Establishing and Integrating a Female Genital Schistosomiasis Control Programme into the Existing Health Care System
Abstract
:1. Introduction
2. The Case of South Africa
2.1. Geography
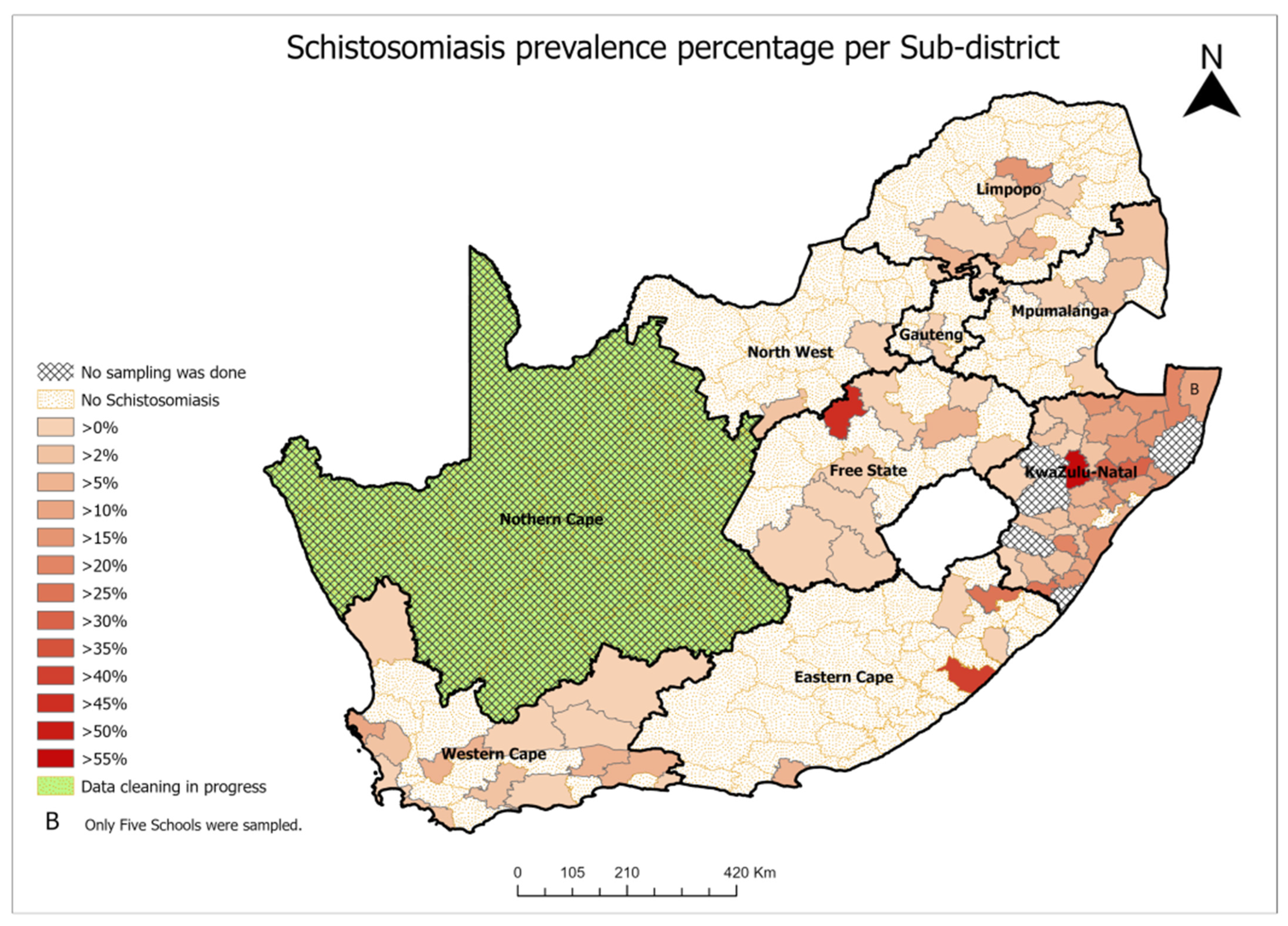
2.2. Schistosomiasis Prevalence
2.3. Policy Actions and Implications
- Action A: Schistosomiasis prevention by establishing a formal control programme and increasing access to treatment:
- Use the opportunities to treat at the endemic primary health care facilities, provide regular treatment for adults, in addition to other community members at risk of infection and school children [59].
- Alongside cervical cancer screening.
- Action B: FGS Screening–FGS screening and diagnosis should be accessible at the following various platforms in the health care system and the community:
- Index and secondary (surrounding) cases’ management—FGS is treated with praziquantel and as individualized disease management. However, hot spot intervention for community members who use the same water source as the index case should be carried out [45].
- Establishment of sentinel sites—At present, only crude extrapolations of the burden of FGS are possible [3]. Therefore, sentinel sites should be established in all municipalities where schistosomiasis is endemic (Figure 1 for South Africa) to establish baseline prevalence of FGS and track progress toward elimination. At these sites, FGS screening and case management should be prioritized alongside cervical cancer screening. Regular monitoring and evaluation surveys every 3 or 5 years are also appropriate for the follow-up of the control progress.
- Action C: Vector Control
- Regular testing of water bodies as schistosomiasis transmission sites through the collection of snails and identification of intermediate hosts of S. haematobium and S. mansoni, followed by examination through snail dissection, crushing, and analysis, e.g., by Polymerase Chain Reaction (PCR) [52];
- Chemical control methods (e.g., molluscicides to kill the snails) are applied in artificial water bodies, such as irrigation channels, ditches, and farm dams, but not in large dams, natural streams, rivers, and lakes [52];
- Environmental control involves the removal of vegetation to remove the snails’ environment, depriving them of the sheltered niches they favour, and where appropriate, ensuring that the water flows faster than their tolerance limit of 0.3 m/s. Ideally, canals should be concrete lined and contoured, to encourage fast flow. Covering open canals near dwellings is a simple way of deterring human contact with the water [52]
- Action D: Water, Sanitation, and Hygiene (WASH)–Provision of Continuous Piped Water
- Identification of households that do not have access to proper sanitation and piped water;
- Installation and maintenance of proper sanitary structures and water pipes to these households;
- Household education on the effective use of sanitary structures and water to improve hygiene; and
- Continuous health promotion campaigns and awareness on the prevention of schistosomiasis and FGS, to influence both domestic and recreational behaviour change.
- Action E: Creating Awareness of Schistosomiasis and FGS
- Training of health promoters, risk communicators, environmental health practitioners, and communicable diseases control coordinators on schistosomiasis and FGS prevention and control;
- Training of community health workers (lay people) on schistosomiasis and FGS prevention and control;
- Training of water and sanitation officials in schistosomiasis and FGS prevention to assist with community awareness during installation and maintenance of sanitary structures and water pipes;
- High power, highly interested stakeholders should be managed closely, be fully engaged, and it is important to make the greatest efforts to satisfy them;
- High power, less interested stakeholders are stakeholders that need to be kept satisfied, but not so much that they become bored with messages;
- Low power, highly interested stakeholders are stakeholders that should be kept informed with adequate information to ensure that no major issues are arising. Stakeholders in this category can often be very helpful with the details of the implementation; and
- Low power, less interested people are stakeholders that need to be monitored, but not bored with excessive communication.
3. Public Health Benefit
4. Ethics/Equity
5. Administrative Feasibility and Budgetary Feasibility
6. Political Feasibility of Donated Medication in South Africa
7. Implementation Challenges and Recommendations
7.1. Adaptive Challenges and Recommendations
7.2. Regulatory Challenges for Praziquantel, Specifically for South Africa and Recommendations
- Praziquantel should be down-regulated from schedule 4 to schedule 1.
- The Department of Health must apply to the South African Health Products Regulatory Authority under Section 21 of the South African Health Product Regulatory Authority for the admission of donated pharmaceuticals.
- The disease is neglected and will require dedicated coordinators. Staff turnover may have an influence on program implementation. Therefore, integration into the existing programmes and improving awareness among health care workers are essential.
- FGS should be incorporated in the “Regulations relating to the surveillance and the control of notifiable medical conditions” [12].
- Registration of the WHO donated praziquantel, Cesol®, Merck, Mexico, should be prioritized instead of using Section 21 of the South African Health Product Regulatory Authority exemption on an annual basis. This can be achieved through strong advocacy and evidence-based motivation.
- Ensure continued availability of praziquantel in all known endemic primary health care facilities, mother and child clinics, cervical cancer screening sites, HPV vaccination programmes, PrEP, and other HIV prevention programmes.
8. Technical Issues and Recommendations
9. Laboratory Analysis for FGS
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kjetland, E.F.; Gwanzura, F.; Ndhlovu, P.D.; Mduluza, T.; Gomo, E.; Mason, P.R.; Midzi, N.; Friis, H.; Gundersen, S.G. Simple clinical manifestations of genital Schistosoma haematobium infection in rural Zimbabwean women. Am. J. Trop. Med. Hyg. 2005, 72, 311–319. [Google Scholar] [CrossRef]
- Jordens, M. The Global Burden of Female Genital Schistosomiasis. infoNTD. 2019. Available online: https://www.infontd.org/resource/global-burden-female-genital-schistosomiasis (accessed on 17 September 2022).
- Christinet, V.; Lazdins-Helds, J.K.; Stothard, J.R.; Reinhard-Rupp, J. Female genital schistosomiasis (FGS): From case reports to a call for concerted action against this neglected gynaecological disease. Int. J. Parasitol. 2016, 46, 395–404. [Google Scholar] [CrossRef] [Green Version]
- Freer, J.B.; Bourke, C.D.; Durhuus, G.H.; Kjetland, E.F.; Prendergast, A.J. Schistosomiasis in the first 1000 days. Lancet Infect. Dis. 2018, 18, e193–e203. [Google Scholar] [CrossRef]
- Galappaththi-Arachchige, H.N.; Holmen, S.; Koukounari, A.; Kleppa, E.; Pillay, P.; Sebitloane, M.; Ndhlovu, P.; Van Lieshout, L.; Vennervald, B.J.; Gundersen, S.G.; et al. Evaluating diagnostic indicators of urogenital Schistosoma haematobium infection in young women: A cross sectional study in rural South Africa. PLoS ONE 2018, 13, e0191459. [Google Scholar] [CrossRef] [Green Version]
- van Bogaert, L.J. Schistosomiasis—An endemic but neglected tropical disease in Limpopo. S. Afr. Med. J. 2010, 100, 788–789. [Google Scholar] [CrossRef] [Green Version]
- Hegertun, I.E.A.; Sulheim Gundersen, K.M.; Kleppa, E.; Zulu, S.G.; Gundersen, S.G.; Taylor, M.; Kvalsvig, J.D.; Kjetland, E.F. S. haematobium as a Common Cause of Genital Morbidity in Girls: A Cross-sectional Study of Children in South Africa. PLoS Negl. Trop. Dis. 2013, 7, e2104. [Google Scholar] [CrossRef] [Green Version]
- Kjetland, E.F.; Ndhlovu, P.D.; Kurewa, E.N.; Midzi, N.; Gomo, E.; Mduluza, T.; Friis, H.; Gundersen, S.G. Prevention of gynecologic contact bleeding and genital sandy patches by childhood anti-schistosomal treatment. Am. J. Trop. Med. Hyg. 2008, 79, 79–83. [Google Scholar] [CrossRef]
- Livingston, M.; Pillay, P.; Zulu, S.G.; Sandvik, L.; Kvalsvig, J.D.; Gagai, S.; Galappaththi-Arachchige, H.; Kleppa, E.; Ndhlovu, P.D.; Vennervald, B.J.; et al. Mapping Schistosoma haematobium for novel interventions against Female Genital Schistosomiasis and associated HIV risk in KwaZulu-Natal, South Africa. Am. J. Trop. Med. Hyg. 2021, 104, 2055–2064. [Google Scholar] [CrossRef]
- Gyapong, J.O.; Gyapong, M.; Yellu, N.; Anakwah, K.; Amofah, G.; Bockarie, M.; Adjei, S. Integration of control of neglected tropical diseases into health-care systems: Challenges and opportunities. Lancet 2010, 375, 160–165. [Google Scholar] [CrossRef]
- Engels, D.; Hotez, P.J.; Ducker, C.; Gyapong, M.; Bustinduy, A.L.; Secor, W.E.; Harrison, W.; Theobald, S.; Thomson, R.; Gamba, V.; et al. Integration of prevention and control measures for female genital schistosomiasis, HIV and cervical cancer. Bull. World Health Organ. 2020, 98, 615–624. [Google Scholar] [CrossRef]
- Department of Health. Regulations Relating to the Surveillance and the Control of Notifiable Medical Conditions; Government Gazette: Pretoria, South Africa, 2017; Volume 630, pp. 1–32.
- Christensen, E.E.; Taylor, M.; Zulu, S.G.; Lillebo, K.; Gundersen, S.G.; Holmen, S.; Kleppa, E.; Vennervald, B.J.; Ndhlovu, P.D.; Kjetland, E.F. Seasonal variations in schistosoma haematobium egg excretion in school-age girls in rural Kwazulu-Natal province, South Africa. S. Afr. Med. J. 2018, 108, 352–355. [Google Scholar] [CrossRef] [Green Version]
- Galappaththi-Arachchige, H.N.; Zulu, S.G.; Kleppa, E.; Lillebo, K.; Qvigstad, E.; Ndhlovu, P.; Vennervald, B.J.; Gundersen, S.G.; Kjetland, E.F.; Taylor, M. Reproductive health problems in rural South African young women: Risk behaviour and risk factors. Reprod. Health 2018, 15, 1–10. [Google Scholar] [CrossRef] [Green Version]
- UNAIDS; World Health Organization. No More Neglect. Female Genital Schistosomiasis and HIV. In Integrating Reproductive Health Interventions to Improve Women’s Lives; WHO: Geneva, Switzerland, 2019; Available online: https://www.unaids.org/sites/default/files/media_asset/female_genital_schistosomiasis_and_hiv_en.pdf (accessed on 12 March 2020).
- Magaisa, K.; Taylor, M.; Kjetland, E.F.; Naidoo, P.J. A review of the control of schistosomiasis in South Africa. S. Afr. J. Sci. 2015, 111, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Swai, B.; Poggensee, G.; Mtweve, S.; Krantz, I. Female genital schistosomiasis as an evidence of a neglected cause for reproductive ill-health: A retrospective histopathological study from Tanzania. BMC Infect. Dis. 2006, 23, 134. [Google Scholar] [CrossRef] [Green Version]
- Toller, A.; Scopin, A.C.; Apfel, V.; Prigenzi, K.C.; Tso, F.K.; Focchi, G.R.; Speck, N.; Ribalta, J. An interesting finding in the uterine cervix: Schistosoma hematobium calcified eggs. Autops. Case Rep. 2015, 5, 41–44. [Google Scholar] [CrossRef]
- Mazigo, H.D.; Samson, A.; Lambert, V.J.; Kosia, A.L.; Ngoma, D.D.; Murphy, R.; Matungwa, D.J. “We know about schistosomiasis but we know nothing about FGS”: A qualitative assessment of knowledge gaps about female genital schistosomiasis among communities living in Schistosoma haematobium endemic districts of Zanzibar and Northwestern Tanzania. PLoS Negl. Trop. Dis. 2021, 15, e0009789. [Google Scholar] [CrossRef]
- Søfteland, S.; Sebitloane, M.H.; Taylor, M.; Roald, B.B.; Holmen, S.; Galappaththi-Arachchige, H.N.; Gundersen, S.G.; Kjetland, E.F. A systematic review of handheld tools in lieu of colposcopy for cervical neoplasia and female genital schistosomiasis. Int. J. Gynecol. Obstet. 2021, 153, 190–199. [Google Scholar] [CrossRef]
- Norseth, H.M.; Ndhlovu, P.D.; Kleppa, E.; Randrianasolo, B.S.; Jourdan, P.M.; Roald, B.; Holmen, S.D.; Gundersen, S.G.; Bagratee, J.; Onsrud, M.; et al. The colposcopic atlas of schistosomiasis in the lower female genital tract based on studies in Malawi, Zimbabwe, Madagascar and South Africa. PLoS Negl. Trop. Dis. 2014, 8, e3229. [Google Scholar] [CrossRef] [Green Version]
- Mbabazi, P.S.; Vwalika, B.; Randrianasolo, B.S.; Roald, B.; Ledzinski, D.; Olowookorun, F.; Hyera, F.; Galappaththi-Arachchige, H.N.; Sebitloane, M.; Taylor, M.; et al. World Health Organisation Female Genital Schistosomiasis. In A Pocket Atlas for Clinical Health-care Professionals; WHO: Geneva, Switzerland, 2015; Volume 2015, ISBN 978 92 4 150929 9. Available online: http://apps.who.int/iris/bitstream/10665/180863/1/9789241509299_eng.pdf (accessed on 20 May 2022).
- Kjetland, E.F.; Kurewa, E.N.; Ndhlovu, P.D.; Midzi, N.; Gwanzura, L.; Mason, P.R.; Gomo, E.; Sandvik, L.; Mduluza, T.; Friis, H.; et al. Female genital schistosomiasis—A differential diagnosis to sexually transmitted disease: Genital itch and vaginal discharge as indicators of genital S. haematobium morbidity in a cross-sectional study in endemic rural Zimbabwe. Trop. Med. Int. Health 2008, 13, 1509–1517. [Google Scholar] [CrossRef]
- Kjetland, E.F.; Ndhlovu, P.D.; Mduluza, T.; Deschoolmeester, V.; Midzi, N.; Gomo, E.; Gwanzura, L.; Mason, P.R.; Vermorken, J.B.; Friis, H.; et al. The effects of genital Schistosoma haematobium on human papillomavirus and the development of neoplasia after 5 years in a Zimbabwean population. A pilot study. Eur. J. Gynec. Oncol. 2010, 31, 169–173. [Google Scholar]
- Kjetland, E.F.; Hegertun, I.E.A.; Baay, M.F.D.; Onsrud, M.; Ndhlovu, P.D.; Taylor, M. Genital schistosomiasis and its unacknowledged role on HIV transmission in the STD intervention studies. Int. J. STD AIDS 2014, 25, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Molvik, M.; Helland, E.; Zulu, S.G.; Kleppa, E.; Lillebo, K.; Gundersen, S.G.; Kvalsvig, J.D.; Taylor, M.; Kjetland, E.F.; Vennervald, B.J. Co-infection with Schistosoma haematobium and soil-transmitted helminths in rural South Africa. S. Afr. J. Sci. 2017, 113, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Zulu, S.G.; Kjetland, E.F.; Gundersen, S.G.; Taylor, M. Prevalence and intensity of neglected tropical diseases (schistosomiasis and soil-transmitted helminths) amongst rural female pupils in Ugu district, KwaZulu-Natal, South Africa. S. Afr. J. Infect. Dis. 2020, 35, 1–7. [Google Scholar] [CrossRef]
- Kildemoes, A.O.; Kjetland, E.F.; Zulu, S.G.; Taylor, M.; Vennervald, B.J. Schistosoma haematobium infection and asymptomatic bacteriuria in young South African females. Acta Trop. 2015, 144, 19–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubaba, O.; Chimbari, M.J.; Soko, W.; Manyangadze, T.; Mukaratirwa, S. Validation of a urine circulating cathodic antigen cassette test for detection of Schistosoma haematobiumin uMkhanyakude district of South Africa. Acta Trop. 2018, 182, 161–165. [Google Scholar] [CrossRef]
- Kabuyaya, M.; Chimbari, M.J.; Manyangadze, T.; Mukaratirwa, S. Schistosomiasis risk factors based on the infection status among school-going children in the Ndumo area, uMkhanyakude district, South Africa. S. Afr. J. Infect. Dis. 2017, 32, 67–72. [Google Scholar] [CrossRef]
- Sacolo-Gwebu, H.; Chimbari, M.; Kalinda, C. Prevalence and risk factors of schistosomiasis and soil-transmitted helminthiases among preschool aged children (1–5 years) in rural KwaZulu-Natal, South Africa: A cross-sectional study. Infect. Dis. Poverty 2019, 8, 47. [Google Scholar] [CrossRef] [Green Version]
- Samie, A.; Nchachi, D.J.; Obi, C.L.; Igumbor, E.O. Prevalence and temporal distribution of Schistosoma haematobium infections in the Vhembe district, Limpopo Province, South Africa. Afr. J. Biotechnol. 2010, 9, 7157–7164. [Google Scholar] [CrossRef]
- Botes, S.N.; Ibirogba, S.B.; McCallum, A.D.; Kahn, D. Schistosoma prevalence in appendicitis. World J. Surg. 2015, 39, 1080–1083. [Google Scholar] [CrossRef]
- National Department of Health. Mpumalanga NTD School Prevalence; National Department of Health: Pretoria, South Africa, 2018.
- National Department of Health. KwaZulu-Natal High Risk NTD School Prevalence; National Department of Health: Pretoria, South Africa, 2016.
- National Department of Health. Eastern Cape NTD School Prevalencel; National Department of Health: Pretoria, South Africa, 2017.
- National Department of Health. Limpopo NTD School Prevalence; National Department of Health: Pretoria, South Africa, 2016.
- National Department of Health. KwaZulu-Natal NTD School Prevalence; National Department of Health: Pretoria, South Africa, 2016.
- National Department of Health. Schistosomiasis and Soil-Transmitted Helminthiasis Mapping in Gauteng Province; National Department of Health: Pretoria, South Africa, 2019.
- National Department of Health. Schistosomiasis and Soil-Transmitted Helminthiasis Mapping in North West Province; National Department of Health: Pretoria, South Africa, 2019.
- Wolmarans, C.; De Kock, K. Eier-ekskresiepatrone as kriterium vir die bepaling van prevalensie en intensiteit van besmetting by manlike en vroulike individue in verskillende ouderdomsgroepe in n skistosoomendemiese gebied in die Limpopoprovinsie. Suid-Afrik. Tydskr. Vir Nat. En Tegnol. 2010, 29, 130–144. [Google Scholar] [CrossRef] [Green Version]
- Firmo, J.O.; Lima Costa, M.F.; Guerra, H.L.; Rocha, R.S. Urban schistosomiasis: Morbidity, sociodemographic characteristics and water contact patterns predictive of infection. Int. J. Epidemiol. 1996, 25, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Kloos, H.; Correa-Oliveira, R.; Oliveira Quites, H.F.; Caetano Souza, M.C.; Gazzinelli, A. Socioeconomic studies of schistosomiasis in Brazil: A review. Acta Trop. 2008, 108, 194–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ndamba, J.; Chidimu, M.G.; Zimba, M.; Gomo, E.; Munjoma, M. An investigation of the schistosomiasis transmission status in Harare. Cent. Afr. J. Med. 1994, 40, 337–342. [Google Scholar] [PubMed]
- World Health Organisation. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030; World Health Organisation: Geneva, Switzerland, 2020; ISBN 9789240010352. [Google Scholar]
- Kjetland, E.F.; Leutscher, P.D.C.; Ndhlovu, P.D. A review of female genital schistosomiasis. Trends Parasitol. 2012, 28, 58–65. [Google Scholar] [CrossRef] [PubMed]
- National Department of Health. Regular Treatment of School-Going Children for Soil-Transmitted Helminth Infections and Bilharzia. In Policy and Implementation Guidelines; The South African National Department of Health: Pretoria, South Africa, 2008; pp. 1–61. [Google Scholar]
- Feldmeier, H.; Poggensee, G.; Krantz, I. A synoptic inventory of needs for research on women and tropical parasitic diseases. II. Gender-related biases in the diagnosis and morbidity assessment of schistosomiasis in women. Acta Trop. 1993, 55, 139–169. [Google Scholar] [CrossRef]
- KwaZulu-Natal Department of Health. Bilharzia Outbreak in KwaZulu-Natal Ethekwini Municipality; National Department of Health: Pretoria, South Africa, 2021.
- KwaZulu-Natal Department of Health. Bilharzia Outbreak in KwaZulu-Natal Zululand District; National Department of Health: Pretoria, South Africa, 2017.
- Berge, S.T.; Kabatereine, N.B.; Gundersen, S.G.; Taylor, M.; Kvalsvig, J.D.; Mkhize-Kwitshana, Z.; Jinabhai, C.C.; Kjetland, E.F. Generic praziquantel in South Africa: The necessity for policy change to avail cheap, safe and efficacious schistosomiasis drugs to the poor, rural population. S. Afr J. Epidemiol. Infect. 2011, 26, 22–25. [Google Scholar] [CrossRef]
- Appleton, C.; Miranda, N. Locating bilharzia transmission sites in South Africa: Guidelines for public health personnel. South. Afr. J. Infect. Dis. 2015, 30, 95–102. [Google Scholar] [CrossRef]
- Fenwick, A. Host-parasite relations and implications for control. Adv. Parasitol. 2009, 68, 247–261. [Google Scholar]
- Dabo, A.; Doucoure, B.; Koita, O.; Diallo, M.; Kouriba, B.; Klinkert, M.Q.; Doumbia, S.; Doumbo, O. Reinfection with Schistosoma haematobium and mansoni despite repeated praziquantel office treatment in Niger, Mali. Med. Trop. 2000, 60, 351–355. [Google Scholar]
- Davis, A. The Professor Gerald Webbe Memorial Lecture: Global control of schistosomiasis. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 609–615. [Google Scholar] [CrossRef]
- Clark, T.E.; Appleton, C.C.; Kvalsvig, J.D. Schistosomiasis and the use of indigenous plant molluscicides: A rural South African perspective. Acta Trop. 1997, 66, 93–107. [Google Scholar] [CrossRef]
- Bengu, M.D.; Dorsamy, V.; Moodley, J. Schistosomiasis infections in South African pregnant women: A review. S. Afr. J. Infect. Dis. 2020, 35, 7. [Google Scholar] [CrossRef]
- van Bogaert, L. Case of invasive adenocarcinoma of the cervix in a human immunodeficiency virus and schistosome co-infected patient. S. Afr. J. Gynaecol. Oncol. 2014, 6, 5–6. [Google Scholar] [CrossRef]
- World Health Organisation. Deworming Adolescent Girls and Women of Reproductive Age: Policy Brief; World Health Organisation: Geneva, Switzerland, 2021. [Google Scholar]
- World Health Organization. WHO Guideline on Control and Elimination of Human Schistosomiasis; World Health Organisation: Geneva, Switzerland, 2022; p. 142. ISBN 978 92 4 004160 8. Available online: https://www.who.int/publications/i/item/9789240041608?msclkid=66e125e4aa9911eca97802b2699b0852 (accessed on 19 June 2022).
- Cribb, D.M.; Clarke, N.E.; Doi, S.A.R.; Nery, S.V. Differential impact of mass and targeted praziquantel delivery on schistosomiasis control in school-aged children: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2019, 13, e0007808. [Google Scholar] [CrossRef]
- Mawa, P.A.; Kincaid-Smith, J.; Tukahebwa, E.M.; Webster, J.P.; Wilson, S. Schistosomiasis Morbidity Hotspots: Roles of the Human Host, the Parasite and Their Interface in the Development of Severe Morbidity. Front. Immunol. 2021, 12, 635869. [Google Scholar] [CrossRef]
- Brodish, P.H.; Singh, K. Association between Schistosoma haematobium exposure and Human Immunodeficiency Virus infection among females in Mozambique. Am. J. Trop. Med. Hyg. 2016, 94, 1040–1044. [Google Scholar] [CrossRef] [Green Version]
- Pinto, S.Z.; Friedman, R.; Van Den Berg, E.J. A case of paediatric bladder bilharzioma in Johannesburg, South Africa. Clin. Case Rep. 2019, 7, 1890–1894. [Google Scholar] [CrossRef] [Green Version]
- van Bogaert, L.-J.J. Biopsy-diagnosed female genital schistosomiasis in rural Limpopo, South Africa. Int. J. Gynaecol. Obstet. 2011, 115, 75–76. [Google Scholar] [CrossRef]
- Yirenya-Tawiah, D.; Amoah, C.; Apea-Kubi, K.A.; Dade, M.; Ackumey, M.; Annang, T.; Mensah, D.Y.; Bosompem, K.M. A survey of female genital schistosomiasis of the lower reproductive tract in the volta basin of ghana. Ghana Med. J. 2011, 45, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Randrianasolo, B.S.; Jourdan, P.M.; Ravoniarimbinina, P.; Ramarokoto, C.E.; Rakotomanana, F.; Ravaoalimalala, V.E.; Gundersen, S.G.; Feldmeier, H.; Vennervald, B.J.; Van Lieshout, L.; et al. Gynecological manifestations, histopathological findings, and schistosoma-specific polymerase chain reaction results among women with schistosoma haematobium infection: A cross-sectional study in Madagascar. J. Infect. Dis. 2015, 212, 275–284. [Google Scholar] [CrossRef] [Green Version]
- Ekpo, U.F.; Odeyemi, O.M.; Sam-Wobo, S.O.; Onunkwor, O.B.; Mogaji, H.O.; Oluwole, A.S.; Abdussalam, H.O.; Stothard, J.R. Female genital schistosomiasis (FGS) in Ogun State, Nigeria: A pilot survey on genital symptoms and clinical findings. Parasitol. Open 2017, 3, e10. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, J.; Pantelias, A.; Williamson, M.; Kjetland, E.F.; Krentel, A.; Gyapong, M.; Mbabazi, P.S.; Djirmay, A.G. Addressing a silent and neglected scourge in sexual and reproductive health in Sub-Saharan Africa by development of training competencies to improve prevention, diagnosis, and treatment of female genital schistosomiasis (FGS) for health workers. Reprod. Health 2022, 19, 20. [Google Scholar] [CrossRef] [PubMed]
- Gyapong, M.; Marfo, B.; Theobold, S.; Hawking, K.; Page, S.; Osei-Atweneboanaa, M.; Stothard, J. The double jeopardy of having female genital schistosomiasis in poorly informed and resourced health systems in Ghana. Trop. Med. Int. Health 2015, 20, 7. [Google Scholar]
- Mind Tools Content Team. Stakeholder Analysis. Available online: https://www.mindtools.com/pages/article/newPPM_07.htm (accessed on 21 August 2021).
- Turner, H.C.; French, M.D.; Montresor, A.; King, C.H.; Rollinson, D.; Toor, J. Economic evaluations of human schistosomiasis interventions: A systematic review and identification of associated research needs. Wellcome Open Res. 2020, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Chisango, T.J.; Ndlovu, B.; Vengesai, A.; Nhidza, A.F.; Sibanda, E.P.; Zhou, D.; Mutapi, F.; Mduluza, T. Benefits of annual chemotherapeutic control of schistosomiasis on the development of protective immunity. BMC Infect. Dis. 2019, 19, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotez, P.J.; Fenwick, A.; Kjetland, E.F. Africa’s 32 Cents Solution for HIV/AIDS. PLoS Negl. Trop. Dis. 2009, 3, e430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SAHPRA. 2. 52 Section 21 Access to Unregistered Medicines; South African Health Products Regulatory Authority: Pretoria, South Africa, 2020; Volume 1965, pp. 1–15. [Google Scholar]
- National Department of Health. Standard Treatment Guidelines and Essential Medicines List for South Africa: Primary Health Care Level, 7th ed.; The South African National Department of Health: Pretoria, South Africa, 2020; pp. 1–561.
- World Health Organization. Prequalification of Medical Products (IVDs, Medicines, Vaccines and Immunization Devices, Vector Control). Available online: https://extranet.who.int/pqweb/medicines/prequalified-lists/finished-pharmaceutical-products?label=&field_medicine_applicant=&field_medicine_fpp_site_value=&search_api_aggregation_1=&field_medicine_pq_date%5Bdate%5D=&field_medicine_pq_date_1%5Bdate%5D=&fi (accessed on 10 August 2022).
- Maphumulo, A.; Mahomed, O.; Vennervald, B.; Gundersen, S.G.; Taylor, M.; Kjetland, E.F. The cost of a school based mass treatment of schistosomiasis in Ugu District, KwaZulu Natal, South Africa in 2012. PLoS ONE 2020, 15, e0232867. [Google Scholar] [CrossRef] [PubMed]
- Treatment Action Campaign. Treatment Action Campaign. Available online: https://www.tac.org.za/our-history/ (accessed on 29 January 2022).
- World Health Organisation. Fourth WHO report on neglected tropical diseases. In Integrating Neglected Tropical Diseases into Global Health and Development; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Kjetland, E.F.; Ndhlovu, P.D.; Gomo, E.; Mduluza, T.; Midzi, N.; Gwanzura, L.; Mason, P.R.; Sandvik, L.; Friis, H.; Gundersen, S.G. Association between genital schistosomiasis and HIV in rural Zimbabwean women. Aids 2006, 20, 593–600. [Google Scholar] [CrossRef] [Green Version]
- Mbabazi, P.S.; Andan, O.; Fitzgerald, D.W.; Chitsulo, L.; Engels, D.; Downs, J.A. Examining the relationship between urogenital schistosomiasis and HIV infection. PLoS Negl. Trop. Dis. 2011, 5, e1396. [Google Scholar] [CrossRef] [Green Version]
- Downs, J.A.; Mguta, C.; Kaatano, G.M.; Mitchell, K.B.; Bang, H.; Simplice, H.; Kalluvya, S.E.; Changalucha, J.M.; Johnson, W.D.; Fitzgerald, D.W. Urogenital schistosomiasis in women of reproductive age in Tanzania’s Lake Victoria region. Am. J. Trop. Med. Hyg. 2011, 84, 364–369. [Google Scholar] [CrossRef] [Green Version]
- Ndeffo Mbah, M.L.; Skrip, L.; Greenhalgh, S.; Hotez, P.; Galvani, A.P. Impact of Schistosoma mansoni on malaria transmission in Sub-Saharan Africa. PLoS Negl. Trop. Dis. 2014, 8, e3234. Available online: http://search.ebscohost.com/login.aspx?direct=true&db=mnh&AN=25329403&site=ehost-live (accessed on 19 March 2018). [CrossRef] [PubMed] [Green Version]
- Bardach, E.S.; Patashnik, E.M. A Practical Guide for Policy Analysis: The Eightfold Path to More Effective Problem Solving, 6th ed.; CQ Press: Washington, DC, USA, 2019; pp. 1–216. Available online: https://us.sagepub.com/en-us/nam/a-practical-guide-for-policy-analysis/book255357 (accessed on 6 May 2021).
- Bergquist, R.; Zhou, X.N.; Rollinson, D.; Reinhard-Rupp, J.; Klohe, K. Elimination of schistosomiasis: The tools required. Infect. Dis. Poverty 2017, 6, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engels, D.; Wang, L.-Y.Y.; Palmer, K.L. Control of schistosomiasis in China. Acta Trop. 2005, 96, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.G.P.; Li, Y.S.; Sleigh, A.C.; McManus, D.P. Schistosomiasis control in the People’s Republic of China. Parasitol. Today 1997, 13, 152–155. [Google Scholar] [CrossRef]
- Xu, J.; Li, S.; Zhang, L.; Bergquist, R.; Dang, H.; Wang, Q.; Lv, S.; Wang, T.; Lin, D.; Liu, J.; et al. Surveillance-based evidence: Elimination of schistosomiasis as a public health problem in the Peoples’ Republic of China. Infect. Dis. Poverty 2020, 9, 39–50. [Google Scholar] [CrossRef]
- Wang, W.; Bergquist, R.; King, C.H.; Yang, K. Elimination of schistosomiasis in china: Current status and future prospects. PLoS Negl. Trop. Dis. 2021, 15, e0009578. [Google Scholar] [CrossRef]
- Xianyi, C.; Liying, W.; Jiming, C.; Xiaonong, Z.; Jiang, Z.; Jiagang, G.; Xiaohua, W.; Engels, D.; Minggang, C. Schistosomiasis control in China: The impact of a 10-year World Bank Loan Project (1992–2001). Bull. World Health Organ. 2005, 83, 43–48. [Google Scholar]
- World Health Organisation. WHO|Helminth Control in School Age Children: A Guide for Managers of Control Programmes; World Health Organization: Geneva, Switzerland, 2016; Available online: https://www.who.int/neglected_diseases/resources/9789241548267/en/ (accessed on 24 May 2019).
- Baan, M.; Galappaththi-Arachchige, H.N.; Gagai, S.; Aurlund, C.G.; Vennervald, B.J.; Taylor, M.; van Lieshout, L.; Kjetland, E.F. The Accuracy of Praziquantel Dose Poles for Mass Treatment of Schistosomiasis in School Girls in KwaZulu-Natal, South Africa. PLoS Negl. Trop. Dis. 2016, 10, e0004623. [Google Scholar] [CrossRef]
- Randjelovic, A.; Frønæs, S.G.; Munsami, M.; Kvalsvig, J.D.; Zulu, S.G.; Gagai, S.; Maphumulo, A.; Sandvik, L.; Gundersen, S.G.; Kjetland, E.F.; et al. A study of hurdles in mass treatment of schistosomiasis in KwaZulu-Natal, South Africa. S. Afri. Fam. Pract. 2015, 57, 57–61. [Google Scholar] [CrossRef]
- Corstjens, P.; de Dood, C.J.; Knopp, S.; Clements, M.N.; Ortu, G.; Umulisa, I.; Ruberanziza, E.; Wittmann, U.; Kariuki, T.; LoVerde, P.; et al. Circulating Anodic Antigen (CAA): A Highly Sensitive Diagnostic Biomarker to Detect Active Schistosoma Infections-Improvement and Use during SCORE. Am. J. Trop. Med. Hyg. 2020, 103, 50–57. [Google Scholar] [CrossRef]
- Sousa, M.S.; van Dam, G.J.; Pinheiro, M.C.C.; de Dood, C.J.; Peralta, J.M.; Peralta, R.H.S.; Daher, E.F.; Corstjens, P.; Bezerra, F.S.M. Performance of an Ultra-Sensitive Assay Targeting the Circulating Anodic Antigen (CAA) for Detection of Schistosoma mansoni Infection in a Low Endemic Area in Brazil. Front. Immunol. 2019, 10, 682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kvalsvig, J.D.; Schutte, C.H. The role of human water contact patterns in the transmission of schistosomiasis in an informal settlement near a major industrial area. Ann. Trop. Med. Parasitol. 1986, 80, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Sharfi, A.R.; Hassan, O. Evaluation of haematuria in Khartoum. East Afr. Med. J. 1994, 71, 29–31. [Google Scholar]
- Ernould, J.C.; Kaman, A.; Labbo, R.; Couret, D.; Chippaux, J.P. Recent urban growth and urinary schistosomiasis in Niamey, Niger. Trop. Med. Int. Health 2000, 5, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.L.; Appleton, C.C. Urban schistosomiasis transmission in Pietermaritzburg, South Africa. S. Afr. J. Epidemiol. Infect. 2005, 20, 103–107. [Google Scholar] [CrossRef] [Green Version]
- Agbolade, O.M.; Agu, N.C.; Adesanya, O.O.; Odejayi, A.O.; Adigun, A.A.; Adesanlu, E.B.; Ogunleye, F.G.; Sodimu, A.O.; Adeshina, S.A.; Bisiriyu, G.O.; et al. Intestinal helminthiases and schistosomiasis among school children in an urban center and some rural communities in southwest Nigeria. Korean J. Parasitol. 2007, 45, 233–238. [Google Scholar] [CrossRef]
- Kloos, H.; Correa-Oliveira, R.; dos Reis, D.C.; Rodrigues, E.W.; Monteiro, L.A.; Gazzinelli, A. The role of population movement in the epidemiology and control of schistosomiasis in Brazil: A preliminary typology of population movement. Memórias Do Inst. Oswaldo Cruz 2010, 105, 578–586. [Google Scholar] [CrossRef]

| Year of Study | Study Area | Province | Number of Schools (Total Number of Participants) | Prevalence of S. haematobium | Prevalence of S. mansoni |
Mean Intensity of S. haematobium Infection (Eggs/ 10 mL Urine) |
|---|---|---|---|---|---|---|
| 2009 | Ugu District [9] | KwaZulu-Natal | 5 (Not reported (NR)) | ≥50% | NR | NR |
| 2009–2010 | Ugu District [26] | KwaZulu-Natal | 15 (726) a | 36.9% | 0% | 20 (range from 1–624) |
| 2009–2010 | Ugu District [7] | KwaZulu-Natal | 18 (970) | 32% | 0% | 52 |
| 2010 | Ugu District [27] | KwaZulu-Natal | 18 (1057) | 32% | 0% | 52 |
| 2010 2011 | Ugu District b,f [13] | KwaZulu-Natal | 18 (108) 18 (76) | 38% and 29.6% c 13.2% f and 11.8% c | NR | 18.4 b and 14.9 c 9 b and 12.9 c |
| 2011 | Ugu District [9] | KwaZulu-Natal | 9 (NR)– | 10–49% | NR | NR |
| 2011 | Ugu District [9] | KwaZulu-Natal | 2 (NR) | <10% | NR | NR |
| 2011 | Ugu, iLembe, and Southern uThungulu (King Cetshwayo) districts [9] | KwaZulu-Natal | 2 (NR) | ≥50% | NR | NR |
| 2011 | Ugu, iLembe, and Southern uThungulu (King Cetshwayo) districts [9] | KwaZulu-Natal | 47 (NR) | 10–49% | NR | NR |
| 2011 | Ugu, iLembe, and Southern uThungulu (King Cetshwayo) districts [9] | KwaZulu-Natal | 13 (NR) | <10% | NR | NR |
| 2011–2013 | iLlembe, Ugu and uThungulu (King Cetshwayo) districts [5] | KwaZulu-Natal | NR (1123) | 26.0% | NR | |
| 2012 | Ugu District [28] | KwaZulu-Natal | 3 (246) and 9 d (873) | 20.4% | NR | 14 |
| 2014 | uMkhanyakude (Jozini Municipality) [29] | KwaZulu-Natal | 10 (420) | 40.2% | NR | NR |
| 2015 | uMkhanyakude District (Ndumo) [30] | KwaZulu-Natal | 10 (320) | 37.5% | NR | NR |
| 2019 | uMkhanyakude District [31] | KwaZulu-Natal | 34 (1143) e | 1.0% | 0.9% | 30.4 |
| 2004 2005 2005 2005 2005 2005 | Vhembe District [32] | Limpopo | NR | >70% f | NR | NR |
| 2005 | Vhembe District [32] | Limpopo | 1 (94) | 36.2% g | NR | NR |
| 2005 | Vhembe District [32] | Limpopo | NR (148) | 42% h | NR | NR |
| 2005 | Vhembe District [32] | Limpopo | 1 (247) | 86% e | NR | NR |
| 2005 | Vhembe District [32] | Limpopo | 1 (191) | 84% e | NR | NR |
| 2005 | Vhembe District [32] | Limpopo | 1 (138) | 78.2% e | NR | NR |
| 2009–2013 | Rob Ferreira Hospital Patients [33] | Mpumalanga | 304 i | 10.2% | NR | NR |
| Policy Actions | Strategic Activities | Stakeholder at the Forefront with Both High Power and High Interest a | Other Stakeholders and Their Interest |
|---|---|---|---|
| Action A: FGS screening |
|
|
|
| Action B: Treatment for Schistosomiasis |
|
|
|
| Action C: Vector Control |
|
|
|
| Action D: Water, Sanitation and Hygiene (WASH) |
|
|
|
| Action E: Creating Awareness of Schistosomiasis and FGS |
|
|
|
| Action | Criteria | |||
|---|---|---|---|---|
| Public Health Impact (Efficacy) | Ethics/Equity | Administrative Feasibility | Budgetary Feasibility | |
| A. Prevention and increased access to treatment | High | High | High | High |
| B. FGS diagnosis | High | High | High | High |
| C. Vector control | High | Medium | Medium | Medium |
| D. Water, sanitation and hygiene (WASH) | High | High | Low | Low |
| E. Creating awareness | High | Medium | High | High |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemungadi, T.G.; Furumele, T.E.; Gugerty, M.K.; Djirmay, A.G.; Naidoo, S.; Kjetland, E.F. Establishing and Integrating a Female Genital Schistosomiasis Control Programme into the Existing Health Care System. Trop. Med. Infect. Dis. 2022, 7, 382. https://doi.org/10.3390/tropicalmed7110382
Nemungadi TG, Furumele TE, Gugerty MK, Djirmay AG, Naidoo S, Kjetland EF. Establishing and Integrating a Female Genital Schistosomiasis Control Programme into the Existing Health Care System. Tropical Medicine and Infectious Disease. 2022; 7(11):382. https://doi.org/10.3390/tropicalmed7110382
Chicago/Turabian StyleNemungadi, Takalani Girly, Tsakani Ernica Furumele, Mary Kay Gugerty, Amadou Garba Djirmay, Saloshni Naidoo, and Eyrun Flörecke Kjetland. 2022. "Establishing and Integrating a Female Genital Schistosomiasis Control Programme into the Existing Health Care System" Tropical Medicine and Infectious Disease 7, no. 11: 382. https://doi.org/10.3390/tropicalmed7110382
APA StyleNemungadi, T. G., Furumele, T. E., Gugerty, M. K., Djirmay, A. G., Naidoo, S., & Kjetland, E. F. (2022). Establishing and Integrating a Female Genital Schistosomiasis Control Programme into the Existing Health Care System. Tropical Medicine and Infectious Disease, 7(11), 382. https://doi.org/10.3390/tropicalmed7110382








