Brenner, K.M.; Higgins, J.J.; Hutchinson, J.M.S.; Harkin, K.R. Evaluations of hydrologic risk factors for canine leptospirosis: 94 cases (2002–2009). Prev. Vet. Med. 2012, 107, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.L.; Smythe, L.D.; Craig, S.B.; Weinstein, P. Climate change, flooding, urbanisation and leptospirosis: Fuelling the fire? Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Reis, R.B.; Ribeiro, G.S.; Felzemburgh, R.D.M.; Santana, F.S.; Mohr, S.; Melendez, A.X.T.O.; Queiroz, A.; Santos, A.C.; Ravines, R.R.; Tassinari, W.S.; et al. Impact of environment and social gradient on Leptospira infection in urban slums. PLoS Negl. Trop. Dis. 2008, 2, e228. [Google Scholar] [CrossRef] [Green Version]
- US Environmental Protection Agency. Reducing Urban Heat Islands: Compendium of Strategies; U.S. Environmental Protection Agency: Washington, DC, USA, 2008. Available online: https://www.epa.gov/heat-islands/heat-island-compendium (accessed on 22 September 2022).
- Oke, T.R. Urban Climates and Global Environmental Change. In Applied Climatology: Principles and Practices; Routledge: New York, NY, USA, 1997; pp. 273–287. [Google Scholar]
- Oke, T.R. Boundary Layer Climates; Routledge: New York, NY, USA, 1987. [Google Scholar]
- Gubler, D.J.; Reiter, P.; Ebi, K.L.; Yap, W.; Nasci, R.; Patz, J.A. Climate variability and change in the United States: Potential impacts on vector-and rodent-borne diseases. Environ. Health Perspect. 2001, 109, 223–233. [Google Scholar]
- United Nations 68% of the World Population Projected to Live in Urban Areas by 2050, Says UN. Available online: https://www.un.org/development/desa/en/news/population/2018-revision-of-world-urbanization-prospects.html (accessed on 29 March 2020).
- WHO Hidden Cities: Unmasking and Overcoming Health Inequities in Urban Settings; World Health Organization: Geneva, Switzerland, 2010.
- Neiderud, C.-J. How urbanization affects the epidemiology of emerging infectious diseases. Infect. Ecol. Epidemiol. 2015, 5, 27060. [Google Scholar] [CrossRef]
- Andersen-Ranberg, E.U.; Pipper, C.; Jensen, P.M. Global patterns of Leptospira prevalence in vertbrate reservoir hosts. J. Wildl. Dis. 2016, 52, 468–477. [Google Scholar] [CrossRef]
- Hassell, J.M.; Begon, M.; Ward, M.J.; Fèvre, E.M. Urbanization and disease emergence: Dynamics at the wildlife-livestock-human interface. Trends Ecol. Evol. 2017, 32, 55–67. [Google Scholar] [CrossRef] [Green Version]
- Bradley, C.A.; Altizer, S. Urbanization and the ecology of wildlife diseases. Trends Ecol. Evol. 2007, 22, 95–102. [Google Scholar] [CrossRef]
- United Nations Human Settlements Programme. State of the World’s Cities 2012/2013; Routledge: New York, NY, USA, 2013. [Google Scholar]
- Ghneim, G.S.; Viers, J.H.; Chomel, B.B.; Kass, P.H.; Descollonges, D.A.; Johnson, M.L. Use of a case-control study and geographic information systems to determine environmental and demographic risk factors for canine leptospirosis. Vet. Res. 2007, 38, 37–50. [Google Scholar] [CrossRef]
- Maciel, E.A.P.; de Carvalho, A.L.F.; Nascimento, S.F.; de Matos, R.B.; Gouveia, E.L.; Reis, M.G.; Ko, A.I. Household transmission of Leptospira infection in urban slum communities. PLoS Negl. Trop. Dis. 2008, 2, e154. [Google Scholar] [CrossRef] [Green Version]
- Raghavan, R.K.; Brenner, K.M.; Higgins, J.J.; Shawn Hutchinson, J.M.; Harkin, K.R. Neighborhood-level socioeconomic and urban land use risk factors of canine leptospirosis: 94 cases (2002–2009). Prev. Vet. Med. 2012, 106, 324–331. [Google Scholar] [CrossRef] [Green Version]
- Alton, G.D.; Berke, O.; Reid-Smith, R.; Ojkic, D.; Prescott, J.F. Increase in seroprevalence of canine leptospirosis and its risk factors, Ontario 1998–2006. Can. J. Vet. Res. 2009, 73, 167–175. [Google Scholar]
- Raghavan, R.; Brenner, K.; Higgins, J.; Van der Merwe, D.; Harkin, K.R. Evaluations of land cover risk factors for canine leptospirosis: 94 cases (2002–2009). Prev. Vet. Med. 2011, 101, 241–249. [Google Scholar] [CrossRef] [Green Version]
- White, A.M.; Zambrana-Torrelio, C.; Allen, T.; Rostal, M.K.; Wright, A.K.; Ball, E.C.; Daszak, P.; Karesh, W.B. Hotspots of canine leptospirosis in the United States of America. Vet. J. 2017, 222, 29–35. [Google Scholar] [CrossRef]
- Ward, M.P.; Guptill, L.F.; Prahl, A.; Wu, C.C. Serovar-specific prevalence and risk factors for leptospirosis among dogs: 90 cases (1997–2002). J. Am. Vet. Med. Assoc. 2004, 224, 1958–1963. [Google Scholar] [CrossRef]
- Walsh, M.G. Rat sightings in New York City are associated with neighborhood sociodemographics, housing characteristics, and proximity to open public space. PeerJ 2014, 2, e533. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.; Bragdon, C.; Olson, C.; Merlino, M.; Bonaparte, S. Characteristics of the built environment and the presence of the Norway rat in New York City: Results from a neighborhood rat surveillance program, 2008–2010. J. Environ. Health 2016, 78, 22–29. [Google Scholar]
- Thiermann, A.B. Incidence of leptospirosis in the Detroit rat population. Am. J. Trop. Med. Hyg. 1977, 26 Pt 1, 970–974. [Google Scholar] [CrossRef]
- Desvars, A.; Michault, A.; Chiroleu, F. Influence of risk factors on renal leptospiral load in naturally infected wild black rats. Acta Trop. 2013, 125, 258–261. [Google Scholar] [CrossRef]
- Glass, G.E.; Korch, G.W.; Childs, J.E. Seasonal and habitat differences in growth rates of wild Rattus norvegicus. J. Mammal. 1988, 69, 587–592. [Google Scholar] [CrossRef]
- Ordeñana, M.A.; Crooks, K.R.; Boydston, E.E.; Fisher, R.N.; Lyren, L.M.; Siudyla, S.; Haas, C.D.; Harris, S.; Hathaway, S.A.; Turschak, G.M.; et al. Effects of urbanization on carnivore species distribution and richness. J. Mammal. 2010, 91, 1322–1331. [Google Scholar] [CrossRef]
- Hennebelle, J.H.; Sykes, J.E.; Foley, J. Risk factors associated with leptospirosis in dogs from northern California: 2001–2010. Vector Borne Zoonotic Dis. 2014, 14, 733–739. [Google Scholar] [CrossRef]
- Prange, S.; Gehrt, S.D.; Wiggers, E.P. Demographic factors contributing to high raccoon densities in urban landscapes. J. Wildl. Manag. 2003, 67, 324. [Google Scholar] [CrossRef]
- Côté, S.D.; Rooney, T.P.; Tremblay, J.-P.; Dussault, C.; Waller, D.M. Ecological impacts of deer overabundance. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 113–147. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, K.; Anderson, T.D.; Maison, R.M.; Wiscomb, G.W.; Pipas, M.J.; Sinnett, D.R.; John, A.B.; Gidlewski, T. Leptospira antibodies detected in wildlife in the USA and the US Virgin Islands. J. Wildl. Dis. 2018, 54, 450–459. [Google Scholar] [CrossRef]
- Richardson, D.J.; Gauthier, J.L. A serosurvey of leptospirosis in Connecticut peridomestic wildlife. Vector-Borne Zoonotic Dis. 2003, 3, 187–193. [Google Scholar] [CrossRef]
- Mitchell, M.A.; Hungeford, L.L.; Nixon, C.; Esker, T.; Sullivan, J.; Koerkenmeier, R.; Dubey, J.P. Serologic survey for selected infectious disease agents in raccoons from Illinois. J. Wildl. Dis. 1999, 35, 347–355. [Google Scholar] [CrossRef]
- Malmlov, A.; Breck, S.; Fry, T.; Duncan, C. Serologic survey for cross-species pathogens in urban coyotes (Canis iatrans), Colorado, USA. J. Wildl. Dis. 2014, 50, 946–950. [Google Scholar] [CrossRef]
- Davis, M.A.; Evermann, J.F.; Petersen, C.R.; VancerSchalie, J.; Besser, T.E.; Huckabee, J.; Daniels, J.B.; Hancock, D.D.; Leslie, M.; Baer, R. Serological survey for antibodies to Leptospira in dogs and raccoons in Washington State. Zoonoses Public Health 2008, 55, 436–442. [Google Scholar] [CrossRef]
- Raizman, E.A.; Dharmarajan, G.; Beasley, J.C.; Wu, C.C.; Pogranichniy, R.M.; Rhodes, O.E. Serologic survey for selected infectious diseases in raccoons (Procyon lotor) in Indiana, USA. J. Wildl. Dis. 2009, 45, 531–536. [Google Scholar] [CrossRef] [Green Version]
- Duncan, C.; Krafsur, G.; Podell, B.; Baeten, L.A.; LeVan, I.; Charles, B.; Ehrhart, E.J. Leptospirosis and tularaemia in raccoons (Procyon lotor) of Larimer County, [corrected] Colorado. Zoonoses Public Health 2012, 59, 29–34. [Google Scholar] [CrossRef]
- Straub, M.H.; Foley, J.E. Cross-sectional evaluation of multiple epidemiological cycles of Leptospira species in peri-urban wildlife in California. J. Am. Vet. Med. Assoc. 2020, 257, 840–848. [Google Scholar] [CrossRef]
- Straub, M.H.; Church, M.; Glueckert, E.; Foley, J.E. Raccoons (Procyon lotor) and striped skunks (Mephitis mephitis) as potential reservoirs of Leptospira spp. in California. Vector Borne Zoonotic Dis. 2020, 20, 418–426. [Google Scholar] [CrossRef]
- Blessington, T.; Schenck, A.P.; Levine, J.F. Frequency of animal leptospirosis in the southern United States and the Implications for human health. South. Med. J. 2020, 113, 240–249. [Google Scholar] [CrossRef]
- Tan, C.G.; Dharmarajan, G.; Beasley, J.; Rhodes, O.; Moore, G.; Wu, C.C.; Lin, T.L. Neglected leptospirosis in raccoons (Procyon lotor) in Indiana, USA. Vet. Q. 2014, 34, 1–10. [Google Scholar] [CrossRef]
- Shearer, K.E.; Harte, M.J.; Ojkic, D.; Delay, J.; Campbell, D. Detection of Leptospira spp. in wildlife reservoir hosts in Ontario through comparison of immunohistochemical and polymerase chain reaction genotyping methods. Can. Vet. J. Rev. Vet. Can. 2014, 55, 240–248. [Google Scholar]
- Allen, S.E.; Ojkic, D.; Jardine, C.M. Prevalence of antibodies to Leptospira in wild mammals trapped on livestock farms in Ontario, Canada. J. Wildl. Dis. 2014, 50, 666–670. [Google Scholar] [CrossRef]
- Britton, A.P.; Redford, T.; Bidulka, J.J.; Scouras, A.P.; Sojonky, K.R.; Zabek, E.; Schwantje, H.; Joseph, T. Beyond rabies: Qre free-ranging skunks (Mephitis mephitis) in British Columbia reservoirs of emerging infection? Transbound. Emerg. Dis. 2017, 64, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, A.E.; Katz, A.R.; Galloway, R.; Stoddard, R.A.; Goldstein, S.M. Feral swine Leptospira seroprevalence survey in Hawaii, USA, 2007–2009. Zoonoses Public Health 2016, 63, 584–587. [Google Scholar] [CrossRef]
- Chatfield, J.; Milleson, M.; Stoddard, R.; Bui, D.M.; Galloway, R. Serosurvey of leptospirosis in feral hogs (Sus scrofa) in Florida. J. Zoo Wildl. Med. 2013, 44, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Linares, M.; Hicks, C.; Bowman, A.S.; Hoet, A.; Stull, J.W. Infectious agents in feral swine in Ohio, USA (2009–2015): A low but evolving risk to agriculture and public health. Vet. Anim. Sci. 2018, 6, 81–85. [Google Scholar] [CrossRef]
- Pedersen, K.; Pabilonia, K.L.; Anderson, T.D.; Bevins, S.N.; Hicks, C.R.; Kloft, J.M.; Deliberto, T.J. Widespread detection of antibodies to Leptospira in feral swine in the United States. Epidemiol. Infect. 2015, 143, 2131–2136. [Google Scholar] [CrossRef]
- Pedersen, K.; Anderson, T.D.; Bevins, S.N.; Pabilonia, K.L.; Whitley, P.N.; Virchow, D.R.; Gidlewski, T. Evidence of leptospirosis in the kidneys and serum of feral swine (Sus scrofa) in the United States. Epidemiol. Infect. 2017, 145, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, K.; Bauer, N.E.; Rodgers, S.; Bazan, L.R.; Mesenbrink, B.T.; Gidlewski, T. Antibodies to various zoonotic pathogens detected in feral swine (Sus scrofa) at abattoirs in Texas, USA. J. Food Prot. 2017, 80, 1239–1242. [Google Scholar] [CrossRef]
- Poudel, A.; Hoque, M.M.; Madere, S.; Bolds, S.; Price, S.; Barua, S.; Adekanmbi, F.; Kalalah, A.; Kitchens, S.; Brown, V.; et al. Molecular and serological prevalence of leptospira spp. in feral pigs (Sus scrofa) and their habitats in Alabama, USA. Pathogens 2020, 9, 857. [Google Scholar] [CrossRef]
- Eskew, E.A.; White, A.M.; Ross, N.; Smith, K.M.; Smith, K.F.; Rodríguez, J.P.; Zambrana-Torrelio, C.; Karesh, W.B.; Daszak, P. United States wildlife and wildlife product imports from 2000–2014. Sci. Data 2020, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Pavlin, B.I.; Schloegel, L.M.; Daszak, P. Risk of Importing Zoonotic Diseases through Wildlife Trade, United States. Emerg. Infect. Dis. 2009, 15, 1721–1726. [Google Scholar] [CrossRef]
- Norman, C.; Stavisky, J.; Westgarth, C. Importing rescue dogs into the UK: Reasons, methods and welfare considerations. Vet. Rec. 2020, 186, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United States Department of Agriculture: How to Bring Dogs into the United States for Commercial Sale or Adoption. Available online: https://www.aphis.usda.gov/aphis/ourfocus/animalwelfare/dog-import-into-us/import-live-dogs-into-us (accessed on 10 November 2022).
- Schuller, S.; Arent, Z.J.; Gilmore, C.; Nally, J. Prevalence of antileptospiral serum antibodies in dogs in Ireland. Vet. Rec. 2015, 177, 126. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.R.; Sangster, L.T.; Sulzer, C.R.; Pursell, A.R.; Ellinghausen, H.C. Infections with Encephalitozoon cuniculi and Leptospira interrogans, serovars grippotyphosa and ballum, in a kennel of foxhounds. J. Am. Vet. Med. Assoc. 1982, 180, 435–437. [Google Scholar] [PubMed]
- Pratt, N.; Conan, A.; Rajeev, S. Leptospira seroprevalence in domestic dogs and cats on the Caribbean island of Saint Kitts. Vet. Med. Int. 2017, 2017, 5904757. [Google Scholar] [CrossRef] [Green Version]
- Altheimer, K.; Jongwattanapisan, P.; Luengyosluechakul, S.; Pusoonthornthum, R.; Prapasarakul, N.; Kurilung, A.; Broens, E.M.; Wagenaar, J.A.; Goris, M.G.A.; Ahmed, A.A.; et al. Leptospira infection and shedding in dogs in Thailand. BMC Vet. Res. 2020, 16, 89–101. [Google Scholar] [CrossRef]
- Sant’anna, R.; Vieira, A.S.; Grapiglia, J.; Lilenbaum, W. High number of asymptomatic dogs as leptospiral carriers in an endemic area indicates a serious public health concern. Epidemiol. Infect. 2017, 145, 1852–1854. [Google Scholar] [CrossRef] [Green Version]
- Sant’Anna, R.; Vieira, A.S.; Oliveira, J.; Lilenbaum, W. Asymptomatic leptospiral infection is associated with canine chronic kidney disease. Comp. Immunol. Microbiol. Infect. Dis. 2019, 62, 64–67. [Google Scholar] [CrossRef]
- Kaila, M.; Marjoniemi, J.; Nokireki, T. Comparative study of rabies antibody titers of dogs vaccinated in Finland and imported street dogs vaccinated abroad. Acta Vet. Scand. 2019, 61, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Perrin, T. The Business of Urban Animals Survey: The facts and statistics on companion animals in Canada. Can. Vet. J. 2009, 50, 48–52. [Google Scholar]
- American Veterinary Medical Association, U.S. Pet Ownership Statistics. Available online: https://www.avma.org/resources-tools/reports-statistics/us-pet-ownership-statistics (accessed on 21 September 2022).
- Uccheddu, S.; De Cataldo, L.; Albertini, M.; Coren, S.; Da Graça Pereira, G.; Haverbeke, A.; Mills, D.S.; Pierantoni, L.; Riemer, S.; Ronconi, L.; et al. Pet humanisation and related grief: Development and validation of a structured questionnaire instrument to evaluate grief in people who have lost a companion dog. Animals 2019, 9, 933. [Google Scholar] [CrossRef] [Green Version]
- Blouin, D.D. Are dogs children, companions, or just animals? Understanding variations in people’s orientations toward animals. Anthrozoos Multidiscip. J. Interact. People Anim. 2013, 26, 279–294. [Google Scholar] [CrossRef]
- Ramon, M.E.; Slater, M.R.; Ward, M.P.; Lopez, R.R. Repeatability of a telephone questionnaire on cat-ownership patterns and pet-owner demographics evaluation in a community in Texas, USA. Prev. Vet. Med. 2008, 85, 23–33. [Google Scholar] [CrossRef] [PubMed]
- LaFerla Jenni, M.; Woodward, P.; Yaglom, H.; Levy, C.; Iverson, S.A.; Kretschmer, M.; Jarrett, N.; Dooley, E.; Narang, J.; Venkat, H. Knowledge, attitudes, and practices among veterinarians during an outbreak of canine leptospirosis—Maricopa County, Arizona, 2017. Prev. Vet. Med. 2019, 172, 104779. [Google Scholar] [CrossRef] [PubMed]
- Cleary, M.; Visentin, D.; Thapa, D.K.; West, S.; Raeburn, T.; Kornhaber, R. The homeless and their animal companions: An integrative review. Adm. Policy Ment. Health Ment. Health Serv. Res. 2020, 47, 47–59. [Google Scholar] [CrossRef]
- Singer, R.S.; Hart, L.A.; Zasloff, R.L. Dilemmas associated with rehousing homeless people who have companion animals. Psychol. Rep. 1995, 77, 851–857. [Google Scholar] [CrossRef]
- Boisier, P.; Rasolomaharo, M.; Ranaivoson, G.; Rasoamanana, B.; Rakoto, L.; Andrianirina, Z.; Andriamahefazafy, B.; Chanteau, S. Urban epidemic of bubonic plague in Majunga, Madagascar: Epidemiological aspects. Trop. Med. Int. Health 1997, 2, 422–427. [Google Scholar] [CrossRef]
- Leibler, J.H.; Zakhour, C.M.; Gadhoke, P.; Gaeta, J.M. Zoonotic and vector-borne infections among urban homeless and marginalized people in the United States and Europe, 1990–2014. Vector-Borne Zoonotic Dis. 2016, 16, 435–444. [Google Scholar] [CrossRef]
- Childs, J.E.; Schwartz, B.S.; Ksiazek, T.G.; Graham, R.R.; LeDuc, J.W.; Glass, G.E. Risk factors associated with antibodies to leptospires in inner-city residents of Baltimore: A protective role for cats. Am. J. Public Health 1992, 82, 597–599. [Google Scholar] [CrossRef] [Green Version]
- French, S.K.; Pearl, D.L.; Lem, M.; Kilborn, S.; Donnelly, B.; Slater, M. Understanding the associations between owner and pet demographics on pet body condition among those experiencing homelessness and housing vulnerability in Canada. Prev. Vet. Med. 2021, 195, 105454. [Google Scholar] [CrossRef]
- Blum Domínguez, S.D.C.; Chi Dzib, M.Y.; Maldonado Velázquez, M.G.; Nuñez Oreza, L.A.; Gómez Solano, M.I.; Caballero Poot, R.I.; Tamay Segovia, P. Detection of reactive canines to Leptospira in Campeche City, Mexico. Rev. Argent. Microbiol. 2013, 45, 34–38. [Google Scholar]
- Ortega-Pacheco, A.; Guzmán-Marín, E.; Acosta-Viana, K.Y.; Vado-Solís, I.; Jiménez-Delgadillo, B.; Cárdenas-Marrufo, M.; Pérez-Osorio, C.; Puerto-Solís, M.; Jiménez-Coello, M. Serological survey of Leptospira interrogans, Toxoplasma gondii and Trypanosoma cruzi in free roaming domestic dogs and cats from a marginated rural area of Yucatan Mexico. Vet. Med. Sci. 2017, 3, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Bergmann Esteves, S.; Moreira Santos, C.; Ferreira Salgado, F.; Paldês Gonçales, A.; Gil Alves Guilloux, A.; Marinelli Martins, C.; Kuribaiashi Hagiwara, M.; Alonso Miotto, B. Efficacy of commercially available vaccines against canine leptospirosis: A systematic review and meta-analysis. Vaccine 2022, 40, 1722–1740. [Google Scholar] [CrossRef] [PubMed]
- Francey, T.; Schweighauser, A.; Reber, A.; Schuller, S. Evaluation of changes in the epidemiology of leptospirosis in dogs after introduction of a quadrivalent antileptospiral vaccine in a highly endemic area. J. Vet. Intern. Med. 2020, 34, 2405–2417. [Google Scholar] [CrossRef] [PubMed]
- Sykes, J.E.; Haake, D.A.; Gamage, C.D.; Mills, W.Z.; Nally, J.E. A global one health perspective on leptospirosis in humans and animals. J. Am. Vet. Med. Assoc. 2022, 260, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Klaasen, H.L.B.M.; Molkenboer, M.J.C.H.; Vrijenhoek, M.P.; Kaashoek, M.J. Duration of immunity in dogs vaccinated against leptospirosis with a bivalent inactivated vaccine. Vet. Microbiol. 2003, 95, 121–132. [Google Scholar] [CrossRef]
- Grosenbaugh, D.A.; Pardo, M.C. Fifteen-month duration of immunity for the serovar Grippotyphosa fraction of a tetravalent canine leptospirosis vaccine. Vet. Rec. 2018, 182, 665. [Google Scholar] [CrossRef]
- Minke, J.M.; Bey, R.; Tronel, J.P.; Latour, S.; Colombet, G.; Yvorel, J.; Cariou, C.; Guiot, A.L.; Cozette, V.; Guigal, P.M. Onset and duration of protective immunity against clinical disease and renal carriage in dogs provided by a bi-valent inactivated leptospirosis vaccine. Vet. Microbiol. 2009, 137, 137–145. [Google Scholar] [CrossRef]
- Sonrier, C.; Branger, C.; Michel, V.; Ruvoën-Clouet, N.; Ganière, J.P.; André-Fontaine, G. Evidence of cross-protection within Leptospira interrogans in an experimental model. Vaccine 2000, 19, 86–94. [Google Scholar] [CrossRef]
- Kohn, B.; Steinicke, K.; Arndt, G.; Gruber, A.D.; Guerra, B.; Jansen, A.; Kaser-Hotz, B.; Klopfleisch, R.; Lotz, F.; Luge, E.; et al. Pulmonary abnormalities in dogs with leptospirosis. J. Vet. Intern. Med. 2010, 24, 1277–1282. [Google Scholar] [CrossRef]
- Ford, R.B.; Larson, L.J.; McClure, K.D.; Schultz, R.D.; Welborn, L.V. 2017 AAHA Canine Vaccination Guidelines. J. Am. Anim. Hosp. Assoc. 2017, 53, 243–251. [Google Scholar] [CrossRef]
- Ellis, J.; Marziani, E.; Aziz, C.; Brown, C.M.; Cohn, L.A.; Lea, C.; Moore, G.E.; Taneja, N. 2022 AAHA Canine Vaccination Guidelines. J. Am. Anim. Hosp. Assoc. 2022, 58, 213–230. [Google Scholar] [CrossRef] [PubMed]
- Malter, K.B.; Tugel, M.E.; Gil-Rodriguez, M.; de la, G.; Jackson, S.W.; Ryan, W.G.; Moore, G.E. Variability in non-core vaccination rates of dogs and cats in veterinary clinics across the United States. Vaccine 2022, 40, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.J.; Stephenson, N.; Foley, J.E.; Toussieng, C.R.; Farver, T.B.; Sykes, J.E.; Fleer, K.A. Incidence rates and risk factors for owner-reported adverse events following vaccination of dogs that did or did not receive a Leptospira vaccine. J. Am. Vet. Med. Assoc. 2015, 247, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.E.; Guptill, L.F.; Ward, M.P.; Glickman, N.W.; Faunt, K.K.; Lewis, H.B.; Glickman, L.T. Adverse events diagnosed within three days of vaccine administration in dogs. J. Am. Vet. Med. Assoc. 2005, 227, 1102–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langston, C.E.; Heuter, K.J. Leptospirosis. A re-emerging zoonotic disease. Vet. Clin. Small Anim. Pract. 2003, 33, 791–807. [Google Scholar] [CrossRef]
- Jessica, V. 7 Scary Diseases Your Dog Can Get from Water. Available online: https://www.petmd.com/dog/slideshows/parasites/7-scary-diseases-your-dog-can-get-water (accessed on 20 September 2022).
- Goeijenbier, M.; Wagenaar, J.; Goris, M.; Martina, B.; Henttonen, H.; Vaheri, A.; Reusken, C.; Hartskeerl, R.; Osterhaus, A.; Van Gorp, E. Rodent-borne hemorrhagic fevers: Under-recognized, widely spread and preventable—epidemiology, diagnostics and treatment. Crit. Rev. Microbiol. 2013, 39, 26–42. [Google Scholar] [CrossRef]
- Reese, J. Is There a Correlation between Rodents and Backyard Poultry? 2018. Available online: https://extension.usu.edu/news_sections/agriculture_and_natural_resources/rodent-chicken (accessed on 19 September 2022).
- Stull, J.W.; Kasten, J.I.; Evason, M.D.; Sherding, R.G.; Hoet, A.E.; O’Quin, J.; Burkhard, M.J.; Weese, J.S. Risk reduction and management strategies to prevent transmission of infectious disease among dogs at dog shows, sporting events, and other canine group settings. J. Am. Vet. Med. Assoc. 2016, 249, 612–627. [Google Scholar] [CrossRef]
- Kogan, L.R.; Rishniw, M. Canine and feline core vaccinations: US veterinarians’ concerns and perceived impact of COVID-19 antivaccination views on veterinary medicine. J. Am. Vet. Med. Assoc. 2022, 260, 1482–1488. [Google Scholar] [CrossRef]
- Kogan, L.R.; Hellyer, P.W.; Rishniw, M. American and Canadian veterinarians’ perceptions on dog and cat core vaccination rates and the impact of the human medicine anti-vaxx movement on veterinary medicine. Can. Vet. J. Rev. Vet. Can. 2021, 62, 247–252. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, A.M.; Stull, J.W.; Moore, G.E. Potential Drivers for the Re-Emergence of Canine Leptospirosis in the United States and Canada. Trop. Med. Infect. Dis. 2022, 7, 377. https://doi.org/10.3390/tropicalmed7110377
Smith AM, Stull JW, Moore GE. Potential Drivers for the Re-Emergence of Canine Leptospirosis in the United States and Canada. Tropical Medicine and Infectious Disease. 2022; 7(11):377. https://doi.org/10.3390/tropicalmed7110377
Chicago/Turabian StyleSmith, Amanda M., Jason W. Stull, and George E. Moore. 2022. "Potential Drivers for the Re-Emergence of Canine Leptospirosis in the United States and Canada" Tropical Medicine and Infectious Disease 7, no. 11: 377. https://doi.org/10.3390/tropicalmed7110377
APA StyleSmith, A. M., Stull, J. W., & Moore, G. E. (2022). Potential Drivers for the Re-Emergence of Canine Leptospirosis in the United States and Canada. Tropical Medicine and Infectious Disease, 7(11), 377. https://doi.org/10.3390/tropicalmed7110377





