Needs Assessment of Southeastern United States Vector Control Agencies: Capacity Improvement Is Greatly Needed to Prevent the Next Vector-Borne Disease Outbreak
Abstract
:1. Introduction
2. Materials and Methods
2.1. Survey Development
2.2. Identification of Recipients
2.3. Distribution
2.4. Statistical Analysis
3. Results
3.1. Survey Responses and Respondent Characteristics
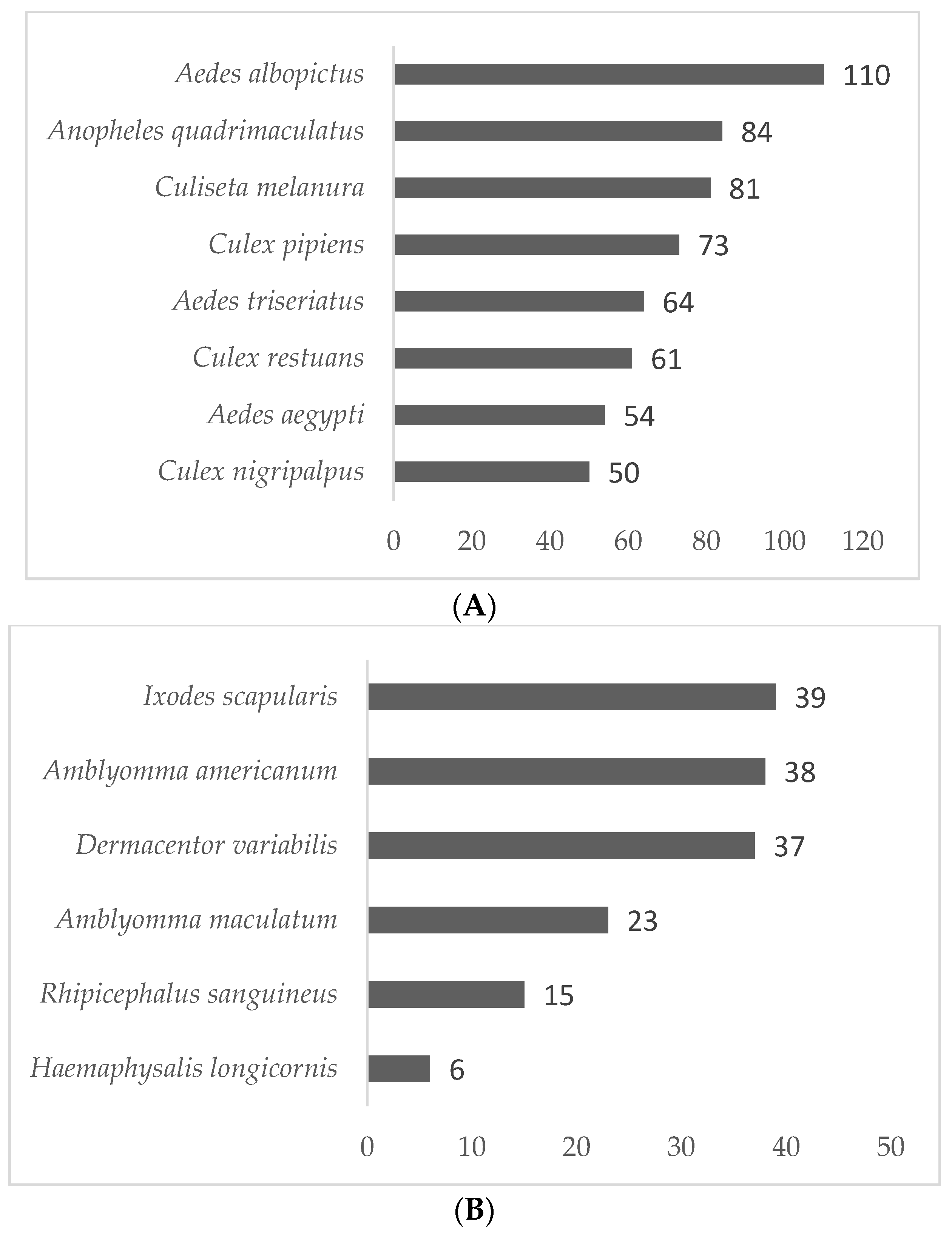
3.2. Vector Surveillance Results
3.3. Vector-Borne Diseases Reported
3.4. Agency Demographic Associated Capabilities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Vector-Borne Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 15 April 2021).
- CDC. West Nile Virus: Statistics & Maps. Available online: https://www.cdc.gov/westnile/statsmaps/index.html (accessed on 15 April 2021).
- CDC. Lyme Disease: Data and Surveillance. Available online: https://www.cdc.gov/lyme/datasurveillance/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Flyme%2Fstats%2Findex.html (accessed on 15 April 2021).
- CDC. Zika Virus: Statistics and Maps. Available online: https://www.cdc.gov/zika/reporting/index.html (accessed on 15 April 2021).
- Wilke, A.B.; Vasquez, C.; Medina, J.; Carvajal, A.; Petrie, W.; Beier, J.C. Community composition and year-round abundance of vector species of mosquitoes make Miami-Dade County, Florida a receptive gateway for arbovirus entry to the United States. Sci. Rep. 2019, 9, 8732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisanzio, D.; Martello, E.; Izenour, K.; Stevens, K.; Kaur, R.; McKenzie, B.A.; Kraemer, M.; Reithinger, R.; Zohdy, S. Arboviral diseases and poverty in Alabama, 2007–2017. PLoS Negl. Trop. Dis. 2021, 15, e0009535. [Google Scholar] [CrossRef] [PubMed]
- DePietro, A. U.S. Poverty Rate by State in 2021. Forbes 2021. Available online: https://www.forbes.com/sites/andrewdepietro/2021/11/04/us-poverty-rate-by-state-in-2021/?sh=33c211351b38 (accessed on 15 April 2021).
- Central, C. This News Bites: More Mosquito Days. Available online: https://medialibrary.climatecentral.org/resources/more-mosquito-days (accessed on 15 April 2021).
- McKenzie, B.A.; Stevens, K.; McKenzie, A.E.; Bozic, J.; Mathias, D.; Zohdy, S. Aedes Vector Surveillance in the Southeastern United States Reveals Growing Threat of Aedes japonicus japonicus (Diptera: Culicidae) and Aedes albopictus. J. Med. Entomol. 2019, 56, 1745–1749. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, A.J.; Eisen, R.J.; Eisen, L.; McAllister, J.; Savage, H.M.; Mutebi, J.-P.; Johansson, M.A. Consensus and uncertainty in the geographic range of Aedes aegypti and Aedes albopictus in the contiguous United States: Multi-model assessment and synthesis. PLoS Comput. Biol. 2019, 15, e1007369. [Google Scholar] [CrossRef]
- Parker, C.; Ramirez, D.; Connelly, C.R. State-wide survey of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Florida. J. Vector Ecol. 2019, 44, 210–215. [Google Scholar] [CrossRef] [Green Version]
- Day, C.A.; Lewandowski, K.; Vonesh, J.R.; Byrd, B.D. Phenology of Rock Pool Mosquitoes in the Southern Appalachian Mountains: Surveys Reveal Apparent Winter Hatching of Aedes japonicus and the Potential For Asymmetrical Stage-Specific Interactions. J. Am. Mosq. Control Assoc. 2020, 36, 216–226. [Google Scholar] [CrossRef]
- Lwande, O.W.; Obanda, V.; Lindström, A.; Ahlm, C.; Evander, M.; Näslund, J.; Bucht, G. Globe-Trotting Aedes aegypti and Aedes albopictus: Risk factors for arbovirus pandemics. Vector-Borne Zoonotic Dis. 2020, 20, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Reeves, L.E.; Medina, J.; Miqueli, E.; Sloyer, K.E.; Petrie, W.; Vasquez, C.; Burkett-Cadena, N.D. Establishment of Aedes (Ochlerotatus) scapularis (Diptera: Culicidae) in mainland Florida, with notes on the Ochlerotatus group in the United States. J. Med. Entomol. 2021, 58, 717–729. [Google Scholar] [CrossRef]
- Eisen, R.J.; Eisen, L. The blacklegged tick, Ixodes scapularis: An increasing public health concern. Trends Parasitol. 2018, 34, 295–309. [Google Scholar] [CrossRef]
- Sonenshine, D.E. Range expansion of tick disease vectors in North America: Implications for spread of tick-borne disease. Int. J. Environ. Res. Public Health 2018, 15, 478. [Google Scholar] [CrossRef] [Green Version]
- Raghavan, R.K.; Peterson, A.T.; Cobos, M.E.; Ganta, R.; Foley, D. Current and future distribution of the lone star tick, Amblyomma americanum (L.)(Acari: Ixodidae) in North America. PLoS ONE 2019, 14, e0209082. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L. The Impacts of Climate Change on Ticks and Tick-Borne Disease Risk. Annu. Rev. Entomol. 2021, 66, 373–388. [Google Scholar] [CrossRef] [PubMed]
- NACCHO. Mosquito Control Capabilities in the U.S. 2017. Available online: https://www.naccho.org/uploads/downloadable-resources/Mosquito-control-in-the-U.S.-Report.pdf (accessed on 15 April 2021).
- Vigilant, M.; Battle-Freeman, C.; Braumuller, K.C.; Riley, R.; Fredregill, C.L. Harris County Public Health Mosquito and Vector Control Division Emergency Response to Hurricane Harvey: Vector-Borne Disease Surveillance and Control. J. Am. Mosq. Control. Assoc. 2020, 36, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Grenadier, A. The Impact of COVID-19 on Local Vector Control Response; National Association of County and City Health Officials (NACCHO): Washington, DC, USA, 2020. [Google Scholar]
- WHO; UNICEF. Framework for a National Vector Control Needs Assessment; CC BY-NC-SA 3.0 IGO; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Dye-Braumuller, K.C.; Kanyangarara, M. Malaria in the USA: How Vulnerable Are We to Future Outbreaks? Curr. Trop. Med. Rep. 2021, 8, 43–51. [Google Scholar] [CrossRef] [PubMed]
- EPA. Controling Adult Mosquitoes. Available online: https://www.epa.gov/mosquitocontrol/controlling-adult-mosquitoes (accessed on 15 April 2021).
- EPA. Causal Analysis/Diagnosis Decision Information System (CADDIS): Insecticides. Available online: https://www.epa.gov/caddis-vol2/insecticides (accessed on 15 April 2021).
- Bunge, J.; McKay, B. In the Fight Against Zika, Insecticides Hit a ‘Dead End’. Wall Str. J. 2017. Available online: https://www.wsj.com/articles/fight-against-zika-nears-dead-end-1483621245 (accessed on 15 April 2021).
- Manual de Organización y Procedimientos de las Unidades de Investigación Entomológica y de Bioensayos; Centro Nacional de Programas Preventivos y Control de Enfermedades: Ciudad de Mexico, Mexico, 2020; Volume 2.
- Guía para la Determinación de la Susceptibilidad/Resistencia y Eficacia Biológica a Insecticidas; Centro Nacional de Programas Preventivos y Control de Enfermedades: Ciudad de Mexico, Mexico, 2020.
- CDC. A National Public Health Framework for the Prevention and Control of Vector-Borne Diseases in Humans; CDC: Atlanta, GA, USA, 2020.
- Eisen, R.J.; Paddock, C.D. Tick and tickborne pathogen surveillance as a public health tool in the United States. J. Med. Entomol. 2020, 58, 1490–1502. [Google Scholar] [CrossRef]
- Mader, E.M.; Ganser, C.; Geiger, A.; Harrington, L.C.; Foley, J.; Smith, R.L.; Mateus-Pinilla, N.; Teel, P.D.; Eisen, R.J. A survey of tick surveillance and control practices in the United States. J. Med. Entomol. 2020, 58, 1503–1512. [Google Scholar] [CrossRef]
- USDA. National Haemaphysalis longicornis (Asian longhorned tick) Situation Report As of 12 May 2021. 2021. Available online: https://www.aphis.usda.gov/animal_health/animal_diseases/tick/downloads/longhorned-tick-sitrep.pdf (accessed on 15 April 2021).
- Connelly, C.; Borchert, J. Mosquito control emergency preparedness and response to natural disasters. J. Am. Mosq. Control Assoc. 2020, 36, 2–4. [Google Scholar] [CrossRef]
- Goddard, J.; Varnado, W.C. Disaster Vector Control in Mississippi After Hurricane Katrina: Lessons Learned. J. Am. Mosq. Control Assoc. 2020, 36, 56–60. [Google Scholar] [CrossRef]
- Weaver, J.R.; Xue, R.D.; Gaines, M.K. Population Outbreaks of Mosquitoes After Hurricanes Matthew and Irma and the Control Efforts in St. Johns County, Northeastern Florida. J. Am. Mosq. Control Assoc. 2020, 36, 28–34. [Google Scholar] [CrossRef]
- King, R.A.; Heinig, R.; Linn, P.; Lucas, K.J. The Impact of Hurricane Irma on Our Community and the Collier Mosquito Control District’s Mission. J. Am. Mosq. Control Assoc. 2020, 36, 11–14. [Google Scholar] [CrossRef]
- CDC. DVBD’s Top Accomplishments in 2020. Available online: https://www.cdc.gov/ncezid/dvbd/stories/accomplishments-2020.html (accessed on 15 April 2021).
- AMCA. Best Practices for Integrated Mosquito Management: A Focused Update; AMCA’s Best Practices for Integrated Mosquito Management Manual; American Mosquito Control Association: Sacramento, CA, USA, 2017. [Google Scholar]
- Wimberly, M.C.; de Beurs, K.M.; Loboda, T.V.; Pan, W.K. Satellite Observations and Malaria: New Opportunities for Research and Applications. Trends Parasitol. 2021, 37, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Mains, J.W.; Kelly, P.H.; Dobson, K.L.; Petrie, W.D.; Dobson, S.L. Localized Control of Aedes aegypti (Diptera: Culicidae) in Miami, FL, via Inundative Releases of Wolbachia-Infected Male Mosquitoes. J. Med. Entomol. 2019, 56, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Waltz, E. First genetically modified mosquitoes released in the United States. Nature 2021, 593, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Guia de Tratamento da Malaria No Brasil; Departamento de Imunização e Doenças Transmissíveis: Brasalia, Brazil, 2019; pp. 39–43.
- Tran, T.T.; Olsen, A.; Viennet, E.; Sleigh, A. Social sustainability of Mesocyclops biological control for dengue in South Vietnam. Acta Trop. 2015, 141, 54–59. [Google Scholar] [CrossRef]
- Chanda, E.; Doggale, C.; Pasquale, H.; Azairwe, R.; Baba, S.; Mnzava, A. Addressing malaria vector control challenges in South Sudan: Proposed recommendations. Malar. J. 2013, 12, 59. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.Y.; Alfaro-Murillo, J.A.; Parpia, A.S.; Asti, L.; Wedlock, P.T.; Hotez, P.J.; Galvani, A.P. The potential economic burden of Zika in the continental United States. PLoS Negl. Trop. Dis. 2017, 11, e0005531. [Google Scholar] [CrossRef]
- Mac, S.; da Silva, S.R.; Sander, B. The economic burden of Lyme disease and the cost-effectiveness of Lyme disease interventions: A scoping review. PLoS ONE 2019, 14, e0210280. [Google Scholar] [CrossRef]


| All Participants | Agency Size > 100,000 Residents 1 | ||
|---|---|---|---|
| Number (%) | Number (%) | p-Value 2; OR 3 (95% CI) | |
| Vector(s) agency controls for: | |||
| Mosquitoes | 136 (96%) | 69 (99%) | 0.136 |
| Ticks | 11 (8%) | 2 (3%) | 0.046; 0.20 (0.04–0.97) |
| Other 4 | 20 (14%) | 9 (13%) | 0.654 |
| Surveillance conducted all-year (vs. summer only) | 63 (49%) | 41 (65%) | <0.001; 3.82 (1.83–7.96) |
| Surveillance type: | |||
| Vector collections | 92 (70%) | 46 (71%) | 0.848 |
| Pathogen testing | 39 (30%) | 27 (42%) | 0.005; 3.14 (1.41–6.97) |
| Adulticides used: | |||
| Malathion | 24 (20%) | 17 (27%) | 0.044; 2.70 (1.03–7.10) |
| Permethrin | 99 (83%) | 49 (79%) | 0.324 |
| Larvicides used: | |||
| Biological control | 81 (66%) | 52 (81%) | <0.001; 4.49 (1.99–10.14) |
| Growth regulators | 76 (62%) | 48 (75%) | 0.002; 3.33 (1.55–7.19) |
| Contact insecticides | 41 (34%) | 27 (42%) | 0.043; 2.24 (1.03–4.89) |
| Stomach insecticides | 61 (50%) | 37 (58%) | 0.056 |
| Insecticide applied at least biweekly | 45 (35%) | 28 (41%) | 0.031; 2.21 (1.08–4.53) |
| Major equipment: | |||
| Organization-owned truck | 106 (81%) | 58 (88%) | 0.057 |
| Organization-owned aerial | 35 (27%) | 24 (36%) | 0.010; 3.03 (1.31–7.03) |
| Contractor | 19 (15%) | 11 (17%) | 0.526 |
| Conducts vector speciation in-house | 81 (56%) | 54 (77%) | <0.001; 6.23 (2.99–12.98) |
| Conducts disease testing in-house | 9 (6%) | 5 (7%) | 0.683 |
| Performs community outreach and education | 120 (83%) | 61 (87%) | 0.169 |
| Conducts GIS or mapping in-house | 94 (64%) | 59 (83%) | <0.001; 5.78 (2.68–12.50) |
| Conducts insecticide resistance testing in-house | 54 (44%) | 37 (66%) | <0.001; 5.50 (2.51–12.02) |
| All Participants | Agency Part of Local Health Department 1 | ||
|---|---|---|---|
| Number (%) | Number (%) | p-Value 2; OR (95% CI) 3 | |
| Vector(s) agency controls for: | |||
| Mosquitos | 136 (96%) | 62 (46%) | N/A |
| Ticks | 11 (8%) | 68 (48%) | 0.034; 5.49 (1.14–26.41) |
| Other 4 | 20 (14%) | 12 (60%) | 0.246 |
| Surveillance conducted all-year (vs. summer only) | 63 (49%) | 29 (46%) | 0.989 |
| Surveillance type: | |||
| Vector collections | 92 (70%) | 40 (43%) | 0.278 |
| Pathogen testing | 39 (30%) | 16 (41%) | 0.409 |
| Adulticides used: | |||
| Malathion | 24 (20%) | 6 (25%) | 0.099 |
| Permethrin | 99 (83%) | 39 (39%) | 0.769 |
| Larvicides used: | |||
| Biological control | 81 (66%) | 34 (42%) | 0.754 |
| Growth regulators | 76 (62%) | 31 (41%) | 0.955 |
| Contact insecticides | 41 (34%) | 18 (44%) | 0.641 |
| Stomach insecticides | 61 (50%) | 19 (31%) | 0.028; 0.44 (0.21–1.71) |
| Insecticide applied at least biweekly | 45 (35%) | 17 (38%) | 0.127 |
| Major equipment: | |||
| Organization-owned truck | 106 (81%) | 39 (37%) | 0.002; 0.23 (0.09–0.60) |
| Organization-owned aerial | 35 (27%) | 13 (37%) | 0.376 |
| Contractor | 19 (15%) | 9 (47%) | 0.714 |
| Conducts vector speciation in-house | 81 (56%) | 34 (42%) | 0.089 |
| Conducts disease testing in-house | 9 (6%) | 5 (56%) | 0.607 |
| Performs community outreach and education | 120 (83%) | 59 (49%) | 0.405 |
| Conducts GIS or mapping in-house | 94 (64%) | 38 (40%) | 0.027; 0.56 (0.23–0.92) |
| Conducts insecticide resistance testing in-house | 54 (44%) | 23 (43%) | 0.126 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dye-Braumuller, K.C.; Gordon, J.R.; Johnson, D.; Morrissey, J.; McCoy, K.; Dinglasan, R.R.; Nolan, M.S. Needs Assessment of Southeastern United States Vector Control Agencies: Capacity Improvement Is Greatly Needed to Prevent the Next Vector-Borne Disease Outbreak. Trop. Med. Infect. Dis. 2022, 7, 73. https://doi.org/10.3390/tropicalmed7050073
Dye-Braumuller KC, Gordon JR, Johnson D, Morrissey J, McCoy K, Dinglasan RR, Nolan MS. Needs Assessment of Southeastern United States Vector Control Agencies: Capacity Improvement Is Greatly Needed to Prevent the Next Vector-Borne Disease Outbreak. Tropical Medicine and Infectious Disease. 2022; 7(5):73. https://doi.org/10.3390/tropicalmed7050073
Chicago/Turabian StyleDye-Braumuller, Kyndall C., Jennifer R. Gordon, Danielle Johnson, Josie Morrissey, Kaci McCoy, Rhoel R. Dinglasan, and Melissa S. Nolan. 2022. "Needs Assessment of Southeastern United States Vector Control Agencies: Capacity Improvement Is Greatly Needed to Prevent the Next Vector-Borne Disease Outbreak" Tropical Medicine and Infectious Disease 7, no. 5: 73. https://doi.org/10.3390/tropicalmed7050073
APA StyleDye-Braumuller, K. C., Gordon, J. R., Johnson, D., Morrissey, J., McCoy, K., Dinglasan, R. R., & Nolan, M. S. (2022). Needs Assessment of Southeastern United States Vector Control Agencies: Capacity Improvement Is Greatly Needed to Prevent the Next Vector-Borne Disease Outbreak. Tropical Medicine and Infectious Disease, 7(5), 73. https://doi.org/10.3390/tropicalmed7050073







