Identification and Characterization of a Potential Antimicrobial Peptide Isolated from Soil Brevibacillus sp. WUL10 and Its Activity against MRSA Pathogens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbial Strains and Culture Conditions
2.2. Bacterial Morphology and Identification
2.3. Determination of Growth Curve and Production Kinetics of Antimicrobial Substances
2.4. Agar Well Diffusion Assay
2.5. Purification of Antimicrobial Peptides
2.6. Sodium Dodecyl Sulfate (SDS)-Polyacrylamide Gel Electrophoresis (PAGE) and Gel Overlay Assay
2.7. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Antimicrobial Peptide
2.8. Stability Study of Pure Antimicrobial Peptide
2.9. Scanning Electron Microscope (SEM)
2.10. Peptide Sequencing and Antimicrobial Activity of the Synthetic Peptide
2.11. Statistical Analysis
3. Results
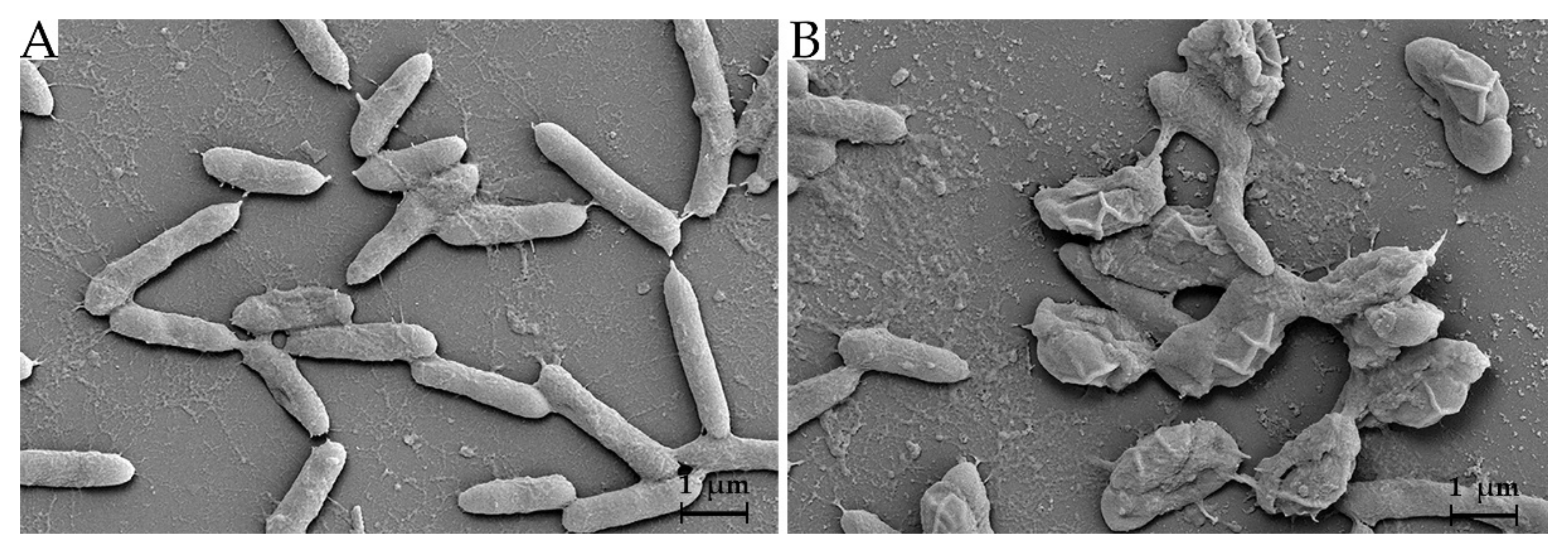
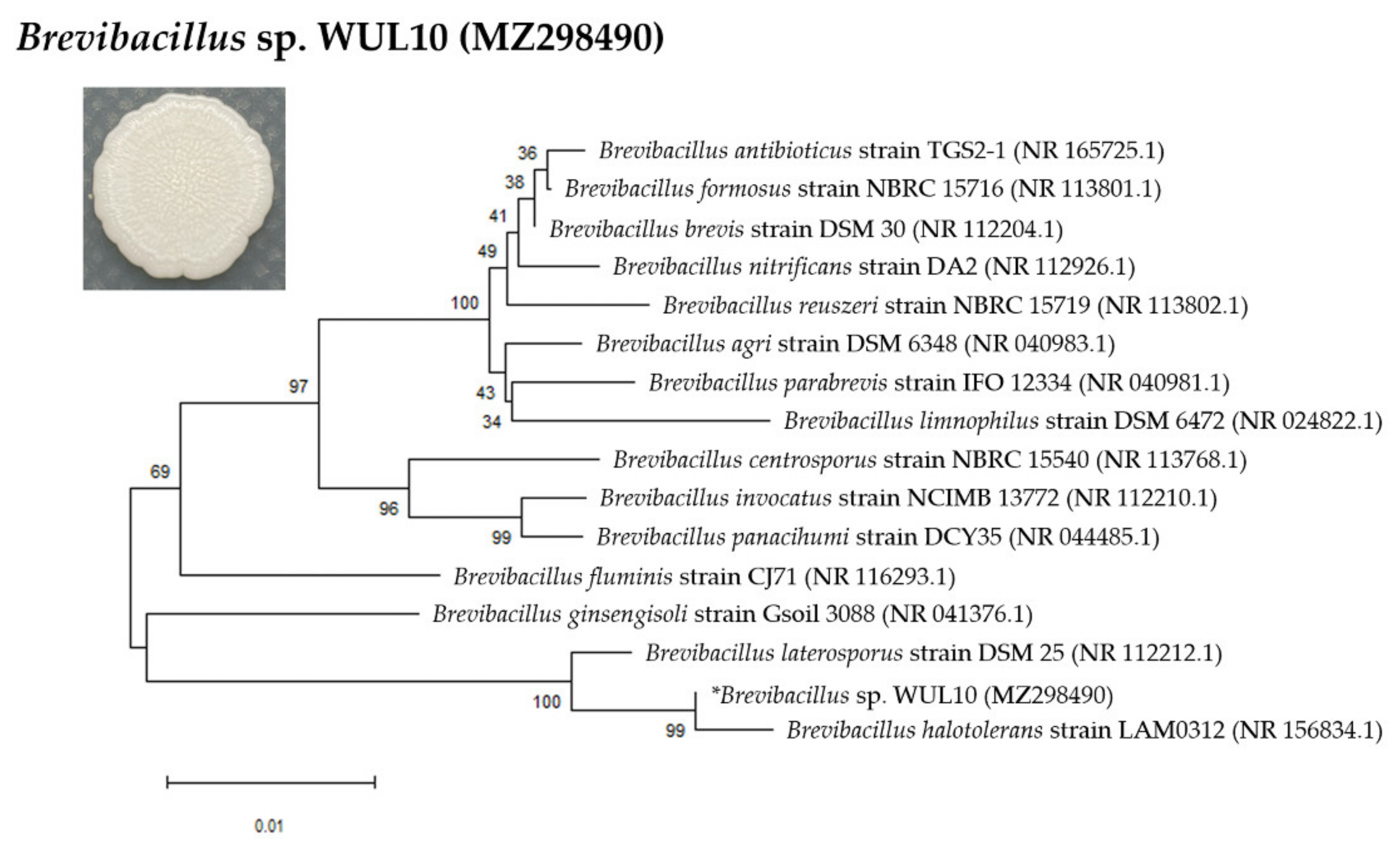
3.1. Cell Morphology and Phylogenetic Analysis
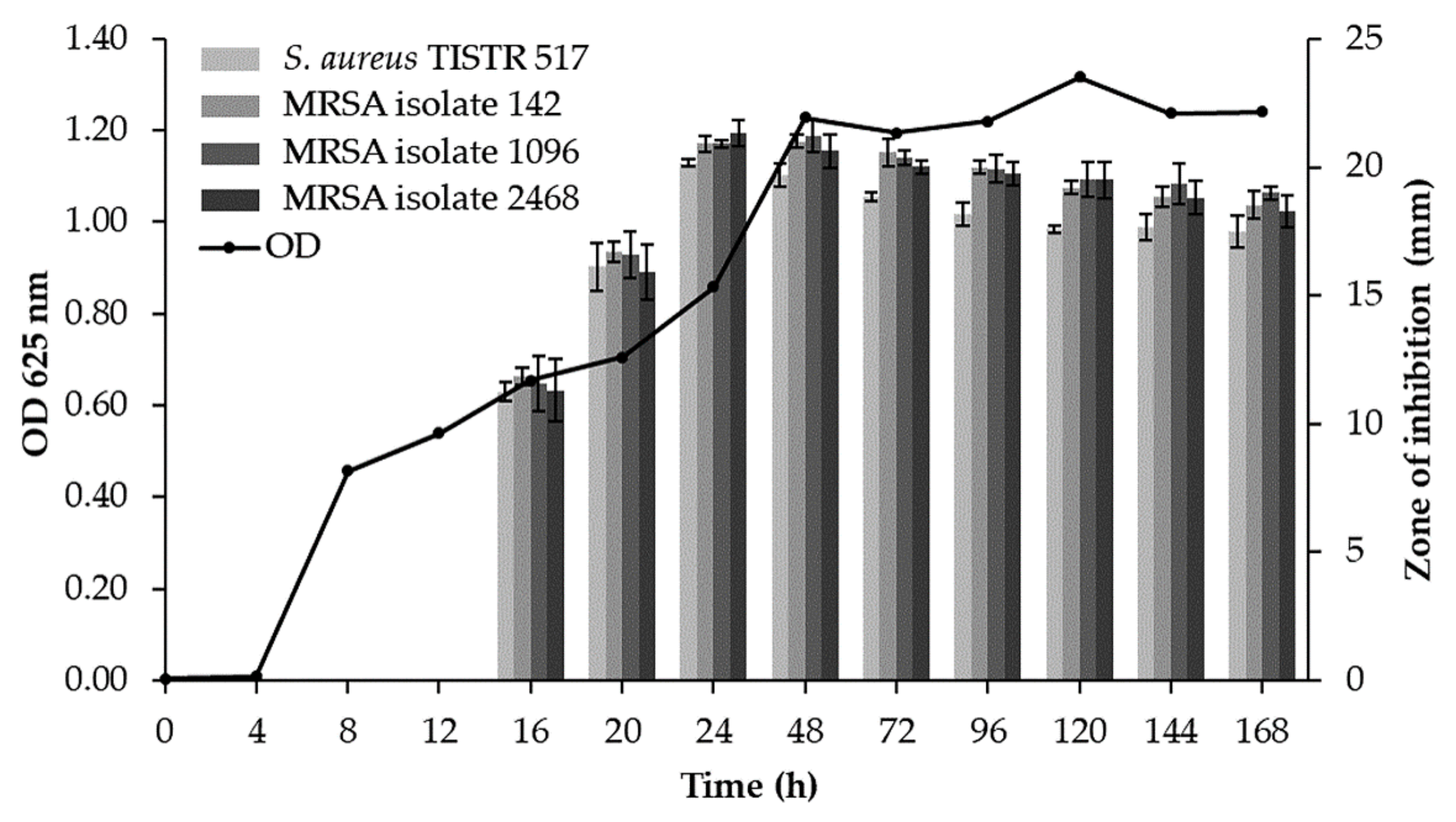
3.2. Production Kinetics of Antimicrobial Substances
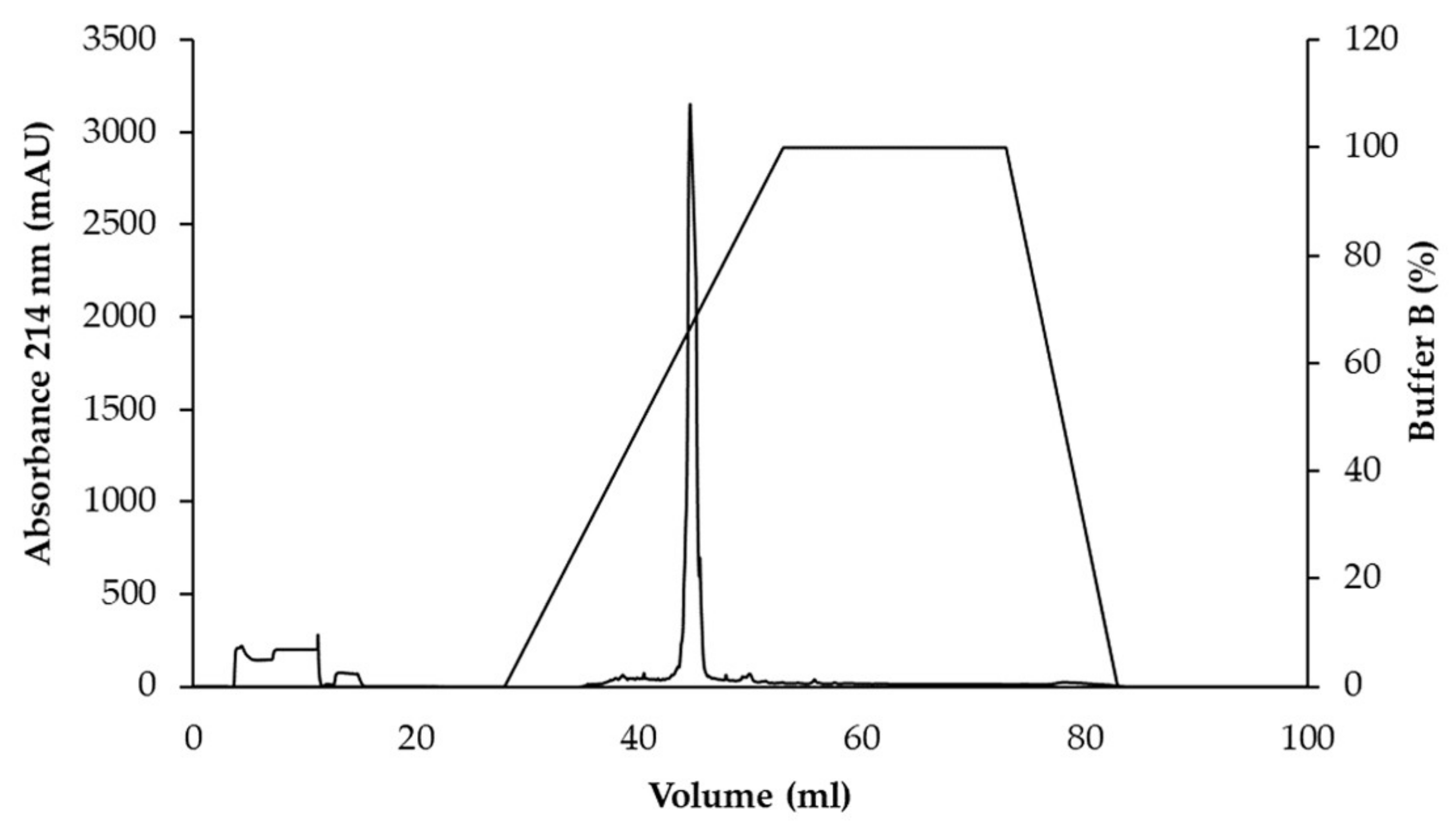
3.3. Purification of Active Antimicrobial Substances
3.4. Determination of the Antimicrobial Activity of Active Substance
3.5. Stability Study of Purified WUL10 Substance
3.6. Effect of Antimicrobial Substance on Bacterial Cells
3.7. Peptide Sequencing, and MIC and MBC Determination of Synthetic Peptide
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paulin, S.; Beyer, P. 2019 Antibacterial Agents in Clinical Development an Analysis of the Antibacterial Clinical Development Pipeline; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; The Review on Antimicrobial Resistance; Government of the United Kingdom: London, UK, 2016. [Google Scholar]
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A review on antibiotic resistance: Alarm bells are ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef] [Green Version]
- McEwen, S.A.; Fedorka-Cray, P.J. Antimicrobial use and resistance in animals. Clin. Infect. Dis. 2002, 34, S93–S106. [Google Scholar] [CrossRef] [Green Version]
- Llarrull, L.I.; Fisher, J.F.; Mobashery, S. Molecular basis and phenotype of methicillin resistance in Staphylococcus aureus and insights into new beta-lactams that meet the challenge. Antimicrob. Agents Chemother. 2009, 53, 4051–4063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowy, F.D. Antimicrobial resistance: The example of Staphylococcus aureus. J. Clin. Investig. 2003, 111, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.; Frees, D.; Ingmer, H. Antibiotic resistance and the MRSA problem. Microbiol. Spectr. 2019, 7, GPP3-0057-2018. [Google Scholar] [CrossRef]
- Beyer, P. Antibacterial Agents in Clinical Development an Analysis of the Antibacterial Clinical Development Pipeline, Including Tuberculosis; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro da Cunha, B.; Fonseca, L.P.; Calado, C.R.C. Antibiotic discovery: Where have we come from, where do we go? Antibiotics 2019, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Quinn, G.A.; Banat, A.M.; Abdelhameed, A.M.; Banat, I.M. Streptomyces from traditional medicine: Sources of new innovations in antibiotic discovery. J. Med. Microbiol. 2020, 69, 1040–1048. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial peptides: Classification, design, application and research progress in multiple fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Blecha, F. Porcine host defense peptides: Expanding repertoire and functions. Dev. Comp. Immunol. 2009, 33, 334–343. [Google Scholar] [CrossRef]
- Al-sahlany, S.T.G.; Altemimi, A.B.; Al-Manhel, A.J.A.; Niamah, A.K.; Lakhssassi, N.; Ibrahim, S.A. Purification of bioactive peptide with antimicrobial properties produced by Saccharomyces cerevisiae. Foods 2020, 9, 324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.Y.; Yan, Z.B.; Meng, Y.M.; Hong, X.Y.; Shao, G.; Ma, J.J.; Cheng, X.R.; Liu, J.; Kang, J.; Fu, C.Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The value of antimicrobial peptides in the age of resistance. Lancet. Infect. Dis. 2020, 20, e216–e230. [Google Scholar] [CrossRef]
- Ray, S.; Patel, N.; Amin, D. Brevibacillus. In Beneficial Microbes in Agro-Ecology; Amaresan, N., Senthil, K.M., Annapurna, K., Kumar, K., Sankaranarayanan, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 149–167. [Google Scholar]
- Panda, A.K.; Bisht, S.S.; DeMondal, S.; Senthil Kumar, N.; Gurusubramanian, G.; Panigrahi, A.K. Brevibacillus as a biological tool: A short review. Antonie Leeuwenhoek 2014, 105, 623–639. [Google Scholar] [CrossRef]
- Songnaka, N.; Lertcanawanichakul, M.; Atipairin, A. Promising anti-MRSA activity of Brevibacillus sp. isolated from soil and strain improvement by UV mutagenesis. Sci. Pharm. 2021, 89, 1. [Google Scholar]
- Sudhir, K.; Glen, S.; Michael, L.; Christina, K.; Koichiro, T. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar]
- Clinical and Laboratory Standards Institute. M100 Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021. [Google Scholar]
- Xi, Q.; Wang, J.; Du, R.; Zhao, F.; Han, Y.; Zhou, Z. Purification and characterization of bacteriocin produced by a strain of Enterococcus faecalis TG2. Appl. Biochem. Biotechnol. 2018, 184, 1106–1119. [Google Scholar] [CrossRef]
- Carolissen-Mackay, V.; Arendse, G.; Hastings, J.W. Purification of bacteriocins of lactic acid bacteria: Problems and pointers. Int. J. Food Microbiol. 1997, 34, 1–16. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. M07 Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Chalasani, A.G.; Dhanarajan, G.; Nema, S.; Sen, R.; Roy, U. An antimicrobial metabolite from Bacillus sp.: Significant activity against pathogenic bacteria including multidrug-resistant clinical strains. Front. Microbiol. 2015, 6, 1335. [Google Scholar] [CrossRef] [Green Version]
- Ma, B.; Zhang, K.; Hendrie, C.; Liang, C.; Li, M.; Doherty-Kirby, A.; Lajoie, G. PEAKS: Powerful software for peptide de novo sequencing by tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 2337–2342. [Google Scholar] [CrossRef]
- Veltri, D.; Kamath, U.; Shehu, A. Deep learning improves antimicrobial peptide recognition. Bioinformatics 2018, 34, 2740–2747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Wang, Y.; Song, Y.; Zhao, B.; Wang, H.; Zhou, S.; Kong, D.; Guo, X.; Li, Y.; He, M.; et al. Brevibacillus halotolerans sp. nov., isolated from saline soil of a paddy field. Int. J. Syst. Evol. Microbiol. 2017, 67, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Maupetit, J.; Derreumaux, P.; Tufféry, P. Improved PEP-FOLD approach for peptide and miniprotein structure prediction. J. Chem. Theory Comput. 2014, 10, 4745–4758. [Google Scholar] [CrossRef] [PubMed]
- Lamiable, A.; Thévenet, P.; Rey, J.; Vavrusa, M.; Derreumaux, P.; Tufféry, P. PEP-FOLD3: Faster de novo structure prediction for linear peptides in solution and in complex. Nucleic Acids Res. 2016, 44, W449–W454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thévenet, P.; Shen, Y.; Maupetit, J.; Guyon, F.; Derreumaux, P.; Tufféry, P. PEP-FOLD: An updated de novo structure prediction server for both linear and disulfide bonded cyclic peptides. Nucleic Acids Res. 2012, 40, W288–W293. [Google Scholar] [CrossRef] [Green Version]
- Gautier, R.; Douguet, D.; Antonny, B.; Drin, G. HELIQUEST: A web server to screen sequences with specific alpha-helical properties. Bioinformatics 2008, 24, 2101–2102. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Pham, J.V.; Yilma, M.A.; Feliz, A.; Majid, M.T.; Maffetone, N.; Walker, J.R.; Kim, E.; Cho, H.J.; Reynolds, J.M.; Song, M.C.; et al. A review of the microbial production of bioactive natural products and biologics. Front. Microbiol. 2019, 10, 1404. [Google Scholar] [CrossRef] [Green Version]
- Katak, R.M.; Rocha, E.M.; Oliveira, J.C.; Muniz, V.A.; Oliveira, M.R.; Ferreira, F.A.S.; Silva, W.R.; Roque, R.A.; de Souza, A.Q.L.; Souza-Neto, J.A.; et al. Larvicidal activities against Aedes aegypti of supernatant and pellet fractions from cultured Bacillus spp. isolated from Amazonian microenvironments. Trop. Med. Infect. Dis. 2021, 6, 104. [Google Scholar]
- Ruiu, L. Brevibacillus laterosporus, a pathogen of invertebrates and a broad-spectrum antimicrobial species. Insects 2013, 4, 476–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glare, T.R.; Durrant, A.; Berry, C.; Palma, L.; Ormskirk, M.M.; Cox, M.P. Phylogenetic determinants of toxin gene distribution in genomes of Brevibacillus laterosporus. Genomics 2020, 112, 1042–1053. [Google Scholar] [CrossRef]
- Yang, X.; Yousef, A.E. Antimicrobial peptides produced by Brevibacillus spp.: Structure, classification and bioactivity: A mini review. World J. Microbiol. Biotechnol. 2018, 34, 57. [Google Scholar] [CrossRef] [PubMed]
- Benfield, A.H.; Henriques, S.T. Mode-of-action of antimicrobial peptides: Membrane disruption vs. intracellular mechanisms. Front. Med. Technol. 2020, 2, 610997. [Google Scholar] [CrossRef]
- Yang, X.; Huang, E.; Yuan, C.; Zhang, L.; Yousef, A.E. Isolation and structural elucidation of brevibacillin, an antimicrobial lipopeptide from Brevibacillus laterosporus that combats drug-resistant gram-positive bacteria. Appl. Environ. Microbiol. 2016, 82, 2763–2772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleem, F.; Ahmad, S.; Yaqoob, Z.; Rasool, S.A. Comparative study of two bacteriocins produced by representative indigenous soil bacteria. Pak. J. Pharm. Sci. 2009, 22, 252–258. [Google Scholar] [PubMed]
- Faheem, F.; Saeed, S.; Rasool, S.A. Studies on Brevicin AF01: A Bacteriocin like inhibitory substance active against Methicillin resistant Staphylococcus aureus. Pak. J. Bot. 2007, 39, 1293–1302. [Google Scholar]
- Aunpad, R.; Na-Bangchang, K. Pumilicin 4, a novel bacteriocin with anti-MRSA and anti-VRE activity produced by newly isolated bacteria Bacillus pumilus strain WAPB4. Curr. Microbiol. 2007, 55, 308–313. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, E.; Yuan, C.; Zhang, L.; Yousef, A.E. Isolation of a Paenibacillus sp. strain and structural elucidation of its broad-spectrum lipopeptide antibiotic. Appl. Environ. Microbiol. 2012, 78, 3156–3165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavaco, M.; Andreu, D.; Castanho, M. The challenge of peptide proteolytic stability studies: Scarce data, difficult readability, and the need for harmonization. Angew. Chem. Int. Ed. Engl. 2020, 60, 2–5. [Google Scholar]
- Maraming, P.; Kah, J.C.Y. Conjugation of peptides to gold nanoparticles. Methods Mol. Biol. 2021, 2355, 9–16. [Google Scholar] [PubMed]
- French, G.L. Bactericidal agents in the treatment of MRSA infections-the potential role of daptomycin. J. Antimicrob. Chemother. 2006, 58, 1107–1117. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2015, 44, D1087–D1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirtskhalava, M.; Amstrong, A.A.; Grigolava, M.; Chubinidze, M.; Alimbarashvili, E.; Vishnepolsky, B.; Gabrielian, A.; Rosenthal, A.; Hurt, D.E.; Tartakovsky, M. DBAASP v3: Database of antimicrobial/cytotoxic activity and structure of peptides as a resource for development of new therapeutics. Nucleic Acids Res. 2021, 49, D288–D297. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, B.S.; Díaz-Roa, A.; Yamane, E.S.; Hayashi, M.A.F.; da Silva, P.I., Jr. Doderlin: Isolation and characterization of a broad-spectrum antimicrobial peptide from Lactobacillus acidophilus. bioRxiv 2022. [Google Scholar] [CrossRef]
- Ovchinnikov, K.V.; Chi, H.; Mehmeti, I.; Holo, H.; Nes, I.F.; Diep, D.B. Novel group of leaderless multipeptide bacteriocins from gram-positive bacteria. Appl. Environ. Microbiol. 2016, 82, 5216–5224. [Google Scholar] [CrossRef] [Green Version]
- Zouhir, A.; Jridi, T.; Nefzi, A.; Ben Hamida, J.; Sebei, K. Inhibition of methicillin-resistant Staphylococcus aureus (MRSA) by antimicrobial peptides (AMPs) and plant essential oils. Pharm. Biol. 2016, 54, 3136–3150. [Google Scholar] [CrossRef] [Green Version]
- Ciandrini, E.; Morroni, G.; Arzeni, D.; Kamysz, W.; Neubauer, D.; Kamysz, E.; Cirioni, O.; Brescini, L.; Baffone, W.; Campana, R. Antimicrobial activity of different antimicrobial peptides (AMPs) against clinical methicillin-resistant Staphylococcus aureus (MRSA). Curr. Top. Med. Chem. 2018, 18, 2116–2126. [Google Scholar] [CrossRef]
- Chowdhury, T.; Mandal, S.M.; Kumari, R.; Ghosh, A.K. Purification and characterization of a novel antimicrobial peptide (QAK) from the hemolymph of Antheraea mylitta. Biochem. Biophys. Res. Commun. 2020, 527, 411–417. [Google Scholar] [CrossRef] [PubMed]



| Samples | Zone of Inhibition (mm ± SD) | |||
|---|---|---|---|---|
| S. aureus TISTR 517 | MRSA Isolate 142 | MRSA Isolate 1096 | MRSA Isolate 2468 | |
| WUL10 | 20.15 ± 0.15 | 20.91 ± 0.29 | 20.91 ± 0.15 | 21.34 ± 0.51 |
| Cefoxitin (30 µg) | 32.68 ± 0.15 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Oxacillin (1 µg) | 29.29 ± 0.39 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Vancomycin (30 µg) | 22.94 ± 0.39 | 24.05 ± 0.15 | 23.96 ± 0.39 | 25.40 ± 0.25 |
| Samples | Total Volume (mL) | Total Protein (mg) | Total Activity (AU) | Specific Activity (AU/mg) | Purification (Fold) | Yield (%) |
|---|---|---|---|---|---|---|
| Cell-free supernatant | 1700 | 253.41 | 68,000 | 268.34 | 1.00 | 100.00 |
| Ammonium sulfate precipitation | 234 | 116.06 | 37,440 | 322.60 | 1.20 | 55.06 |
| Cation exchange chromatography | 285 | 10.33 | 22,800 | 2207.75 | 8.23 | 33.53 |
| Reversed-phase chromatography | 70 | 4.31 | 22,400 | 5201.83 | 19.39 | 32.94 |
| WUL10 | Cefoxitin | Vancomycin | ||||
|---|---|---|---|---|---|---|
| Strains | MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) |
| S. aureus TISTR 517 | 1 | 1 | 2 | 2 | 2 | 2 |
| MRSA isolate 142 | 1 | 2 | N/D | N/D | 2 | 2 |
| MRSA isolate 1096 | 1 | 2 | N/D | N/D | 2 | 2 |
| MRSA isolate 2468 | 1 | 1 | N/D | N/D | 2 | 2 |
| Zone of Inhibition (mm ± SD) | ||||
|---|---|---|---|---|
| S. aureus TISTR 517 | MRSA Isolate 142 | MRSA Isolate 1096 | MRSA Isolate 2468 | |
| Cefoxitin (30 µg) | 32.77 ± 0.55 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Vancomycin (30 µg) | 22.48 ± 0.23 | 25.61 ± 0.52 | 25.70 ± 0.55 | 26.75 ± 0.18 |
| 1× MIC WUL10 (0.1 µg) | 0.00 ± 0.00 | 0.00 ± 0.00 | 9.70 ± 0.51 | 0.00 ± 0.00 |
| 5× MIC WUL10 (0.5 µg) | 15.91 ± 0.25 | 17.10 ± 0.14 | 18.97 ± 0.39 | 19.43 ± 0.50 |
| 10× MIC WUL10 (1.0 µg) | 19.24 ± 0.15 | 20.37 ± 0.26 | 20.73 ± 0.25 | 21.81 ± 0.30 |
| 20× MIC WUL10 (2.0 µg) | 21.80 ± 0.15 | 23.03 ± 0.30 | 23.84 ± 0.39 | 25.22 ± 0.39 |
| Conditions | % Remaining Activity | |
|---|---|---|
| S. aureus TISTR 517 | MRSA Isolate 2468 | |
| Untreated sample | 100.00 ± 1.21 | 100.00 ± 1.53 |
| Sample at 60 °C, 1 h | 98.80 ± 1.20 | 99.25 ± 1.77 |
| Sample at 80 °C, 1 h | 98.39 ± 1.84 | 99.22 ± 0.67 |
| Sample at 100 °C, 1 h | 97.58 ± 1.19 * | 98.48 ± 1.32 |
| Sample at 121 °C, 15 min | 96.77 ± 0.66 * | 97.72 ± 2.27 |
| pH 1 | 100.81 ± 0.70 | 100.03 ± 2.29 |
| pH 2 | 100.41 ± 0.70 | 101.17 ± 2.31 |
| pH 3 | 100.81 ± 0.70 | 100.03 ± 2.29 |
| pH 4 | 100.41 ± 1.40 | 101.17 ± 2.31 |
| pH 5 | 100.41 ± 1.40 | 99.25 ± 1.76 |
| pH 6 | 99.19 ± 0.70 | 98.49 ± 1.71 |
| pH 7 | 100.57 ± 0.78 | 99.92 ± 1.26 |
| pH 8 | 97.96 ± 1.40 | 99.23 ± 0.66 |
| pH 9 | 97.96 ± 1.40 | 99.23 ± 0.67 |
| pH 10 | 97.56 ± 2.11 | 98.47 ± 1.33 |
| pH 11 | 97.96 ± 1.40 | 100.00 ± 1.16 |
| pH 12 | 96.74 ± 1.40 * | 98.08 ± 1.32 |
| pH 13 | 96.33 ± 1.22 * | 98.85 ± 0.01 * |
| pH 14 | 96.73 ± 0.72 * | 98.46 ± 0.66 * |
| Sample + Proteinase K (1 mg/mL) | 96.68 ± 0.71 * | 95.77 ± 0.64 * |
| Sample + Lysozyme (1 mg/mL) | 101.25 ± 1.25 | 101.16 ± 1.16 |
| Sample + Trypsin (1 mg/mL) | 97.93 ± 0.71 * | 98.47 ± 0.64 * |
| Sample + α-chymotrypsin (1 mg/mL) | 97.12 ± 1.43 * | 96.59 ± 0.08 * |
| Sample + 1% SDS | 96.30 ± 1.23 * | 92.03 ± 1.25 * |
| Sample + 1% Triton X-100 | 101.65 ± 1.89 | 100.03 ± 2.97 |
| Proteinase K (1 mg/mL) | 0.00 ± 0.00 * | 0.00 ± 0.00 * |
| Lysozyme (1 mg/mL) | 0.00 ± 0.00 * | 0.00 ± 0.00 * |
| Trypsin (1 mg/mL) | 0.00 ± 0.00 * | 0.00 ± 0.00 * |
| α-chymotrypsin (1 mg/mL) | 0.00 ± 0.00 * | 0.00 ± 0.00 * |
| 1% SDS | 95.47 ± 0.71 * | 91.28 ± 1.83 * |
| 1% Triton X-100 | 0.00 ± 0.00 * | 0.00 ± 0.00 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atipairin, A.; Songnaka, N.; Krobthong, S.; Yingchutrakul, Y.; Chinnawong, T.; Wanganuttara, T. Identification and Characterization of a Potential Antimicrobial Peptide Isolated from Soil Brevibacillus sp. WUL10 and Its Activity against MRSA Pathogens. Trop. Med. Infect. Dis. 2022, 7, 93. https://doi.org/10.3390/tropicalmed7060093
Atipairin A, Songnaka N, Krobthong S, Yingchutrakul Y, Chinnawong T, Wanganuttara T. Identification and Characterization of a Potential Antimicrobial Peptide Isolated from Soil Brevibacillus sp. WUL10 and Its Activity against MRSA Pathogens. Tropical Medicine and Infectious Disease. 2022; 7(6):93. https://doi.org/10.3390/tropicalmed7060093
Chicago/Turabian StyleAtipairin, Apichart, Nuttapon Songnaka, Sucheewin Krobthong, Yodying Yingchutrakul, Thapanee Chinnawong, and Thamonwan Wanganuttara. 2022. "Identification and Characterization of a Potential Antimicrobial Peptide Isolated from Soil Brevibacillus sp. WUL10 and Its Activity against MRSA Pathogens" Tropical Medicine and Infectious Disease 7, no. 6: 93. https://doi.org/10.3390/tropicalmed7060093
APA StyleAtipairin, A., Songnaka, N., Krobthong, S., Yingchutrakul, Y., Chinnawong, T., & Wanganuttara, T. (2022). Identification and Characterization of a Potential Antimicrobial Peptide Isolated from Soil Brevibacillus sp. WUL10 and Its Activity against MRSA Pathogens. Tropical Medicine and Infectious Disease, 7(6), 93. https://doi.org/10.3390/tropicalmed7060093










