When a Neglected Tropical Disease Goes Global: Knowledge, Attitudes and Practices of Italian Physicians towards Monkeypox, Preliminary Results
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Questionnaire
- Main demographic data: age, gender, seniority as medical professional, medical background (i.e., working as OP, PH, GP, or other medical professional); the Italian region where the professional mainly worked and lived.
- Knowledge Test. Participants received a total of 24 statements on MPX (e.g., “Typically, one out of 3 women is affected by migraine”; TRUE). A summary score (Knowledge Score; KS) was then calculated by adding +1 to a sum score for every correct answer, whereas a wrong indication or a missing/“don’t know” answer added 0 to the sum score (potential range 0 to 24).
- Risk perception. Participants were requested to rate the perceived severity (CMPX) and the perceived frequency (FMPX) of MPX in Italian population by means of a fully labeled 5-point Likert scale (range: from “not significant” to “very significant”). According to Yates [49], perceived risk can be defined as a function of the perceived probability of an event and its expected consequences, and a Risk Perception Score (RPS) was therefore calculated as the product of CMPX and FMPX (potential range 1 to 25).
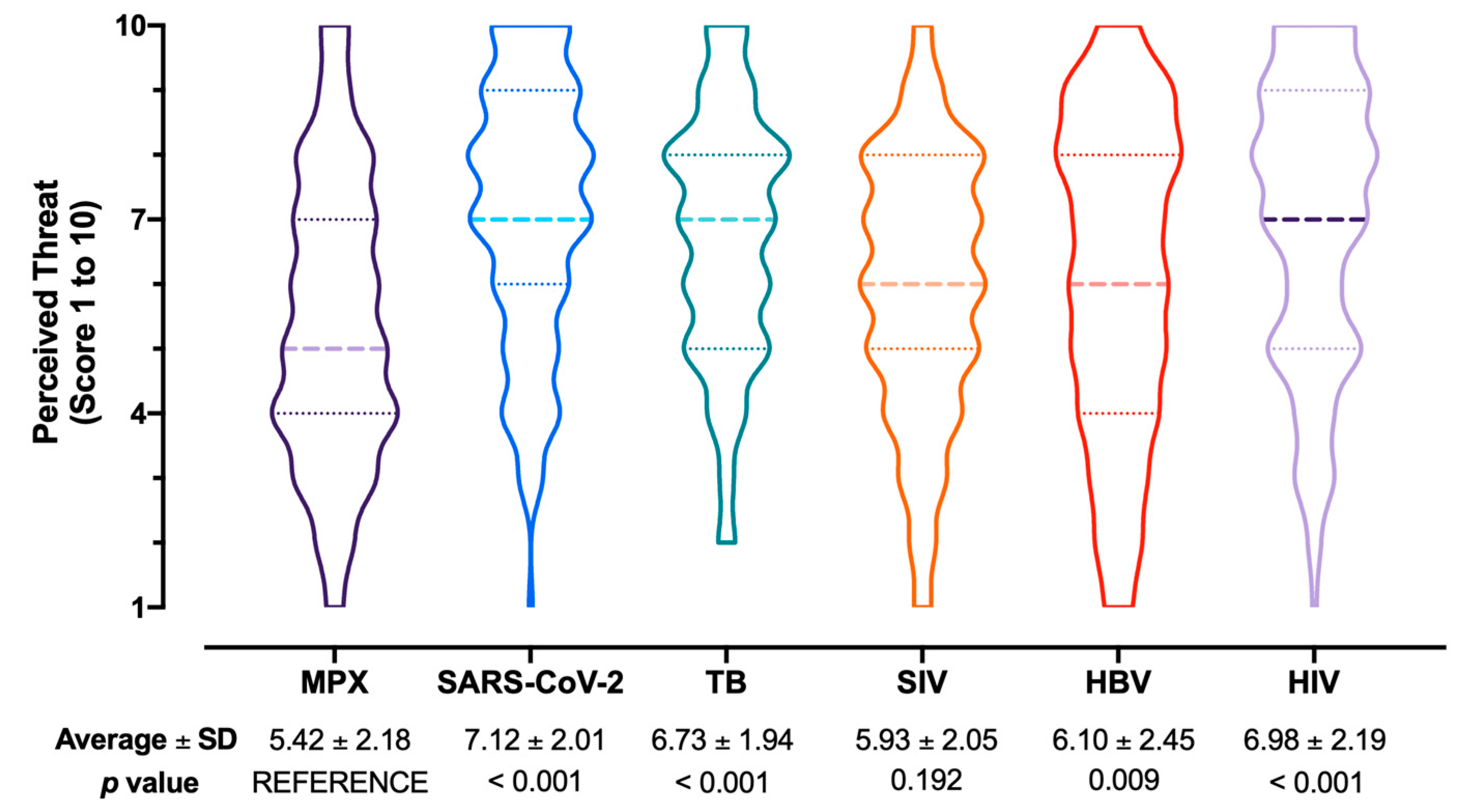
- Attitudes and Practices. The attitude towards VARV vaccine in order to prevent MPX infection was initially inquired, focusing on both the personal acceptance and the use in the general population. Both attitudes were reported in a full scale of 1 (totally disagree) to 5 (totally agree). Medical professionals were then requested to similarly rate how important they perceived the capability of the vaccine to avoid natural infection and complications, and about their willingness to pay, both as a personal expense (i.e., how much they would accept to pay for a MPX vaccine), and from a Public Health point of view (i.e., the optimal price for a MPX vaccine). Respondents were then requested to rate through a full Likert scale 1 (totally disagree) to 5 (totally agree) whether they perceived or not MPX as a likely occurrence during daily activities in the following months, whether they perceived or not MPX as potentially affecting daily working activities, and whether they were confident of not to be able to recognize a MPX case. Respondents were then asked to rate how difficult they perceived the management of different infectious diseases in the Italian settings, and more precisely: MPX, seasonal influenza virus (SIV), SARS-CoV-2, Hepatitis B virus (HBV), tuberculosis (TB), Human Immunodeficiency Virus (HIV). All of the aforementioned disorders were rated 1 (not difficult) to 10 (very difficult). Eventually, participants were requested to report whether they had received or not VARV vaccine (vaccination mandate for all Italian newborns was enforced until 1977, then suspended and eventually abolished in 1981), SIV vaccine during previous influenza season (i.e., 2021), and SARS-CoV-2 vaccine (at least 2 doses).
2.3. Ethical Considerations
2.4. Data Analysis
3. Results
3.1. Descriptive Analysis
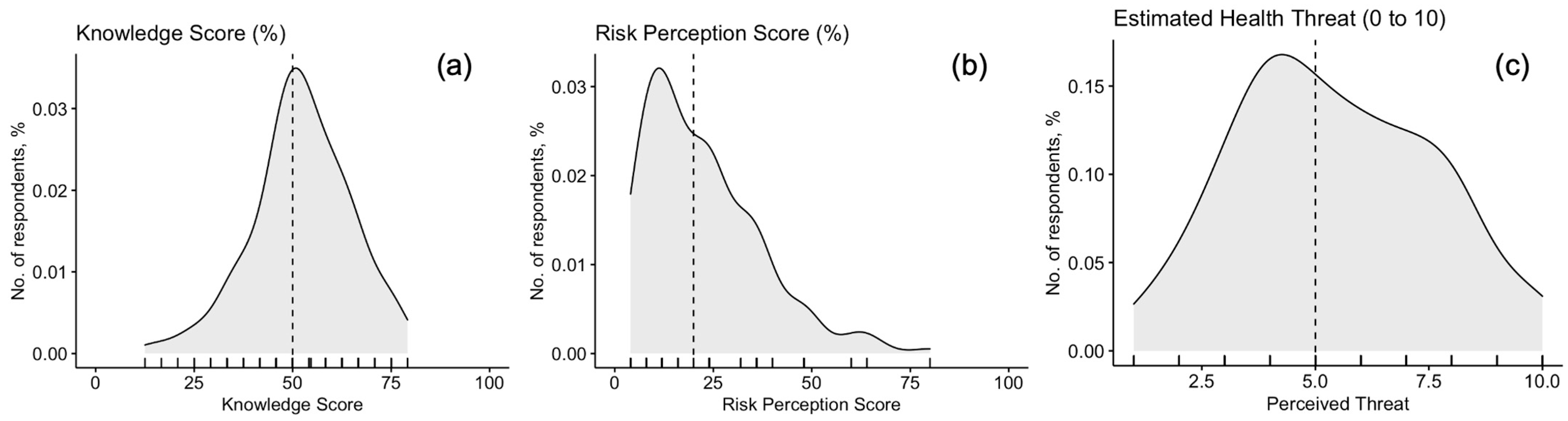
3.2. Knowledge Test
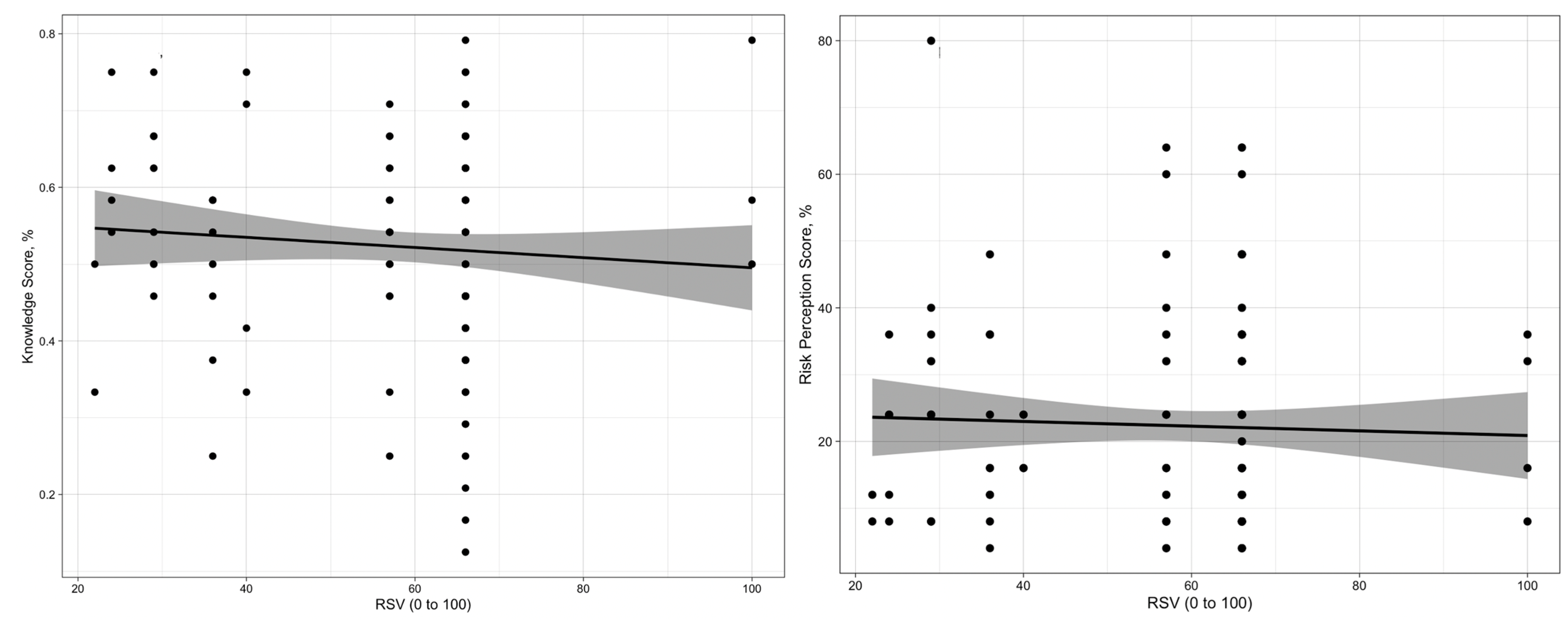
3.3. Risk Perception
3.4. Attitudes and Practices towards MPX Vaccination
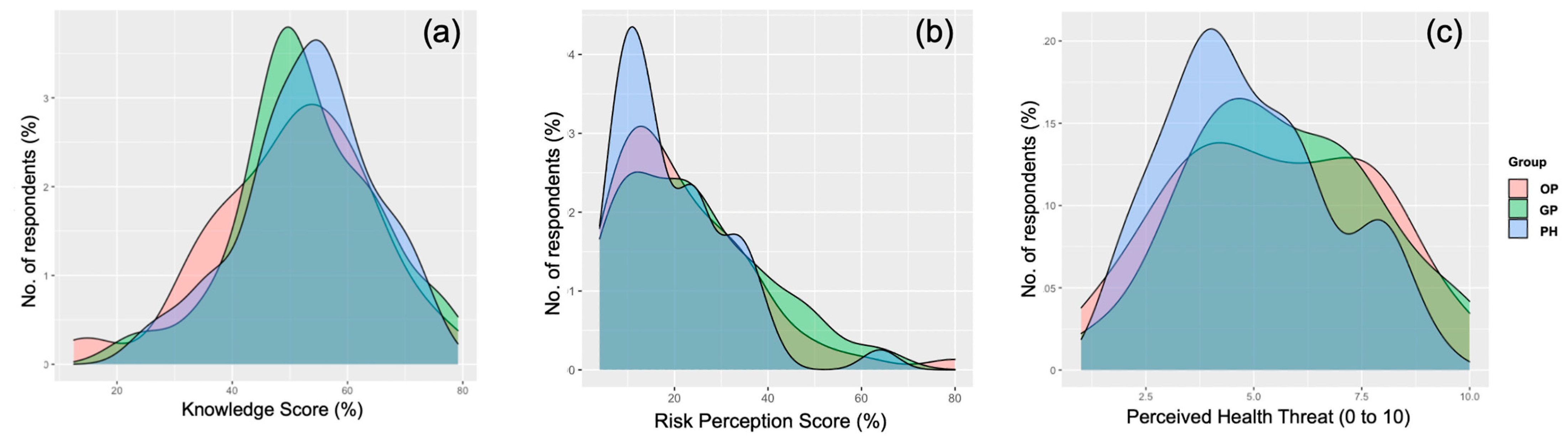
3.5. Univariate Analysis
3.6. Multivariable Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variable | Respondents | p-Value | |
|---|---|---|---|
| Included in the Analyses (No./163, %) | Not Included in the Analyses (No./283, %) | ||
| Male Gender | 57, 35.0% | 92, 32.5% | 0.670 |
| Age (years; average ± SD) | 42.9 ± 10.0 | 42.3 ± 9.8 | 0.512 |
| Seniority (years; average ± SD) | 16.3 ± 10.3 | 15.7 ± 9.9 | 0.593 |
| Living in … | 0.795 | ||
| Northern Italy 1 | 90, 55.2% | 154, 54.4% | |
| Central Italy 2 | 41, 25.2% | 63, 22.3% | |
| Southern Italy/Islands 3 | 28, 17.2% | 58, 20.5% | |
| Other EU country | 4, 2.4% | 8, 2.8% | |
| Knowledge Score (%; average ± SD) | 51.8 ± 13.9 | 50.9 ± 13.9 | 0.560 |
| Risk Perception Score (%; average ± SD) | 22.3 ± 14.6 | 22.6 ± 14.5 | 0.834 |
| Perceived Burden (0 to 10, average ± SD) | 5.4 ± 2.2 | 5.6 ± 2.1 | 0.342 |
| Favorable/Highly favorable to using smallpox vaccination against MPX | 96, 58.9% | 177, 62.5% | 0.446 |
| Favorable/Highly favorable to receive smallpox vaccination against MPX | 105, 64.4% | 191, 64.0% | 0.508 |
| Group | Knowledge Score (%) | Perceived Burden (0 to 10) | Risk Perception Score (%) | |||
|---|---|---|---|---|---|---|
| Average ± SD | p-Value | Average ± SD | p-Value | Average ± SD | p-Value | |
| Occupational Physicians | 50.0 ± 15.9 | 0.404 | 23.1 ± 15.5 | 0.509 | 5.6 ± 2.3 | 0.413 |
| General Practitioners | 52.3 ± 11.6 | 0.914 | 23.1 ± 15.2 | 0.464 | 5.5 ± 2.2 | 0.498 |
| Public Health professionals | 53.2 ± 11.4 | REF. | 20.1 ± 12.4 | REF. | 5.1 ± 2.0 | REF. |
| Total | 51.8 ± 13.0 | - | 22.3 ± 14.6 | - | 5.4 ± 2.2 | - |
| Statement | Correct Answer | Total (No./163) |
|---|---|---|
| MPX is caused by a newly discovered virus | FALSE | 154, 95.1% |
| MPX virus circulates only among primates, including humans | FALSE | 72, 44.4% |
| In most cases, MPX evolves in an uncomplicated influenza-like illness | FALSE | 79, 48.5% |
| MPX infections are associated with typical skin lesions | TRUE | 140, 85.9% |
| Asymptomatic individuals are critical in circulating MPX | FALSE | 50, 24.7% |
| Until recently, European cases of MPX have been mostly travel-associated | TRUE | 134, 82.2% |
| An effective vaccine against MPX is to date available | TRUE | 98, 60.1% |
| Effective drugs targeting MPX virus are to date available | TRUE | 83, 51.2% |
| Recipients of VARV vaccine do not need further vaccination shots to be protected against MPX | FALSE | 53, 32.5% |
| MPX may be transmitted … | ||
| … through the respiratory system | FALSE | 0, - |
| … through respiratory droplets | FALSE | 2, 1.2% |
| … through direct contagion | FALSE | 16, 9.8% |
| … through body fluids | FALSE | 17, 10.4% |
| … all of the above | TRUE | 128, 78.5% |
| Don’t know | ||
| The case-fatality ratio of MPX usually ranges between… | ||
| … 4% and 11% | TRUE | 118, 72.4% |
| … 14% and 19% | FALSE | 11, 6.7% |
| … 20% and 30% | FALSE | 2, 1.2% |
| … 30% and 40% | FALSE | 5, 3.1% |
| Don’t know | - | 27, 16.6% |
| Globally, MPX in the last decade has caused around … | ||
| … 1000 cases or less | FALSE | 46, 28.4% |
| … 1000 to 10,000 cases | FALSE | 61, 37.7% |
| … 10,000 cases or more | TRUE | 20, 12.3% |
| Don’t know | - | 35, 22.2% |
| MPX infection is associated with a high rate of systemic complications | TRUE | 34, 20.9% |
| MPX causes a less severe illness in children (age < 14 y.o.) than in adults | FALSE | 56, 34.4% |
| MPX infection is usually associated with a … lymphadenopathy. | ||
| … typical, cervical and/or inguinal … | TRUE | 94, 57.7% |
| … typical, in axillary and/or groin nodes … | FALSE | 34, 20.9% |
| … not noticeable | FALSE | 9, 5.5% |
| Don’t know | - | 26, 16.0% |
| The skin rash associated with MPX is typically asynchronous | FALSE | 46, 28.2% |
| Surface extension and profusion of MPX-associated skin lesions are of prognostic value | TRUE | 70, 42.9% |
| MPX-associated skin lesions may be differentially diagnosed as … according to their stage | ||
| Varicella/Varicella-Zoster | FALSE | 23, 14.2% |
| Typhus | FALSE | 2, 1.2% |
| Molluscum contagiosum/water warts | FALSE | 5, 3.1% |
| Syphilis | FALSE | 0, - |
| Herpes simplex | FALSE | 0, - |
| All of the above | TRUE | 132, 81.5% |
| Standard preventive measures are effective in preventing MPX infection | TRUE | 122, 74.8% |
| A clinical case characterized by: (1) atypical skin rash; (2) lymphadenopathy (cervical and/or inguinal); (3) history of travel to countries endemic for MPX | ||
| Confirmed MPX case | FALSE | 9, 5.5% |
| Probable MPX case | TRUE | 127, 77,9% |
| Doubtful MPX case | FALSE | 23, 14.1% |
| Don’t know | - | 4, 2.5% |
| A clinical case characterized by: (1) generalized or localized skin rash, either maculopapular or vesiculopustular; (2) umbilicated skin lesions; (3) lymphadenopathy | ||
| Confirmed MPX case | FALSE | 23, 14.1% |
| Probable MPX case | TRUE | 69, 42.3% |
| Doubtful MPX case | FALSE | 60, 36.8% |
| Don’t know | - | 11, 6.7% |
| The case-fatality ratio of smallpox usually ranged between… | ||
| … 4% and 11% | FALSE | 47, 28.8 |
| … 14% and 19% | FALSE | 22, 13.5% |
| … 20% and 30% | FALSE | 32, 19.6% |
| … 30% and 40% | TRUE | 29, 17.8% |
| Don’t know | - | 33, 20.2% |
| MPX is able to survive for several days on contaminated surfaces | TRUE | 69, 42.3% |
References
- McFadden, G. Poxvirus Tropism. Nat. Rev. Microbiol. 2005, 3, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Babkin, I.V.; Babkina, I.N.; Tikunova, N.V. An Update of Orthopoxvirus Molecular Evolution. Viruses 2022, 14, 388. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.E.; Hughes, J.M.; McCollum, A.M.; Damon, I.K. Human Monkeypox. Clin. Infect. Dis. 2014, 58, 260–267. [Google Scholar] [CrossRef] [Green Version]
- von Magnus, P.; Andersen, E.K.; Petersen, K.B.; Birch-Andersen, A. A Pox-like Disease in Cynomolgus Monkey. Acta Pathol. Microbiol. Scand. 1959, 46, 156–176. [Google Scholar] [CrossRef]
- Arita, I.; Henderson, D.A. Smallpox and Monkeypox in Non-Human Primates. Bull. World Health Organ. 1968, 39, 277. [Google Scholar]
- Rezza, G. Emergence of Human Monkeypox in West Africa. Lancet Infect. Dis. 2019, 19, 872–879. [Google Scholar] [CrossRef]
- Ladnyj, D.; Ziegler, P.; Kima, E. A Human Infection Caused by Monkeypox Virus in Basankusu Territory, Democratic Republic of the Congo *. Bull. Org. Mond. Sante 1972, 46, 4. [Google Scholar]
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The Changing Epidemiology of Human Monkeypox—A Potential Threat? A Systematic Review. PLoS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef]
- Beer, E.M.; Bhargavi Rao, V. A Systematic Review of the Epidemiology of Human Monkeypox Outbreaks and Implications for Outbreak Strategy. PLoS Negl. Trop. Dis. 2019, 13, e0007791. [Google Scholar] [CrossRef] [Green Version]
- Sejvar, J.J.; Chowdary, Y.; Schomogyi, M.; Stevens, J.; Patel, J.; Karem, K.; Fischer, M.; Kuehnert, M.J.; Zaki, S.R.; Paddock, C.D.; et al. Human Monkeypox Infection: A Family Cluster in the Midwestern United States. J. Infect. Dis. 2004, 190, 1833–1840. [Google Scholar] [CrossRef]
- Simpson, K.; Heymann, D.; Brown, C.S.; Edmunds, W.J.; Elsgaard, J.; Fine, P.; Hochrein, H.; Hoff, N.A.; Green, A.; Ihekweazu, C.; et al. Human Monkeypox—After 40 Years, an Unintended Consequence of Smallpox Eradication. Vaccine 2020, 38, 5077–5081. [Google Scholar] [CrossRef] [PubMed]
- Rimoin, A.W.; Mulembakani, P.M.; Johnston, S.C.; Smith, J.O.L.; Kisalu, N.K.; Kinkela, T.L.; Blumberg, S.; Thomassen, H.A.; Pike, B.L.; Fair, J.N.; et al. Major Increase in Human Monkeypox Incidence 30 Years after Smallpox Vaccination Campaigns Cease in the Democratic Republic of Congo. Proc. Natl. Acad. Sci. USA 2010, 107, 16262–16267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alakunle, E.; Moens, U.; Nchinda, G.; Okeke, M.I. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses 2020, 12, 1257. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.Y.; Ajisegiri, W.S.; Costantino, V.; Chughtai, A.A.; MacIntyre, C.R. Reemergence of Human Monkeypox and Declining Population Immunity in the Context of Urbanization, Nigeria, 2017–2020. Emerg. Infect. Dis. 2021, 27, 1007–1014. [Google Scholar] [CrossRef]
- Hobson, G.; Adamson, J.; Adler, H.; Firth, R.; Gould, S.; Houlihan, C.; Johnson, C.; Porter, D.; Rampling, T.; Ratcliffe, L.; et al. Family Cluster of Three Cases of Monkeypox Imported from Nigeria to the United Kingdom, May 2021. Euro Surveill 2021, 26, 2100745. [Google Scholar] [CrossRef]
- Adler, H.; Gould, S.; Hine, P.; Snell, L.B.; Wong, W.; Houlihan, C.F.; Osborne, J.C.; Rampling, T.; Beadsworth, M.B.; Duncan, C.J.; et al. Clinical Features and Management of Human Monkeypox: A Retrospective Observational Study in the UK. Lancet Infect. Dis. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Mauldin, M.R.; McCollum, A.M.; Nakazawa, Y.J.; Mandra, A.; Whitehouse, E.R.; Davidson, W.; Zhao, H.; Gao, J.; Li, Y.; Doty, J.; et al. Exportation of Monkeypox Virus From the African Continent. J. Infect. Dis. 2022, 225, 1367–1376. [Google Scholar] [CrossRef]
- Rao, A.K.; Schulte, J.; Chen, T.-H.; Hughes, C.M.; Davidson, W.; Neff, J.M.; Markarian, M.; Delea, K.C.; Wada, S.; Liddell, A.; et al. Monkeypox in a Traveler Returning from Nigeria—Dallas, Texas, July 2021. Morb. Mortal. Wkly. Rev. 2022, 71, 509–516. [Google Scholar] [CrossRef]
- Petersen, E.; Kantele, A.; Koopmans, M.; Asogun, D.; Yinka-Ogunleye, A.; Ihekweazu, C.; Zumla, A. Human Monkeypox: Epidemiologic and Clinical Characteristics, Diagnosis, and Prevention. Infect. Dis. Clin. N. Am. 2019, 33, 1027–1043. [Google Scholar] [CrossRef] [Green Version]
- Erez, N.; Achdout, H.; Milrot, E.; Schwartz, Y.; Wiener-Well, Y.; Paran, N.; Politi, B.; Tamir, H.; Israely, T.; Weiss, S.; et al. Diagnosis of Imported Monkeypox, Israel, 2018. Emerg. Infect. Dis. 2019, 25, 980–983. [Google Scholar] [CrossRef] [Green Version]
- Velavan, T.P.; Meyer, C.G. Monkeypox 2022 Outbreak: An Update. Trop. Med. Int. Health 2022, 27, 604–605. [Google Scholar] [CrossRef]
- Dye, C.; Kraemer, M.U.G. Investigating the Monkeypox Outbreak. BMJ 2022, 377, o1314. [Google Scholar] [CrossRef] [PubMed]
- Zumla, A.; Valdoleiros, S.R.; Haider, N.; Asogun, D.; Ntoumi, F.; Petersen, E.; Kock, R. Monkeypox Outbreaks Outside Endemic Regions: Scientific and Social Priorities. Lancet Infect. Dis. 2022, 22, 929–931. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Disease Outbreak News: Multi-Country Monkeypox Outbreak in Non-Endemic Countries Outbreak at Glance; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- European Centre for Disease Prevention (ECDC). Monkeypox Multi-Country Outbreak Key Messages; European Centre for Disease Prevention: Stockholm, Sweden, 2022. [Google Scholar]
- European Centre for Disease Prevention and Control (ECDC); World Health Organization (WHO). Joint ECDC-WHO Regional Office for Europe Monkeypox Surveillance Bulletin2; European Centre for Disease Prevention and Control: Stockholm, Sweden; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- World Health Organization (WHO) Multi-Country Monkeypox Outbreak: Situation Update Outbreak at a Glance Description of the Outbreak; World Health Organization: Geneva, Switzerland, 2022.
- Riccò, M. When a Neglected Tropical Disease Goes Global: Early Estimates from the Monkeypox Outbreak, the First 1054 cases. Acta Biomed 2022, 93. epub ahead of print. [Google Scholar]
- Ilic, I.; Ilic, M. Historical Review: Towards the 50th Anniversary of the Last Major Smallpox Outbreak (Yugoslavia, 1972). Travel Med. Infect. Dis. 2022, 48, 102327. [Google Scholar] [CrossRef] [PubMed]
- Ihekweazu, C.; Yinka-Ogunleye, A.; Lule, S.; Ibrahim, A. Importance of Epidemiological Research of Monkeypox: Is Incidence Increasing? Expert Rev. Anti-Infect. Ther. 2020, 18, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.; Bergna, A.; della Ventura, C.; Tarkowki, M.; Riva, A.; Moschese, D.; Rizzardini, G.; Antinori, S.; Zehender, G.; First Monkeypox Genome Sequence from Italy. First Monkeypox Genome Sequence from Italy-Genome Reports-Virological. Available online: https://virological.org/t/first-monkeypox-genome-sequence-from-italy/824/print (accessed on 30 May 2022).
- Isidro, J.; Borges, V.; Pinto, M.; Ferreira, R.; Sobral, D.; Nuner, A.; Dourado Santos, J.; Borrego, M.J.; Nuncio, S.; Pelerito, A.; et al. First Draft Genome Sequence of Monkeypox Virus Associated with the Suspected Multi-Country Outbreak, May 2022 (Confirmed Case in Portugal). Available online: https://virological.org/t/first-draft-genome-sequence-of-monkeypox-virus-associated-with-the-suspected-multi-country-outbreak-may-2022-confirmed-case-in-portugal/799 (accessed on 30 May 2022).
- Harapan, H.; Setiawan, A.M.; Yufika, A.; Anwar, S.; Wahyuni, S.; Asrizal, F.W.; Sufri, M.R.; Putra, R.P.; Wijayanti, N.P.; Salwiyadi, S.; et al. Knowledge of Human Monkeypox Viral Infection among General Practitioners: A Cross-Sectional Study in Indonesia. Pathog. Glob. Health 2020, 114, 68–75. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Informal Consultation on Monkeypox 2017—Current Status in West and Central Africa (WHO/WHE/IHM/2018.3); World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Harapan, H.; Setiawan, A.M.; Yufika, A.; Anwar, S.; Wahyuni, S.; Asrizal, F.W.; Sufri, M.R.; Putra, R.P.; Wijayanti, N.P.; Salwiyadi, S.; et al. Confidence in Managing Human Monkeypox Cases in Asia: A Cross-Sectional Survey among General Practitioners in Indonesia. Acta Trop. 2020, 206, 105450. [Google Scholar] [CrossRef]
- Harapan, H.; Wagner, A.L.; Yufika, A.; Setiawan, A.M.; Anwar, S.; Wahyuni, S.; Asrizal, F.W.; Sufri, M.R.; Putra, R.P.; Wijayanti, N.P.; et al. Acceptance and Willingness to Pay for a Hypothetical Vaccine against Monkeypox Viral Infection among Frontline Physicians: A Cross-Sectional Study in Indonesia. Vaccine 2020, 38, 6800–6806. [Google Scholar] [CrossRef]
- Betsch, C.; Wicker, S. Personal Attitudes and Misconceptions, Not Official Recommendations Guide Occupational Physicians’ Vaccination Decisions. Vaccine 2014, 32, 4478–4484. [Google Scholar] [CrossRef]
- Riccò, M.; Ferraro, P.; Camisa, V.; di Palma, P.; Minutolo, G.; Ranzieri, S.; Zaffina, S.; Baldassarre, A.; Restivo, V. Managing of Migraine in the Workplaces: Knowledge, Attitudes and Practices of Italian Occupational Physicians. Medicina (Kaunas) 2022, 58, 686. [Google Scholar] [CrossRef]
- Riccò, M.; Gualerzi, G.; Ranzieri, S.; Ferraro, P.; Bragazzi, N.L. Knowledge, Attitudes, Practices (KAP) of Italian Occupational Physicians towards Tick Borne Encephalitis. Trop. Med. Infect. Dis. 2020, 5, 117. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtsche, P.C.; Vandenbroucke, J.P. STROBE Initiative Strengthening the Reporting of Observational Studies in Epidemiology (StroBE) Statement: Guidelines for Reporting Observational Studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- di Giulio, D.B.; Eckburg, P.B. Human Monkeypox: An Emerging Zoonosis. Lancet Infect. Dis. 2004, 4, 15–25. [Google Scholar] [CrossRef]
- Rao, A.K.; Petersen, B.W.; Whitehill, F.; Razeq, J.H.; Isaacs, S.N.; Merchlinsky, M.J.; Campos-Outcalt, D.; Morgan, R.L.; Damon, I.; Sánchez, P.J.; et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for Preexposure Vaccination of Persons at Risk for Occupational Exposure to Orthopoxviruses: Recommendations of the Advisory Committee on Immunization Practices-United States, 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 1–9. [Google Scholar] [CrossRef]
- Berthet, N.; Descorps-Declère, S.; Besombes, C.; Curaudeau, M.; Nkili Meyong, A.A.; Selekon, B.; Labouba, I.; Gonofio, E.C.; Ouilibona, R.S.; Simo Tchetgna, H.D.; et al. Genomic History of Human Monkey Pox Infections in the Central African Republic between 2001 and 2018. Sci. Rep. 2021, 11, 13085. [Google Scholar] [CrossRef]
- Mbala, P.K.; Huggins, J.W.; Riu-Rovira, T.; Ahuka, S.M.; Mulembakani, P.; Rimoin, A.W.; Martin, J.W.; Muyembe, J.J.T. Maternal and Fetal Outcomes among Pregnant Women with Human Monkeypox Infection in the Democratic Republic of Congo. J. Infect. Dis. 2017, 216, 824–828. [Google Scholar] [CrossRef]
- Whitehouse, E.R.; Bonwitt, J.; Hughes, C.M.; Lushima, R.S.; Likafi, T.; Nguete, B.; Kabamba, J.; Monroe, B.; Doty, J.B.; Nakazawa, Y.; et al. Clinical and Epidemiological Findings from Enhanced Monkeypox Surveillance in Tshuapa Province, Democratic Republic of the Congo during 2011–2015. J. Infect. Dis. 2021, 223, 1870–1878. [Google Scholar] [CrossRef]
- Reynolds, M.G.; Yorita, K.L.; Kuehnert, M.J.; Davidson, W.B.; Huhn, G.D.; Holman, R.C.; Damon, I.K. Clinical Manifestations of Human Monkeypox Influenced by Route of Infection. J. Infect. Dis. 2006, 194, 773–780. [Google Scholar] [CrossRef]
- Heymann, D.L.; Simpson, K. The Evolving Epidemiology of Human Monkeypox: Questions Still to Be Answered. J. Infect. Dis. 2021, 223, 1839–1841. [Google Scholar] [CrossRef]
- Sivapalasingam, S.; Kennedy, J.S.; Borkowsky, W.; Valentine, F.; Zhan, M.X.; Pazoles, P.; Paolino, A.; Ennis, F.A.; Steigbigel, N.H. Immunological Memory after Exposure to Variola Virus, Monkeypox Virus, and Vaccinia Virus. J. Infect. Dis. 2007, 195, 1151–1159. [Google Scholar] [CrossRef] [Green Version]
- Yates, F.J.; Stone, E.R. The Risk Construct. In Risk-Taking Behaviour; Yates, F.J., Ed.; John Wiley & Sons.: Chichester, UK, 1992; pp. 1–25. ISBN 0471922501. [Google Scholar]
- Riccò, M.; Ferraro, P.; Peruzzi, S.; Balzarini, F.; Ranzieri, S. Mandate or Not Mandate: Knowledge, Attitudes, and Practices of Italian Occupational Physicians towards SARS-CoV-2 Immunization at the Beginning of Vaccination Campaign. Vaccines 2021, 9, 889. [Google Scholar] [CrossRef] [PubMed]
- Ricco, M.; Gualerzi, G.; Ranzieri, S.; Ferraro, P. West Nile Virus Infection: Before Involving Occupational Physicians in Active Surveillance, Make Sure They Are More Aware. Infect. Dis. Now. 2021, 51, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Kaynarcalidan, O.; Moreno Mascaraque, S.; Drexler, I. Vaccinia Virus: From Crude Smallpox Vaccines to Elaborate Viral Vector Vaccine Design. Biomedicines 2021, 9, 1780. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Cattani, S.; Casagranda, F.; Gualerzi, G.; Signorelli, C. Knowledge, Attitudes, Beliefs and Practices of Occupational Physicians towards Vaccinations of Health Care Workers: A Cross Sectional Pilot Study in North-Eastern Italy. Int. J. Occup. Med. Environ. Health 2017, 30, 775–790. [Google Scholar] [CrossRef]
- Riccò, M.; Vezzosi, L.; Gualerzi, G.; Bragazzi, N.L.; Balzarini, F. Pertussis Immunization in Healthcare Workers Working in Pediatric Settings: Knowledge, Attitudes and Practices (KAP) of Occupational Physicians. J. Prev. Med. Hyg. 2020, 61, E66. [Google Scholar] [CrossRef]
- Gilchuk, I.; Gilchuk, P.; Sapparapu, G.; Lampley, R.; Singh, V.; Kose, N.; Blum, D.L.; Hughes, L.J.; Satheshkumar, P.S.; Townsend, M.B.; et al. Cross-Neutralizing and Protective Human Antibody Specificities to Poxvirus Infections. Cell 2016, 167, 684–694.e9. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Li, G.; Liszewski, M.K.; Atkinson, J.P.; Jahrling, P.B.; Feng, Z.; Schriewer, J.; Buck, C.; Wang, C.; Lefkowitz, E.J.; et al. Virulence Differences between Monkeypox Virus Isolates from West Africa and the Congo Basin. Virology 2005, 340, 46–63. [Google Scholar] [CrossRef] [Green Version]
- Falato, R.; Ricciardi, S.; Franco, G. Influenza Risk Perception and Vaccination Attitude in Medical and Nursing Students during the Vaccination Campaigns of 2007/2008 (Seasonal Influenza) and 2009/2010 (H1N1 Influenza). Med. Del. Lav. 2011, 102, 208–215. [Google Scholar]
- Ives, J.; Greenfield, S.; Parry, J.M.; Draper, H.; Gratus, C.; Petts, J.I.; Sorell, T.; Wilson, S. Healthcare Workers’ Attitudes to Working during Pandemic Influenza: A Qualitative Study. BMC Public Health 2009, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- La Torre, G.; Semyonov, L.; Mannocci, A.; Boccia, A. Knowledge, Attitude, and Behaviour of Public Health Doctors towards Pandemic Influenza Compared to the General Population in Italy. Scand. J. Soc. Med. 2012, 40, 69–75. [Google Scholar] [CrossRef]
- Santos, A.J.; Kislaya, I.; Machado, A.; Nunes, B. Beliefs and Attitudes towards the Influenza Vaccine in High-Risk Individuals. Epidemiol. Infect. 2017, 145, 1786–1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riccò, M.; Cattani, S.; Casagranda, F.; Gualerzi, G.; Signorelli, C. Knowledge, Attitudes, Beliefs and Practices of Occupational Physicians towards Seasonal Influenza Vaccination: A Cross-Sectional Study from North-Eastern Italy. J. Prev. Med. Hyg. 2017, 58, E141–E154. [Google Scholar] [PubMed]
- Petersen, E.; Koopmans, M.; Go, U.; Hamer, D.H.; Petrosillo, N.; Castelli, F.; Storgaard, M.; al Khalili, S.; Simonsen, L. Comparing SARS-CoV-2 with SARS-CoV and Influenza Pandemics. Lancet 2020, 20, e238–e244. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC); World Health Organization (WHO) Regional Office for Europe. Monkeypox, Joint Epidemiological Overview; European Centre for Disease Prevention and Control: Stockholm, Sweden; World Health Organization (WHO) Regional Office for Europe: Geneva, Switzerland, 2022. [Google Scholar]
- Shchelkunov, S.N.; Totmenin, A.V.; Safronov, P.F.; Mikheev, M.V.; Gutorov, V.V.; Ryazankina, O.I.; Petrov, N.A.; Babkin, I.V.; Uvarova, E.A.; Sandakhchiev, L.S.; et al. Analysis of the Monkeypox Virus Genome. Virology 2002, 297, 172–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, J.R.; Isaacs, S.N. Monkeypox Virus and Insights into Its Immunomodulatory Proteins. Immunol. Rev. 2008, 225, 96–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De, P.; Chakraborty, I.; Karna, B.; Mazumder, N. Brief Review on Repurposed Drugs and Vaccines for Possible Treatment of COVID-19. Eur. J. Pharmacol. 2021, 898, 173977. [Google Scholar] [CrossRef] [PubMed]
- WHO. Advisory Commitee on Variola Virus Research. In Proceedings of the Report of the Twenty-Third Meeting Virtual Meeting, Geneva, Switzerland, 3–4 November 2021. [Google Scholar]
- Loulergue, P.; Moulin, F.; Vidal-Trecan, G.; Absi, Z.; Demontpion, C.; Menager, C.; Gorodetsky, M.; Gendrel, D.; Guillevin, L.; Launay, O. Knowledge, Attitudes and Vaccination Coverage of Healthcare Workers Regarding Occupational Vaccinations. Vaccine 2009, 27, 4240–4243. [Google Scholar] [CrossRef]
- European Centre For Disease Prevention and Control (ECDC). Vaccine Hesitancy among Healthcare Workers and Their Patients in Europe; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2015; ISBN 9789291937226. [Google Scholar]
- MacDonald, N.E.; Eskola, J.; Liang, X.; Chaudhuri, M.; Dube, E.; Gellin, B.; Goldstein, S.; Larson, H.; Manzo, M.L.; Reingold, A.; et al. Vaccine Hesitancy: Definition, Scope and Determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Poland, G.A. Vaccination Policies for Healthcare Workers in Europe. Vaccine 2014, 32, 4876–4880. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Theodoridou, K.; Ledda, C.; Rapisarda, V.; Theodoridou, M. Vaccination of Healthcare Workers: Is Mandatory Vaccination Needed? Expert Rev. Vaccines 2019, 18, 5–13. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Gargalianos, P.; Nikolaidis, P.; Katerelos, P.; Tedoma, N.; Maltezos, E.; Lazanas, M. Attitudes towards Mandatory Vaccination and Vaccination Coverage against Vaccine-Preventable Diseases among Health-Care Workers in Tertiary-Care Hospitals. J. Infect. 2012, 64, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Montagna, M.T.; Napoli, C.; Tafuri, S.; Agodi, A.; Auxilia, F.; Casini, B.; Coscia, M.F.; D’Errico, M.M.; Ferrante, M.; Fortunato, A.; et al. Knowledge about Tuberculosis among Undergraduate Health Care Students in 15 Italian Universities: A Cross-Sectional Study. BMC Public Health 2014, 14, 970. [Google Scholar] [CrossRef] [PubMed]
- Bakhache, P.; Rodrigo, C.; Davie, S.; Ahuja, A.; Sudovar, B.; Crudup, T.; Rose, M. Health Care Providers’ and Parents’ Attitudes toward Administration of New Infant Vaccines-A Multinational Survey. Eur. J. Pediatrics 2013, 172, 485–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, C.; Maillard, A.; Bodelet, C.; Claudel, A.-L.; Gaillat, J.; Delory, T. Hesitancy towards COVID-19 Vaccination among Healthcare Workers: A Multi-Centric Survey in France. Vaccines 2021, 9, 547. [Google Scholar] [CrossRef] [PubMed]
- Graffigna, G.; Palamenghi, L.; Barello, S.; Stefania, B. “Cultivating” Acceptance of a COVID-19 Vaccination Program: Lessons from Italy. Vaccine 2020, 38, 7585–7586. [Google Scholar] [CrossRef]
- Verger, P.; Scronias, D.; Dauby, N.; Adedzi, K.A.; Gobert, C.; Bergeat, M.; Gagneur, A.; Dubé, E. Attitudes of Healthcare Workers towards COVID-19 Vaccination: A Survey in France and French-Speaking Parts of Belgium and Canada, 2020. Euro Surveill 2021, 26, 2002047. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Wicker, S.; Borg, M.; Heininger, U.; Puro, V.; Theodoridou, M.; Poland, G.A. Vaccination Policies for Health-Care Workers in Acute Health-Care Facilities in Europe. Vaccine 2011, 29, 9557–9562. [Google Scholar] [CrossRef]
- Brunelli, L.; Antinolfi, F.; Malacarne, F.; Cocconi, R.; Brusaferro, S. A Wide Range of Strategies to Cope with Healthcare Workers’ Vaccine Hesitancy in A North-Eastern Italian Region: Are They Enough? Healthcare 2020, 9, 4. [Google Scholar] [CrossRef]
- Heiervang, E.; Goodman, R. Advantages and Limitations of Web-Based Surveys: Evidence from a Child Mental Health Survey. Soc. Psychiat. Epidemiol. 2011, 46, 69–76. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, S.; Lei, W.; Zhao, Y.; Liu, H.; Yao, D.; Xu, Y.; Lv, Q.; Hao, G.; Xu, Y.; et al. Knowledge, Attitudes, and Practices Regarding Zika: Paper and Internet Based Survey in Zhejiang, China. JMIR Public Health Surveill. 2017, 3, e81. [Google Scholar] [CrossRef]
- Riccò, M.; Vezzosi, L.; Balzarini, F. Challenges Faced by the Italian Medical Workforce. Lancet 2020, 395, e55–e56. [Google Scholar] [CrossRef]
- Vicarelli, G.; Pavolini, E. Health Workforce Governance in Italy. Health Policy 2015, 119, 1606–1612. [Google Scholar] [CrossRef] [PubMed]
- Harapan, H.; Wagner, A.L.; Yufika, A.; Winardi, W.; Anwar, S.; Gan, A.K.; Setiawan, A.M.; Rajamoorthy, Y.; Sofyan, H.; Vo, T.Q.; et al. Willingness-to-Pay for a COVID-19 Vaccine and Its Associated Determinants in Indonesia. Hum. Vaccines Immunother. 2020, 16, 2074–3080. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Valente, M.; Marchesi, F. Are Symptoms Associated with SARS-CoV-2 Infections Evolving over Time? Infect. Dis. Now 2022, 52, 110–112. [Google Scholar] [CrossRef]
- Bragazzi, N.; Kong, J.D.; Mahroum, N.; Tsigalou, C.; Khasimy-Farah, R.; Converti, M.; Wu, J. Epidemiological Trends and Clinical Features of the Ongoing Monkeypox Epidemic: A Preliminary Pooled Data Analysis and Literature Review. J. Med. Virol. 2022. epub ahead of print. [Google Scholar]
- Manzoli, L.; Sotgiu, G.; Magnavita, N.; Durando, P.; Barchitta, M.; Carducci, A.; Conversano, M.; de Pasquale, G.; Dini, G.; Firenze, A.; et al. Evidence-Based Approach for Continuous Improvement of Occupational Health. Epidemiol. Prev. 2015, 39, 81–85. [Google Scholar]
- Minhaj, F.S.; Ogale, Y.P.; Whitehill, F.; Schultz, J.; Foote, M.; Davidson, W.; Hughes, C.M.; Wilkins, K.; Bachmann, L.; Chatelain, R.; et al. Morbidity and Mortality Weekly Report Monkeypox Outbreak-Nine States, May 2022. Morb. Mortal. Wkly. Rev. 2022, 71, 1–6. [Google Scholar]
- Perez Duque, M.; Ribeiro, S.; Vieira Martins, J.; Casaca, P.; Pinto Leite, P.; Tavares, M.; Mansinho, K.; Duque, L.M.; Fernandes, C.; Cordeiro, R.; et al. Ongoing Monkeypox Virus Outbreak, Portugal, 29 April to 23 May 2022. Eurosurveillance 2022, 27, 2200424. [Google Scholar] [CrossRef]
- Antinori, A.; Mazzotta, V.; Vita, S.; Carletti, F.; Tacconi, D.; Lapini, L.E.; D’Abramo, A.; Cicalini, S.; Lapa, D.; Pittalis, S.; et al. Epidemiological, Clinical and Virological Characteristics of Four Cases of Monkeypox Support Transmission through Sexual Contact, Italy, May 2022. Euro. Surveill. 2022, 27, 2200421. [Google Scholar] [CrossRef]

| Variable | No./163 | Average ± SD |
|---|---|---|
| Gender | ||
| Male | 57, 35.0% | |
| Female | 106, 65.0% | |
| Age (years) | 42.9 ± 10.0 | |
| Age ≥ 50 years | 35, 21.5% | |
| Seniority (years) | 16.3 ± 10.3 | |
| Seniority ≥ 20 years | 51, 31.3% | |
| Working as … | ||
| Occupational Physician | 49, 30.1% | |
| General Practitioner | 73, 44.8% | |
| Public Health Professional | 41, 25.2% | |
| Living in … | ||
| Northern Italy 1 | 90, 55.2% | |
| Central Italy 2 | 41, 25.2% | |
| Southern Italy/Islands 3 | 28, 17.2% | |
| Other EU country | 4, 2.4% | |
| Previously vaccinated against smallpox | 35, 21.5% | |
| Previous knowledge of MPX | 44, 27.0% | |
| Any University-level formation on smallpox | 69, 42.3% | |
| Acknowledging MPX infection in Europe as … | ||
| … frequent/very frequent | 6, 3.7% | |
| … severe/very severe | 35, 21.5% | |
| Perceiving MPX as a likely occurrence during daily activity (agree/totally agree) | 49, 30.1% | |
| Perceiving MPX as potentially affecting daily working activities (agree/totally agree) | 53, 32.5% | |
| Confident to be able to recognize a MPX case (agree/totally agree) | 27, 16.6% | |
| General Knowledge Score (%) | 51.8 ± 13.0 | |
| General Knowledge Score > median (50.0%) | 81, 49.7% | |
| Risk Perception Score | 22.3 ± 14.6 | |
| Risk Perception Score > median (20.0%) | 80, 49.1% | |
| Favorable/Highly favorable to using smallpox vaccination against MPX | 96, 58.9% | |
| Favorable/Highly favorable to receive smallpox vaccination against MPX | 105, 64.4% | |
| Vaccinated against SARS-CoV-2 during 2022 | 163, 100% | |
| Vaccinated against Seasonal Influenza during 2021 | 137, 84.0% | |
| Acknowledging as significant/very significant aspects for candidate MPX vaccines … | ||
| … avoiding natural infection | 147, 90.2% | |
| … avoiding complications | 148, 90.8% | |
| Willingness to pay for vaccine | ||
| Not interested | 52, 31.9% | |
| <10€ per shot | 39, 23.9% | |
| 10–49€ per shot | 33, 20.2% | |
| 50–99€ per shot | 21, 12.9% | |
| ≥100€ per shot | 18, 11.0% | |
| Optimal price for vaccine | ||
| It should be offered at no cost | 107, 65.6% | |
| <10€ per shot | 26, 16.0% | |
| 10–49€ per shot | 24, 14.7% | |
| 50–99€ per shot | 4, 2.5% | |
| ≥100€ per shot | 2, 1.2% |
| Variable | Attitude towards VARV Vaccination | p-Value | ||
|---|---|---|---|---|
| Somewhat Agree (No./93, %) | Somewhat Disagree (No./63, %) | adjOR (95%CI) | ||
| Male Gender | 35, 36.5% | 22, 32.8% | 0.756 | - |
| Age > 50 years | 14, 14.6% | 21, 31.3% | 0.018 | 2.224 (0.252; 19.645) |
| Seniority > 20 years | 23, 24.0% | 28, 41.8% | 0.025 | 0.723 (0.176; 2.978) |
| Working as … | 0.322 | - | ||
| Occupational Physician | 32, 33.3% | 17, 25.4% | ||
| General Practitioner | 43, 44.8% | 30, 44.8% | ||
| Public Health Professional | 21, 21.9% | 20, 29.9% | ||
| Living in … | 0.401 | - | ||
| Northern Italy 1 | 55, 57.3% | 32, 52.2% | ||
| Central Italy 2 | 21, 21.9% | 20, 29.9% | ||
| Southern Italy/Islands 3 | 16, 16.7% | 12, 17.9% | ||
| Other EU country | 4, 4.2% | 0, - | ||
| Previously vaccinated against smallpox | 13, 13.5% | 22, 32.8% | 0.006 | 0.213 (0.037; 1.223) |
| Previous knowledge of MPX | 24, 25.0% | 20, 29.9% | 0.612 | - |
| Any University-level formation on smallpox | 42, 43.8% | 27, 40.3% | 0.781 | - |
| Acknowledging MPX infection in Europe as … | ||||
| … frequent/very frequent | 3, 3.1% | 3, 4.5% | 0.977 | - |
| … severe/very severe | 22, 22.9% | 13, 19.4% | 0.731 | - |
| Perceiving MPX as a likely occurrence during daily activity (agree/totally agree) | 31, 32.3% | 18, 26.9% | 0.569 | |
| Perceiving MPX as potentially affecting daily working activities (agree/totally agree) | 30, 31.3% | 23, 34.3% | 0.808 | - |
| Confident to be able to recognize a MPX case (agree/totally agree) | 18, 18.8% | 9, 13.4% | 0.494 | - |
| Knowledge Score, > median (50.0%) | 45, 46.9% | 36, 53.7% | 0.483 | - |
| Risk Perception Score, > median (20.0%) | 55, 57.3% | 25, 37.3% | 0.019 | 0.846 (0.348; 2.059) |
| Favorable/Highly favorable to receive smallpox vaccination against MPX | 86, 89.6% | 19, 28.4% | < 0.001 | 21.416 (7.290; 62.914) |
| Vaccinated against Seasonal Influenza during 2021 | 90, 93.8% | 47, 70.1% | < 0.001 | 6.443 (1.798; 23.093) |
| Willingness to pay for vaccine | ||||
| Not interested to pay | 18, 18.8% | 34, 50.7% | < 0.001 | 1.047 (0.348; 3.154) |
| It should be offered at no cost | 59, 61.5% | 48, 71.6% | 0.238 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riccò, M.; Ferraro, P.; Camisa, V.; Satta, E.; Zaniboni, A.; Ranzieri, S.; Baldassarre, A.; Zaffina, S.; Marchesi, F. When a Neglected Tropical Disease Goes Global: Knowledge, Attitudes and Practices of Italian Physicians towards Monkeypox, Preliminary Results. Trop. Med. Infect. Dis. 2022, 7, 135. https://doi.org/10.3390/tropicalmed7070135
Riccò M, Ferraro P, Camisa V, Satta E, Zaniboni A, Ranzieri S, Baldassarre A, Zaffina S, Marchesi F. When a Neglected Tropical Disease Goes Global: Knowledge, Attitudes and Practices of Italian Physicians towards Monkeypox, Preliminary Results. Tropical Medicine and Infectious Disease. 2022; 7(7):135. https://doi.org/10.3390/tropicalmed7070135
Chicago/Turabian StyleRiccò, Matteo, Pietro Ferraro, Vincenzo Camisa, Elia Satta, Alessandro Zaniboni, Silvia Ranzieri, Antonio Baldassarre, Salvatore Zaffina, and Federico Marchesi. 2022. "When a Neglected Tropical Disease Goes Global: Knowledge, Attitudes and Practices of Italian Physicians towards Monkeypox, Preliminary Results" Tropical Medicine and Infectious Disease 7, no. 7: 135. https://doi.org/10.3390/tropicalmed7070135
APA StyleRiccò, M., Ferraro, P., Camisa, V., Satta, E., Zaniboni, A., Ranzieri, S., Baldassarre, A., Zaffina, S., & Marchesi, F. (2022). When a Neglected Tropical Disease Goes Global: Knowledge, Attitudes and Practices of Italian Physicians towards Monkeypox, Preliminary Results. Tropical Medicine and Infectious Disease, 7(7), 135. https://doi.org/10.3390/tropicalmed7070135










