Nano-Encapsulated Melatonin: A Promising Mucosal Adjuvant in Intranasal Immunization against Chronic Experimental T. gondii Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Parasite
2.2. Antigen Preparation
2.3. Preparation of Plain and MLT-SLNs
2.4. Characterization of the Prepared Nanoparticles
2.4.1. Colloidal Characterization
2.4.2. Morphological Characterization
2.4.3. Determination of Entrapment Efficiency and Adjuvant Loading
2.4.4. In Vitro Release Study and Release Kinetics
2.4.5. Determination of the Stability of the Prepared RA-SLNs
2.5. Animal Grouping and Vaccination Schedule
| Group I Control group | Each mouse received sterile PBS. |
| Group II MLT-SLNs group | Each mouse received 100 mg/kg/dose of MLT in a dispersion of SLNs [28]. |
| Group III TLA group | Each mouse received 20 µg/ dose of TLA in PBS [7] |
| Group IV TLA/plain SLNs group | Each mouse received 20 µg/ dose of TLA in PBS and 130 µg lipid/dose of plain SLNs [29] |
| Group V TLA/MLT-SLNs group | Each mouse received a suspension containing 20 µg/dose of TLA in PBS and 100 mg/kg/dose of MLT in a dispersion of SLNs. |
2.6. Vaccine Evaluation
2.6.1. Parasitological Study
Tachyzoite Invasion and Replication Assay
Brain Cyst Count and Size
Ultrastructural Changes
2.6.2. Immunological Study
Measurement of Total IgG in Serum
Measurement of Secretory IgA in the Intestinal Wash
Measurement of the Interferon-Gamma (IFN γ) Level
2.6.3. Biochemical Study (Oxidative Stress Biomarker)
Superoxide Dismutase (SOD)
Malondialdehyde (MDA) (Lipid Peroxide) Assay
Total Antioxidant Capacity Assay (TAC)
2.7. Histopathological Examination
2.8. Statistical Analysis of the Data
3. Results
3.1. Characterization of Plain and Adjuvant-Loaded SLNs
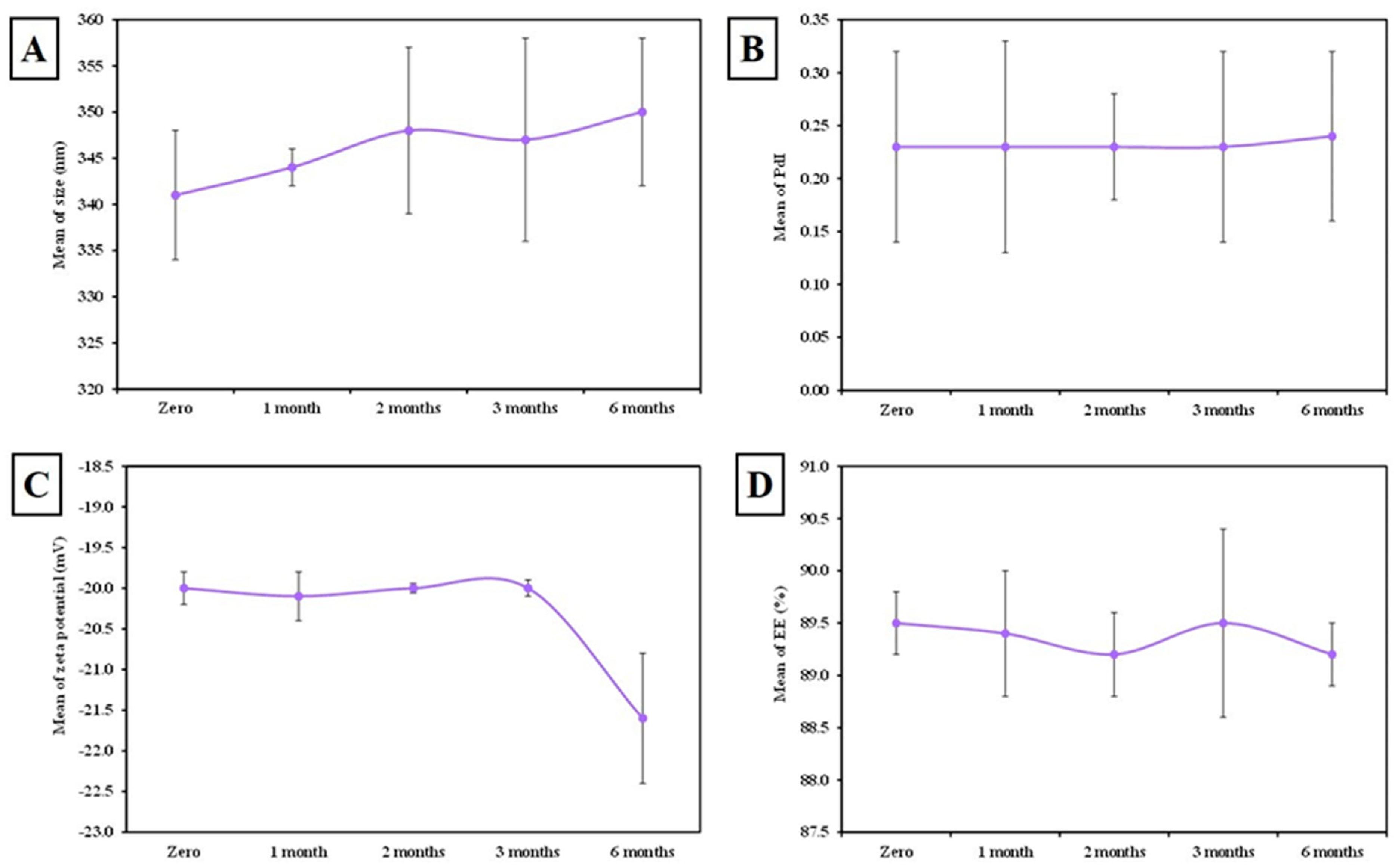
3.1.1. Colloidal Characterization (Figure 2)

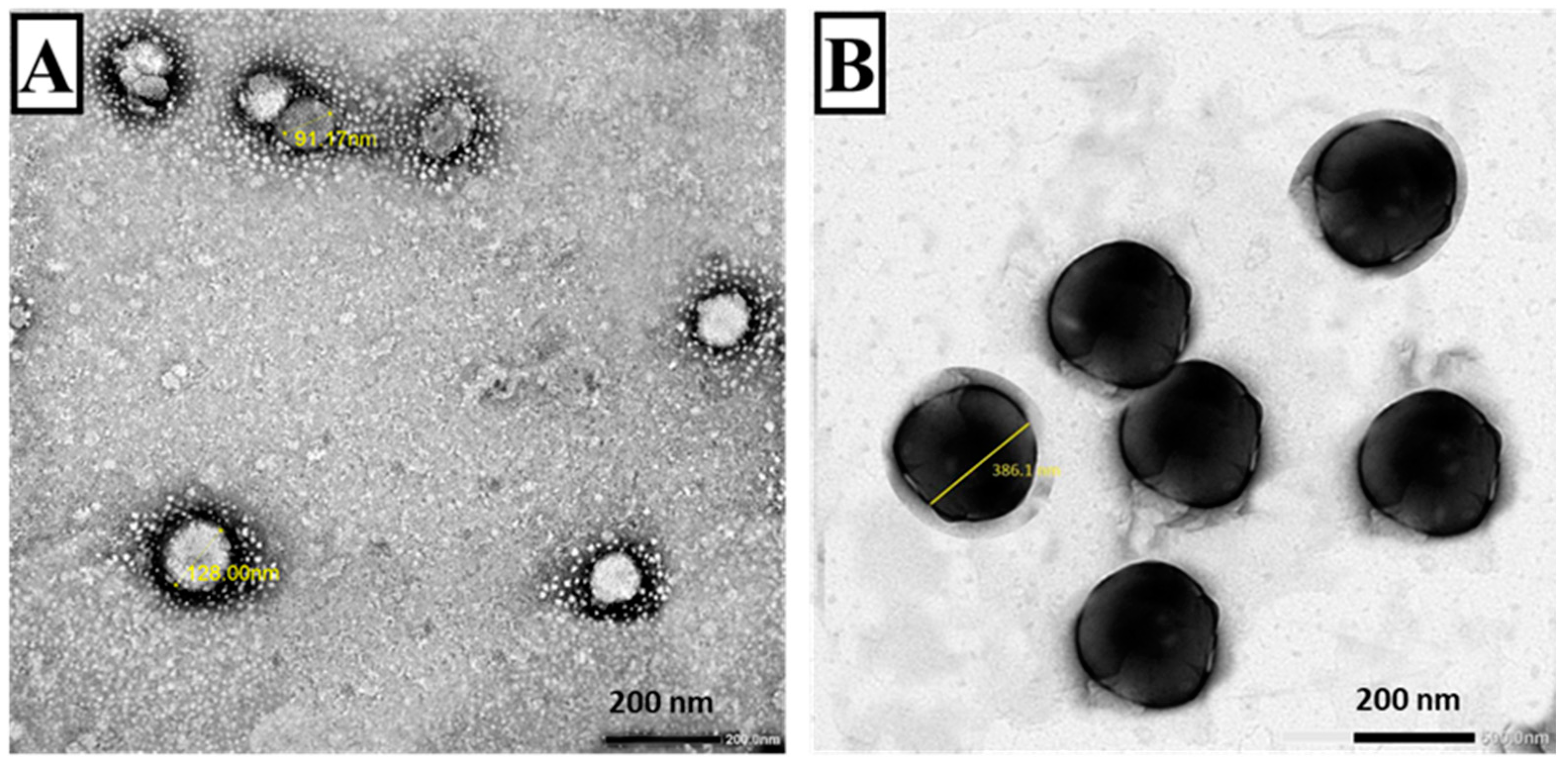
3.1.2. TEM (Figure 3)
3.1.3. Entrapment Efficiency and Adjuvant Loading
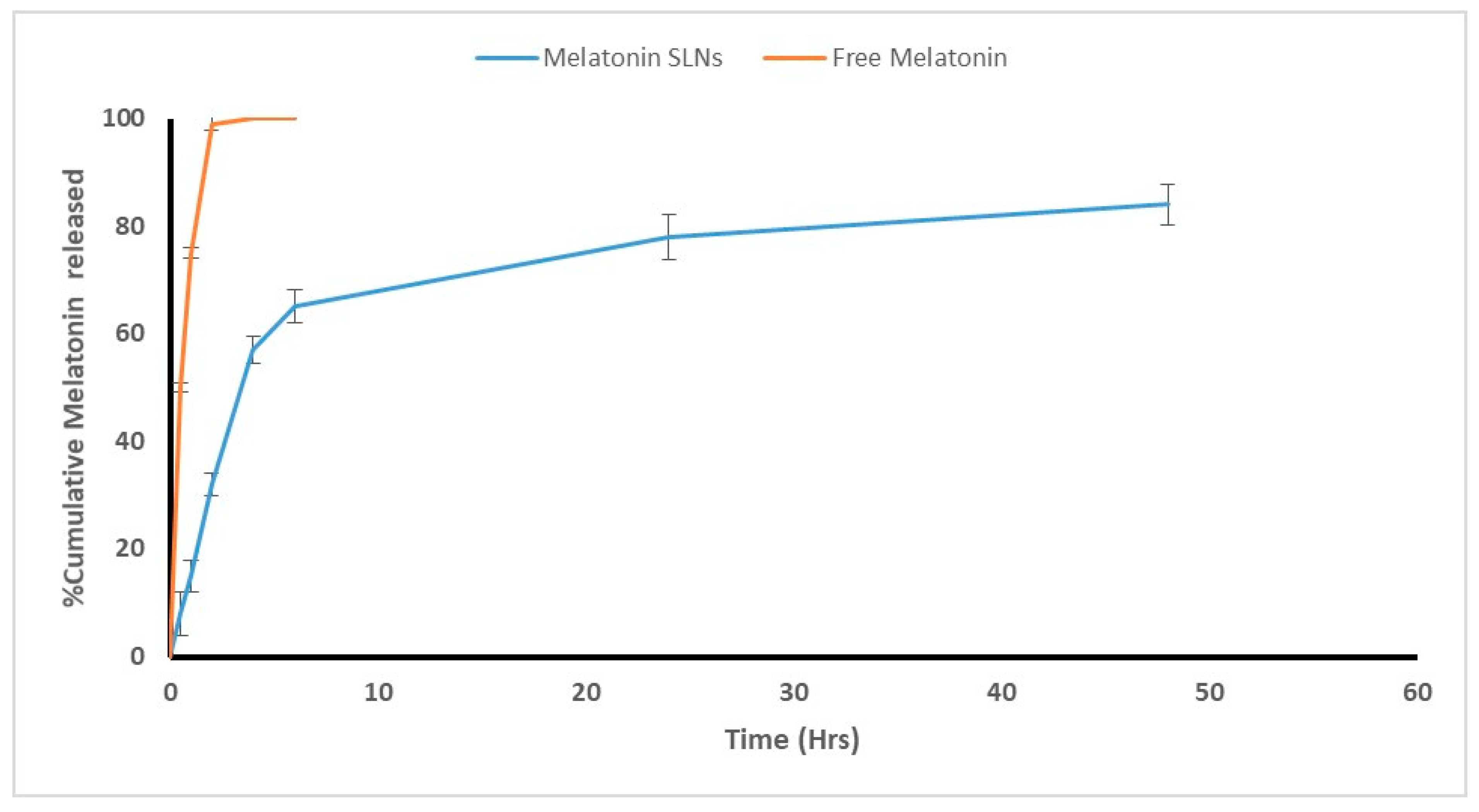
3.1.4. In Vitro Adjuvant Release
3.1.5. The Stability of MLT-Loaded SLNs
3.2. Vaccine Evaluation
3.2.1. Parasitological Study
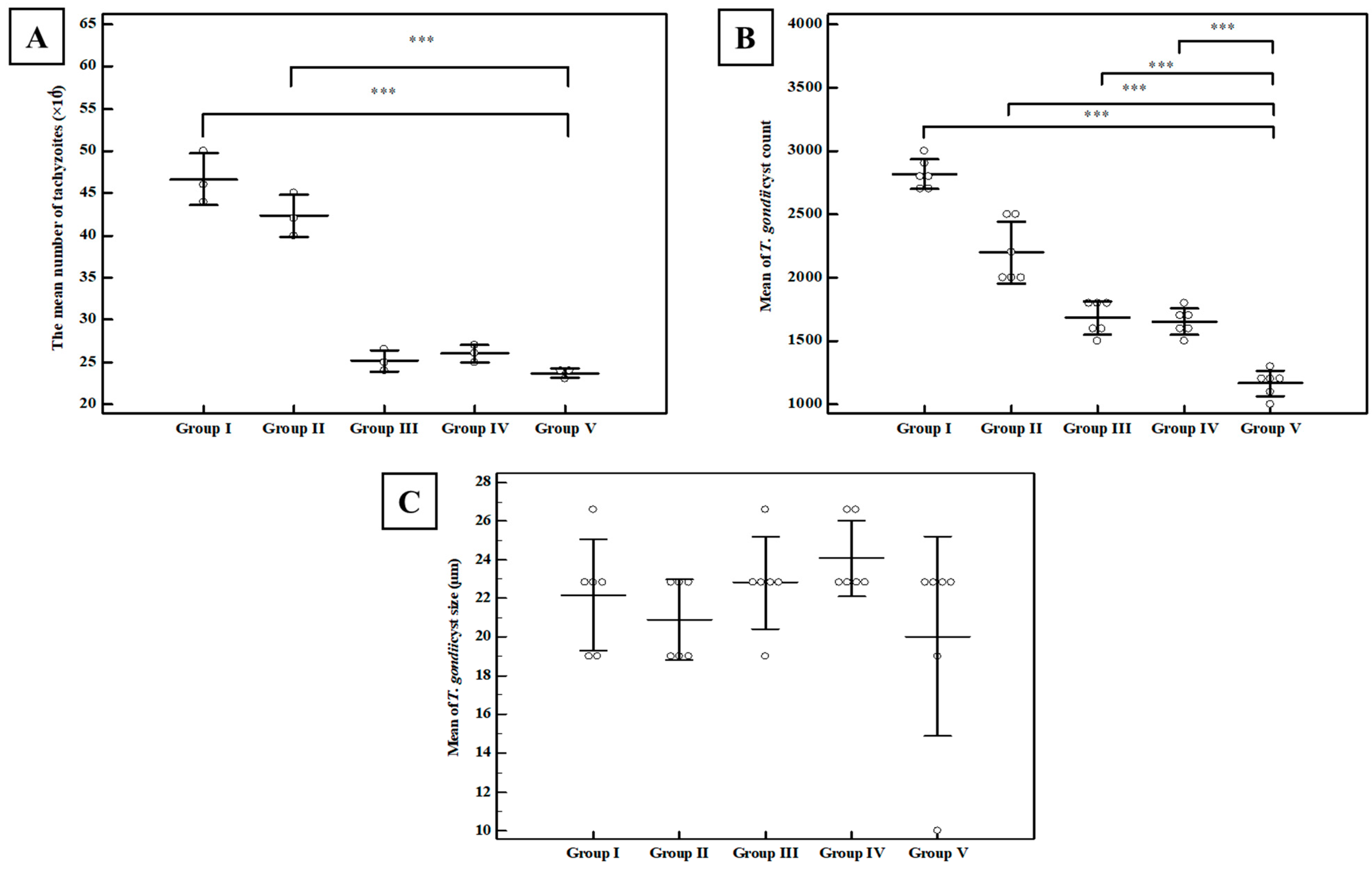
Tachyzoite Invasion and Replication Assay
Brain Cyst Count and Cyst Size
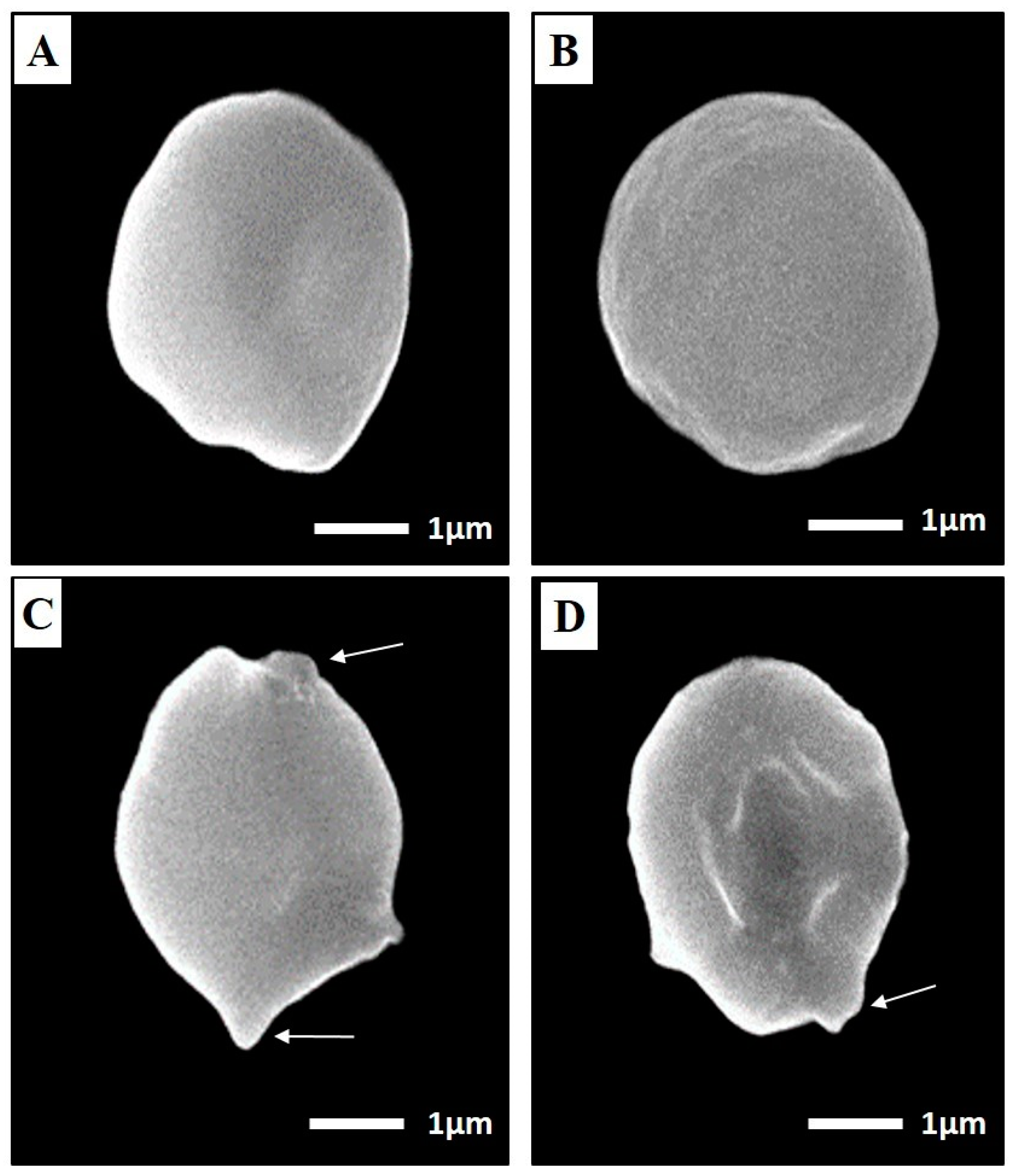
SEM of Toxoplasma Cyst
3.2.2. Immunological Results
Total Ig G in Serum
Secretory IgA in the Intestinal Wash
Interferon-Gamma (IFN γ) Level
3.2.3. Biochemical Results
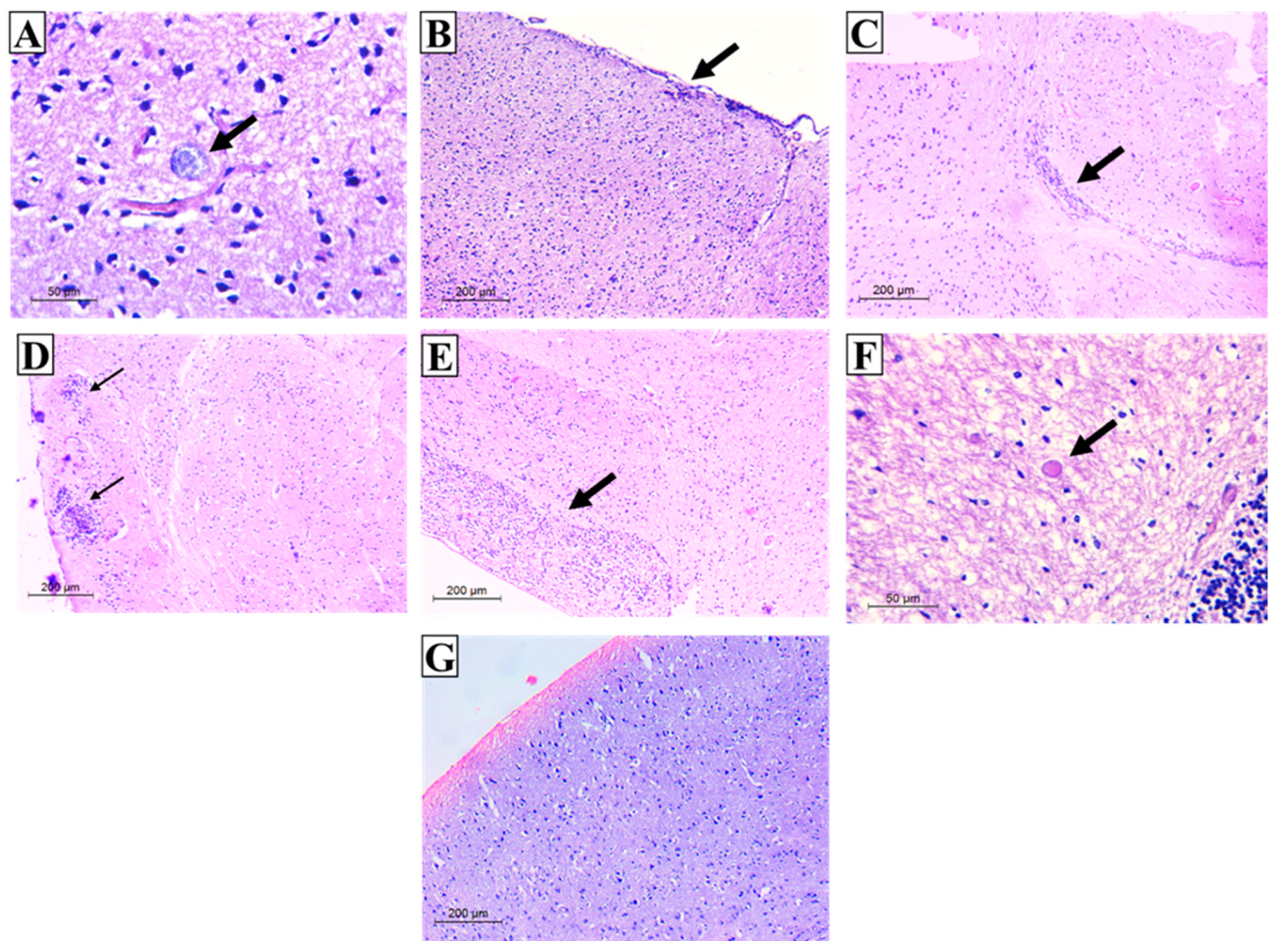
3.3. Histopathological Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Milne, G.; Webster, J.P.; Walker, M. Toxoplasma gondii: An Underestimated Threat? Trends Parasitol. 2020, 36, 959–969. [Google Scholar] [CrossRef]
- Jones, J.L.; Dubey, J.P. Foodborne Toxoplasmosis. Clin. Infect. Dis. 2012, 55, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-B.; Wang, J.-L.; Gui, Q.; Zou, Y.; Chen, K.; Liu, Q.; Liang, Q.-L.; Zhu, X.-Q.; Zhou, D.-H. Immunization with a Live-Attenuated RH:ΔNPT1 Strain of Toxoplasma gondii Induces Strong Protective Immunity Against Toxoplasmosis in Mice. Front. Microbiol. 2019, 10, 1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boog, C.J. Principles of vaccination and possible development strategies for rational design. Immunol. Lett. 2009, 122, 104–107. [Google Scholar] [CrossRef]
- El-Malky, M.; Shaohong, L.; Kumagai, T.; Yabu, Y.; Noureldin, M.S.; Saudy, N.; Maruyama, H.; Ohta, N. Protective Effect of Vaccination with Toxoplasma lysate Antigen and CpG as an Adjuvant against Toxoplasma gondii in Susceptible C57BL/6 Mice. Microbiol. Immunol. 2005, 49, 639–646. [Google Scholar] [CrossRef]
- Wagner, A.; Schabussova, I.; Ruttkowski, B.; Peschke, R.; Kur, J.; Kundi, M.; Joachim, A.; Wiedermann, U. Prime-Boost Vaccination with Toxoplasma Lysate Antigen, but Not with a Mixture of Recombinant Protein Antigens, Leads to Reduction of Brain Cyst Formation in BALB/c Mice. PLoS ONE 2015, 10, e0126334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Malky, M.A.; Al-Harthi, S.A.; Mohamed, R.T.; El Bali, M.A.; Saudy, N.S. Vaccination with Toxoplasma lysate antigen and CpG oligodeoxynucleotides: Comparison of immune responses in intranasal versus intramuscular administrations. Parasitol. Res. 2014, 113, 2277–2284. [Google Scholar] [CrossRef]
- Daryani, A.; Hosseini, A.Z.; Dalimi, A. Immune responses against excreted/secreted antigens of Toxoplasma gondii tachyzoites in the murine model. Vet. Parasitol. 2003, 113, 123–134. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Wang, S. Effect of vaccine administration modality on immunogenicity and efficacy. Expert Rev. Vaccines 2015, 14, 1509–1523. [Google Scholar] [CrossRef] [Green Version]
- Pavot, V.; Rochereau, N.; Genin, C.; Verrier, B.; Paul, S. New insights in mucosal vaccine development. Vaccine 2012, 30, 142–154. [Google Scholar] [CrossRef]
- Otczyk, D.C.; Cripps, A.W. Mucosal immunization: A realistic alternative. Hum. Vaccines 2010, 6, 978–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, D.J.M.; Huo, Z.; Barnett, S.; Kromann, I.; Giemza, R.; Galiza, E.; Woodrow, M.; Thierry-Carstensen, B.; Andersen, P.; Novicki, D.; et al. Transient Facial Nerve Paralysis (Bell’s Palsy) following Intranasal Delivery of a Genetically Detoxified Mutant of Escherichia coli Heat Labile Toxin. PLoS ONE 2009, 4, e6999. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Medina-Fatimi, A.; Nichols, R.; Tendler, D.; Michetti, M.; Simon, J.; Kelly, C.P.; Monath, T.P. Safety and efficacy of low dose Escherichia coli enterotoxin adjuvant for urease based oral immunisation against Helicobacter pylori in healthy volunteers. Gut 2002, 51, 634–640. [Google Scholar] [CrossRef] [Green Version]
- Thompson, A.L.; Staats, H.F. Cytokines: The Future of Intranasal Vaccine Adjuvants. J. Immunol. Res. 2011, 2011, 289597. [Google Scholar] [CrossRef] [Green Version]
- Ajith, Y.; Dimri, U.; Dixit, S.K.; Singh, S.K.; Gopalakrishnan, A.; Madhesh, E.; Rajesh, J.B.; Sangeetha, S.G. Immunomodulatory basis of antioxidant therapy and its future prospects: An appraisal. Inflammopharmacology 2017, 25, 487–498. [Google Scholar] [CrossRef]
- Motta-Teixeira, L.C.; Machado-Nils, A.V.; Battagello, D.S.; Diniz, G.B.; Andrade-Silva, J.; Silva, S., Jr.; Matos, R.A.; Amaral, F.G.D.; Xavier, G.F.; Bittencourt, J.C.; et al. The absence of maternal pineal melatonin rhythm during pregnancy and lactation impairs offspring physical growth, neurodevelopment, and behavior. Horm. Behav. 2018, 105, 146–156. [Google Scholar] [CrossRef]
- Regodón, S.; Ramos, A.; Morgado, S.; Tarazona, R.; Martín-Palomino, P.; Rosado, J.A.; del Prado Míguez, M. Melatonin enhances the immune response to vaccination against A1 and C strains of Dichelobacter nodosus. Vaccine 2009, 27, 1566–1570. [Google Scholar] [CrossRef]
- De Muro, R.L.; Nafziger, A.N.; Blask, D.E.; Menhinick, A.M.; Bertino, J.S., Jr. The absolute bioavailability of oral melatonin. J. Clin. Pharmacol. 2000, 40, 781–784. [Google Scholar] [CrossRef]
- Hafner, A.; Lovrić, J.; Voinovich, D.; Filipović-Grcić, J. Melatonin-loaded lecithin/chitosan nanoparticles: Physicochemical characterisation and permeability through Caco-2 cell monolayers. Int. J. Pharm. 2009, 381, 205–213. [Google Scholar] [CrossRef]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Schöler, N.; Krause, K.; Kayser, O.; Müller, R.H.; Borner, K.; Hahn, H.; Liesenfeld, O. Atovaquone Nanosuspensions Show Excellent Therapeutic Effect in a New Murine Model of Reactivated Toxoplasmosis. Antimicrob. Agents Chemother. 2001, 45, 1771–1779. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Makled, S.; Nafee, N.; Boraie, N. Nebulized solid lipid nanoparticles for the potential treatment of pulmonary hypertension via targeted delivery of phosphodiesterase-5-inhibitor. Int. J. Pharm. 2017, 517, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Eissa, M.M.; El-Moslemany, R.M.; Ramadan, A.A.; Amer, E.I.; El-Azzouni, M.Z.; El-Khordagui, L.K. Miltefosine Lipid Nanocapsules for Single Dose Oral Treatment of Schistosomiasis Mansoni: A Preclinical Study. PLoS ONE 2015, 10, e0141788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, Y.-C.; Chung, J.-F. Physicochemical properties of nevirapine-loaded solid lipid nanoparticles and nanostructured lipid carriers. Colloids Surf. B Biointerfaces 2011, 83, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, L.; Dong, Z.; Xie, S.; Chen, X.; Lu, M.; Wang, X.; Li, X.; Zhou, W. Preparation and stability study of norfloxacin-loaded solid lipid nanoparticle suspensions. Colloids Surf. B Biointerfaces 2012, 98, 105–111. [Google Scholar] [CrossRef]
- Baghban Rahimi, S.; Mohebbi, A.; Vakilzadeh, G.; Biglari, P.; Razeghi Jahromi, S.; Mohebi, S.R.; Shirian, S.; Gorji, A.; Ghaemi, A. Enhancement of therapeutic DNA vaccine potency by melatonin through inhibiting VEGF expression and induction of antitumor immunity mediated by CD8+ T cells. Arch. Virol. 2018, 163, 587–597. [Google Scholar] [CrossRef]
- Shirai, S.; Kawai, A.; Shibuya, M.; Munakata, L.; Omata, D.; Suzuki, R.; Yoshioka, Y. Lipid Nanoparticle Acts as a Potential Adjuvant for Influenza Split Vaccine without Inducing Inflammatory Responses. Vaccines 2020, 8, 433. [Google Scholar] [CrossRef]
- Sánchez, V.R.; Fenoy, I.M.; Picchio, M.S.; Soto, A.; Arcon, N.; Goldman, A.; Martin, V. Homologous prime-boost strategy with TgPI-1 improves the immune response and protects highly susceptible mice against chronic Toxoplasma gondii infection. Acta Trop. 2015, 150, 159–165. [Google Scholar] [CrossRef]
- Eissa, M.M.; Barakat, A.M.; Amer, E.I.; Younis, L.K. Could miltefosine be used as a therapy for toxoplasmosis? Exp. Parasitol. 2015, 157, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Draper, H.H.; Hadley, M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musumeci, G. Past, present and future: Overview on histology and histopathology. J. Histol. Histopathol. 2014, 1, 5. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, C.M.; Plotkin, S.A. Impact of Vaccines; Health, Economic and Social Perspectives. Front. Microbiol. 2020, 11, 1526. [Google Scholar] [CrossRef] [PubMed]
- Facciolà, A.; Visalli, G.; Laganà, P.; La Fauci, V.; Squeri, R.; Pellicanò, G.F.; Nunnari, G.; Trovato, M.; Di Pietro, A. The new era of vaccines: The “nanovaccinology”. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7163–7182. [Google Scholar] [CrossRef]
- Gowder, S. Antioxidants and Vaccines. Int. J. Vaccines Vaccin. 2016, 2, 00020. [Google Scholar] [CrossRef] [Green Version]
- Mwanza-Lisulo, M.; Kelly, P. Potential for use of retinoic acid as an oral vaccine adjuvant. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140145. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Yang, G.-H.; Zhang, L.-L.; Wang, J.; Wang, J.-F. Melatonin as Immune Potentiator for Enhancing Subunit Vaccine Efficacy against Bovine Viral Diarrhea Virus. Vaccines 2021, 9, 1039. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Gupta, S.; Narang, R.K.; Budhiraja, R.D. Amoxicillin Loaded Chitosan–Alginate Polyelectrolyte Complex Nanoparticles as Mucopenetrating Delivery System for H. Pylori. Sci. Pharm. 2011, 79, 673–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emami, J.; Mohiti, H.; Hamishehkar, H.; Varshosaz, J. Formulation and optimization of solid lipid nanoparticle formulation for pulmonary delivery of budesonide using Taguchi and Box-Behnken design. Res. Pharm. Sci. 2015, 10, 17–33. [Google Scholar] [PubMed]
- Pathak, P.; Nagarsenker, M. Formulation and Evaluation of Lidocaine Lipid Nanosystems for Dermal Delivery. AAPS PharmSciTech 2009, 10, 985–992. [Google Scholar] [CrossRef]
- Ding, Y.; Nielsen, K.A.; Nielsen, B.P.; Boje, N.W.; Muller, R.H.; Pyo, S.M. Lipid-drug-conjugate (LDC) solid lipid nanoparticles (SLN) for the delivery of nicotine to the oral cavity—Optimization of nicotine loading efficiency. Eur. J. Pharm. Biopharm. 2018, 128, 10–17. [Google Scholar] [CrossRef]
- Wiśniewska, M. The temperature effect on electrokinetic properties of the silica–polyvinyl alcohol (PVA) system. Colloid Polym. Sci. 2011, 289, 341–344. [Google Scholar] [CrossRef] [Green Version]
- Charão, M.F.; Baierle, M.; Gauer, B.; Goethel, G.; Fracasso, R.; Paese, K.; Brucker, N.; Moro, A.M.; Bubols, G.B.; Dias, B.B.; et al. Protective effects of melatonin-loaded lipid-core nanocapsules on paraquat-induced cytotoxicity and genotoxicity in a pulmonary cell line. Mutat. Res. Toxicol. Environ. Mutagen. 2015, 784–785, 1–9. [Google Scholar] [CrossRef]
- Shokrzadeh, M.; Ghassemi-Barghi, N. Melatonin Loading Chitosan-Tripolyphosphate Nanoparticles: Application in Attenuating Etoposide-Induced Genotoxicity in HepG2 Cells. Pharmacology 2018, 102, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Yang, Q.; Bagby, T.R.; Forrest, M.L. Lymphatic drug delivery using engineered liposomes and solid lipid nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 901–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, M.; Pathak, K. Development and statistical optimization of solid lipid nanoparticles of simvastatin by using 2(3) full-factorial design. AAPS PharmSciTech 2010, 11, 489–496. [Google Scholar] [CrossRef] [Green Version]
- Ekambaram, P.; Sathali, A.A. Formulation and evaluation of solid lipid nanoparticles of ramipril. J. Young Pharm. 2011, 3, 216–220. [Google Scholar] [CrossRef] [Green Version]
- Gaur, P.K.; Mishra, S.; Bajpai, M.; Mishra, A. Enhanced oral bioavailability of efavirenz by solid lipid nanoparticles: In vitro drug release and pharmacokinetics studies. Biomed. Res. Int. 2014, 2014, 363404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.D.A.; Jain, A.; Pandit, J.K.; Chakraborty, S. Formulation and evaluation of solid lipid nanoparticles of a water soluble drug: Zidovudine. Chem. Pharm. Bull. 2010, 58, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, I.; Garg, R.; van Drunen Littel-van den Hurk, S. Selection of adjuvants for vaccines targeting specific pathogens. Expert Rev. Vaccines 2019, 18, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.P.B.S.; Moreira, J.N.; Silva, R.; Almeida, H.; Sousa Lobo, J.M.; Silva, A.C. In Vitro Studies on Nasal Formulations of Nanostructured Lipid Carriers (NLC) and Solid Lipid Nanoparticles (SLN). Pharmaceuticals 2021, 14, 711. [Google Scholar] [CrossRef] [PubMed]
- Foroutan, M.; Ghaffarifar, F.; Sharifi, Z.; Dalimi, A.; Jorjani, O. Rhoptry antigens as Toxoplasma gondii vaccine target. Clin. Exp. Vaccine Res. 2019, 8, 4–26. [Google Scholar] [CrossRef]
- Garcia, J.L.; Innes, E.A.; Katzer, F. Current progress toward vaccines against Toxoplasma gondii. Vaccine 2014, 4, 23–37. [Google Scholar] [CrossRef] [Green Version]
- Schijns, V.E.; Lavelle, E.C. Trends in vaccine adjuvants. Expert Rev. Vaccines 2011, 10, 539–550. [Google Scholar] [CrossRef]
- Chen, K.; Cerutti, A. Vaccination strategies to promote mucosal antibody responses. Immunity 2010, 33, 479–491. [Google Scholar] [CrossRef] [Green Version]
- Mack, D.G.; McLeod, R. Human Toxoplasma gondii-specific secretory immunoglobulin A reduces T. gondii infection of enterocytes in vitro. J. Clin. Investig. 1992, 90, 2585–2592. [Google Scholar] [CrossRef] [Green Version]
- Mineo, J.R.; McLeod, R.; Mack, D.; Smith, J.; Khan, I.A.; Ely, K.H.; Kasper, L.H. Antibodies to Toxoplasma gondii major surface protein (SAG-1, P30) inhibit infection of host cells and are produced in murine intestine after peroral infection. J. Immunol. 1993, 150, 3951–3964. [Google Scholar]
- Sayles, P.C.; Gibson, G.W.; Johnson, L.L. B Cells Are Essential for Vaccination-Induced Resistance to Virulent Toxoplasma gondii. Infect. Immun. 2000, 68, 1026–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Yin, H.; Li, Y.; Zhao, L.; Sun, X.; Cong, H. Vaccination with recombinant adenovirus expressing multi-stage antigens of Toxoplasma gondii by the mucosal route induces higher systemic cellular and local mucosal immune responses than with other vaccination routes. Parasite 2017, 24, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malvey, E.N.; Jenkins, M.; Mueller, D. Peripheral immune tolerance blocks clonal expansion but fails to prevent the differentiation of Th1 cells. J. Immunol. 1998, 161, 2168–2177. [Google Scholar]
- Sanchez, S.G.; Besteiro, S. The pathogenicity and virulence of Toxoplasma gondii. Virulence 2021, 12, 3095–3114. [Google Scholar] [CrossRef]
- Dardé, M.L. Genetic analysis of the diversity in Toxoplasma gondii. Ann. Ist. Super Sanita. 2004, 40, 57–63. [Google Scholar]
- Regodón, S.R.A.; Míguez, M.P.; Carrillo-Vico, A.; Rosado, J.A.; Jardín, I. Vaccination prepartum enhances the beneficial effects of melatonin on the immune response and reduces platelet responsiveness in sheep. BMC Vet. Res. 2012, 8, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.Z.; Gao, Q.; Wang, M.; Hou, J.L.; Zhang, F.K.; Hu, L.Y.; Zhu, X.Q. Protective Efficacy Against Acute and Chronic Toxoplasma gondii Infection Induced by Immunization With the DNA Vaccine TgDOC2C. Front. Microbiol. 2018, 9, 2965. [Google Scholar] [CrossRef] [Green Version]
- Ju, C.-H.; Chockalingam, A.; Leifer, C.A. Early Response of Mucosal Epithelial Cells during Toxoplasma gondii Infection. J. Immunol. 2009, 183, 7420–7427. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, M.; Pedron, T.; Tournebize, R.; Olivo-Marin, J.-C.; Sansonetti, P.J.; Phalipon, A. Anti-Inflammatory Role for Intracellular Dimeric Immunoglobulin A by Neutralization of Lipopolysaccharide in Epithelial Cells. Immunity 2003, 18, 739–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacPherson, A.J.; McCoy, K.D.; Johansen, F.-E.; Brandtzaeg, P. The immune geography of IgA induction and function. Mucosal Immunol. 2007, 1, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Cerutti, A.; Cols, M.; Gentile, M.; Cassis, L.; Barra, C.M.; He, B.; Puga, I.; Chen, K. Regulation of mucosal IgA responses: Lessons from primary immunodeficiencies. Ann. N. Y. Acad. Sci. 2011, 1238, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, H.; Zamri, N.B.; Sekine, S.; Fukuyama, Y.; Tokuhara, D.; Gilbert, R.S.; Fukuiwa, T.; Fujihashi, K.; Sata, T.; Tashiro, M.; et al. A novel combined adjuvant for nasal delivery elicits mucosal immunity to influenza in aging. Vaccine 2012, 30, 803–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimier-Poisson, I.; Aline, F.; Mévélec, M.-N.; Beauvillain, C.; Buzoni-Gatel, D.; Bout, D. Protective Mucosal Th2 Immune Response against Toxoplasma gondii by Murine Mesenteric Lymph Node Dendritic Cells. Infect. Immun. 2003, 71, 5254–5265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhardwaj, P.; Bhatia, E.; Sharma, S.; Ahamad, N.; Banerjee, R. Advancements in prophylactic and therapeutic nanovaccines. Acta Biomater. 2020, 108, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Yarovinsky, F. Innate immunity to Toxoplasma gondii infection. Nat. Rev. Immunol. 2014, 14, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, M.-A.; Olias, P.; Sibley, L.D. Toxoplasma Effectors Targeting Host Signaling and Transcription. Clin. Microbiol. Rev. 2017, 30, 615–645. [Google Scholar] [CrossRef] [Green Version]
- Martens, S.; Parvanova, I.; Zerrahn, J.; Griffiths, G.; Schell, G.; Reichmann, G.; Howard, J.C. Disruption of Toxoplasma gondii Parasitophorous Vacuoles by the Mouse p47-Resistance GTPases. PLoS Pathog. 2005, 1, e24. [Google Scholar] [CrossRef] [Green Version]
- Gigley, J.P.; Bhadra, R.; Khan, I.A. CD8 T Cells and Toxoplasma gondii: A New Paradigm. J. Parasitol. Res. 2011, 2011, 243796. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, A.; Hannah, R.; Lutshumba, J.; Ochiai, E.; Weiss, L.M.; Suzuki, Y. Penetration of CD8(+) Cytotoxic T Cells into Large Target, Tissue Cysts of Toxoplasma gondii, Leads to Its Elimination. Am. J. Pathol. 2019, 189, 1594–1607. [Google Scholar] [CrossRef] [Green Version]
- Bahrami, S.; Shahriari, A.; Tavalla, M.; Azadmanesh, S.; Hamidinejat, H. Blood Levels of Oxidant/Antioxidant Parameters in Rats Infected with Toxoplasma gondii. Oxidative Med. Cell. Longev. 2016, 2016, 8045969. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wuliji, O.; Li, W.; Jiang, Z.-G.; Ghanbari, H.A. Oxidative Stress and Neurodegenerative Disorders. Int. J. Mol. Sci. 2013, 14, 24438–24475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrillo-Vico, A.; Lardone, P.J.; Álvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J.M. Melatonin: Buffering the Immune System. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras, M.; Rubio, C.P.; De La Fuente, J.; Villar, M.; Merino, O.; Gualito, J.M.; Cerón, J.J. Changes in Serum Biomarkers of Oxidative Stress in Cattle Vaccinated with Tick Recombinant Antigens: A Pilot Study. Vaccines 2020, 9, 5. [Google Scholar] [CrossRef]
- Tomás-Zapico, C.; Coto-Montes, A. A proposed mechanism to explain the stimulatory effect of melatonin on antioxidative enzymes. J. Pineal Res. 2005, 39, 99–104. [Google Scholar] [CrossRef]
- Türkoğlu, Ş.A.; Yaman, K.; Orallar, H.; Camsari, C.; Karabork, S.; Ayaz, E. Acute toxoplasmosis and antioxidant levels in the liver, kidney and brain of rats. Ann. Parasitol. 2018, 64, 241–247. [Google Scholar] [PubMed]
- Watson, B.D. Evaluation of the concomitance of lipid peroxidation in experimental models of cerebral ischemia and stroke. Prog. Brain Res. 1993, 96, 69–95. [Google Scholar] [CrossRef] [PubMed]
- Ondrejkova, A.; Suli, J.; Harvanova, J.; Ondrejka, R.; Prokes, M. Antioxidative Protection of Squalene Adjuvant and Rabies Vaccine with Adjuvant. Biol. Pharm. Bull. 2017, 40, 1029–1034. [Google Scholar] [CrossRef] [Green Version]
- Karaman, U.; Celik, T.; Kiran, T.R.; Colak, C.; Daldal, N.U. Malondialdehyde, glutathione, and nitric oxide levels in Toxoplasma gondii seropositive patients. Korean J. Parasitol. 2008, 46, 293–295. [Google Scholar] [CrossRef] [Green Version]
- Fatollahzadeh, M.; Eskandarian, A.; Darani, H.Y.; Pagheh, A.S.; Ahmadpour, E. Evaluation of Th17 immune responses of recombinant DNA vaccine encoding GRA14 and ROP13 genes against Toxoplasma gondii in BALB/c mice. Infect. Genet. Evol. 2021, 96, 105150. [Google Scholar] [CrossRef]
- Morvaridzadeh, M.; Sadeghi, E.; Agah, S.; Nachvak, S.M.; Fazelian, S.; Moradi, F.; Persad, E.; Heshmati, J. Effect of melatonin supplementation on oxidative stress parameters: A systematic review and meta-analysis. Pharmacol. Res. 2020, 161, 105210. [Google Scholar] [CrossRef] [PubMed]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory Response in the CNS: Friend or Foe? Mol. Neurobiol. 2017, 54, 8071–8089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dincel, G.C.; Atmaca, H.T. Role of oxidative stress in the pathophysiology of Toxoplasma gondii infection. Int. J. Immunopathol. Pharmacol. 2016, 29, 226–240. [Google Scholar] [CrossRef] [Green Version]
- Greenlund, L.J.D.T.; Johnson, E.M., Jr. Superoxide dismutase delays neuronal apoptosis: A role for reactive oxygen species in programmed neuronal death. Neuron 1995, 14, 303–315. [Google Scholar] [CrossRef] [PubMed]


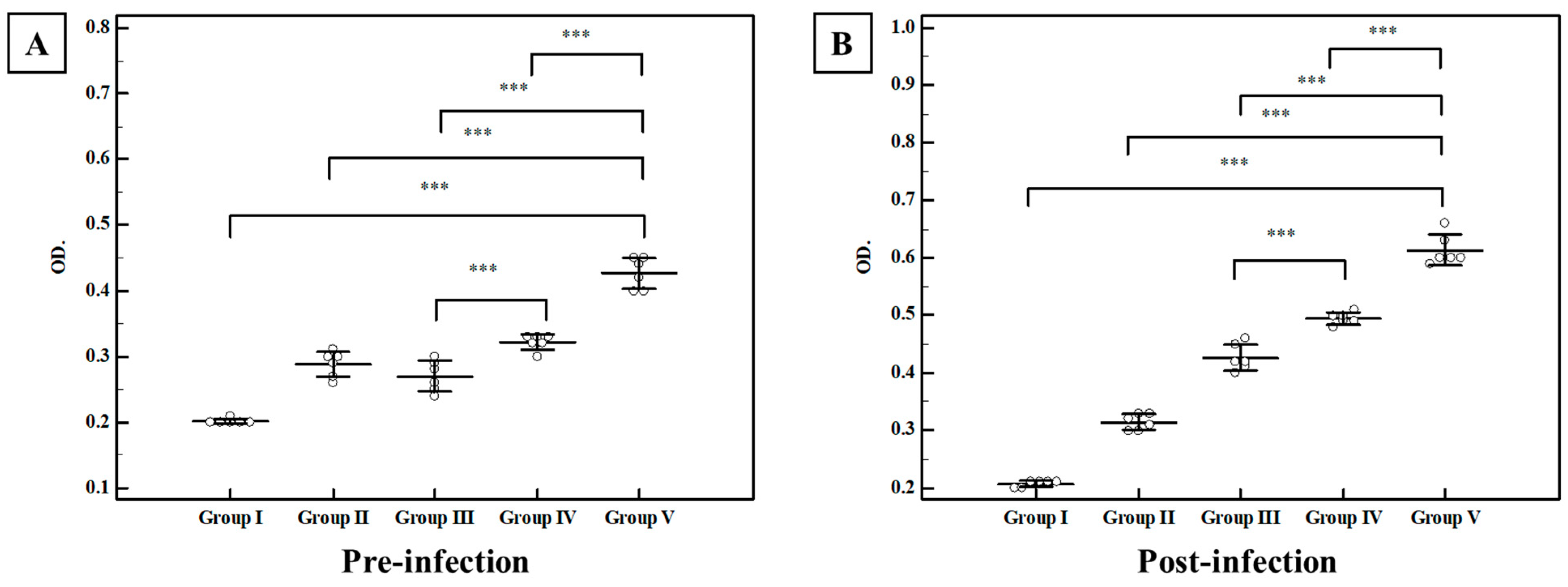

| Group I Control (n = 6) | Group II MLT-SLNs (n = 6) | Group III TLA (n = 6) | Group IV TLA/SLNs (n = 6) | Group V TLA/MLT-SLNs (n = 6) | Fp | |
|---|---|---|---|---|---|---|
| SOD | ||||||
| Pre-infection | 1415 ± 9.7 | 1433 a ± 14.0 | 1426 ± 4.5 | 1429 ± 2.8 | 1722 abcd± 11.7 | <0.001 * |
| Post-infection | 1541 ± 16.4 | 1780 a ± 16.7 | 1753 a ± 9.4 | 1673 abc ± 12.5 | 2223 abcd ± 27.4 | <0.001 * |
| MDA | ||||||
| Pre-infection | 16.92 ± 0.70 | 17.22 ± 1.04 | 16.98 ± 0.55 | 16.97 ± 0.22 | 16.85 ± 0.38 | 0.889 |
| Post-infection | 29.70 ± 0.59 | 9.73 a ± 0.96 | 22.88 ab ± 0.81 | 23.0 ab ± 0.24 | 11.88 abcd ± 0.26 | <0.001 * |
| TAC | ||||||
| Pre-infection | 0.992 ± 0.026 | 1.445 a ± 0.020 | 1.452 a ± 0.027 | 1.332 abc ± 0.017 | 1.495 abcd ± 0.010 | <0.001 * |
| Post-infection | 1.258 ± 0.022 | 1.565 a ± 0.022 | 1.518 ab ± 0.016 | 1.513 ab ± 0.015 | 1.597 abcd ± 0.012 | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Said, D.E.; Amer, E.I.; Sheta, E.; Makled, S.; Diab, H.E.; Arafa, F.M. Nano-Encapsulated Melatonin: A Promising Mucosal Adjuvant in Intranasal Immunization against Chronic Experimental T. gondii Infection. Trop. Med. Infect. Dis. 2022, 7, 401. https://doi.org/10.3390/tropicalmed7120401
Said DE, Amer EI, Sheta E, Makled S, Diab HE, Arafa FM. Nano-Encapsulated Melatonin: A Promising Mucosal Adjuvant in Intranasal Immunization against Chronic Experimental T. gondii Infection. Tropical Medicine and Infectious Disease. 2022; 7(12):401. https://doi.org/10.3390/tropicalmed7120401
Chicago/Turabian StyleSaid, Doaa E., Eglal I. Amer, Eman Sheta, Shaimaa Makled, Hala E. Diab, and Fadwa M. Arafa. 2022. "Nano-Encapsulated Melatonin: A Promising Mucosal Adjuvant in Intranasal Immunization against Chronic Experimental T. gondii Infection" Tropical Medicine and Infectious Disease 7, no. 12: 401. https://doi.org/10.3390/tropicalmed7120401
APA StyleSaid, D. E., Amer, E. I., Sheta, E., Makled, S., Diab, H. E., & Arafa, F. M. (2022). Nano-Encapsulated Melatonin: A Promising Mucosal Adjuvant in Intranasal Immunization against Chronic Experimental T. gondii Infection. Tropical Medicine and Infectious Disease, 7(12), 401. https://doi.org/10.3390/tropicalmed7120401







