Cardiovascular Manifestation of the BNT162b2 mRNA COVID-19 Vaccine in Adolescents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Diary Card
2.3. Definition of Cardiovascular Manifestation
- Chest pain/pericarditis
- Dyspnea/orthopnea
- Palpitation
- Hypertension/hypotension
- Tachycardia/bradycardia
- Shock/cardiogenic shock
- Abnormal ECG or abnormal rhythm or ECG change
- Bundle branch block
- Decreased ejection fraction
- Diastolic dysfunction
- Elevation in at least one cardiac biomarker (troponin-T, CK-MB)/myocarditis
2.4. Definition of Myocarditis [18]
2.5. Definition of Pericarditis [18]
2.6. Cardiac Enzymes
2.7. Echocardiography Protocol
2.8. Sample Size Calculation
2.9. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Cardiovascular Findings
3.3. Evaluation of Patients with Elevated Biomarkers or Positive Lab Assessments
3.4. Evaluation of Patients Developing Abnormal ECG Post-Vaccination
3.5. Evaluation of Patients with Serial Echocardiographic Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skowronski, D.M.; De Serres, G. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2021, 384, 1576–1577. [Google Scholar] [PubMed]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of two RNA-based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.E.; Gargano, J.W.; Marin, M.; Wallace, M.; Curran, K.G.; Chamberland, M.; McClung, N.; Campos-Outcalt, D.; Morgan, R.L.; Mbaeyi, S.; et al. The Advisory Committee on Immunization Practice’s Interim Recommendation for use Pfizer-BioNTech COVID-19 vaccine–United States, December 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 1922–1924. [Google Scholar] [CrossRef]
- Faix, D.J.; Gordon, D.M.; Perry, L.N.; Raymond-Loher, I.; Tati, N.; Lin, G.; DiPietro, G.; Selmani, A.; Decker, M.D. Prospective safety surveillance study of ACAM2000 smallpox vaccine in deploying military personnel. Vaccine 2020, 38, 7323–7330. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, A.; Narasimhan, M.; Li, Q.-Z.; Mahimainathan, L.; Hitto, I.; Fuda, F.; Batra, K.; Jiang, X.; Zhu, C.; Schoggins, J.; et al. In-Depth Evaluation of a Case of Presumed Myocarditis After the Second Dose of COVID-19 mRNA Vaccine. Circulation 2021, 144, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, D.; Cooper, L.T.; Blauwet, L.A. Sex and Gender Differences in Myocarditis and Dilated Cardiomyopathy. Curr. Probl. Cardiol. 2013, 38, 7–46. [Google Scholar] [CrossRef] [PubMed]
- Witberg, G.; Barda, N.; Hoss, S.; Richter, I.; Wiessman, M.; Aviv, Y.; Grinberg, T.; Auster, O.; Dagan, N.; Balicer, R.D.; et al. Myocarditis after Covid-19 Vaccination in a Large Health Care Organization. N. Engl. J. Med. 2021, 385, 2132–2139. [Google Scholar] [CrossRef]
- Mevorach, D.; Anis, E.; Cedar, N.; Bromberg, M.; Haas, E.J.; Nadir, E.; Olsha-Castell, S.; Arad, D.; Hasin, T.; Levi, N.; et al. Myocarditis after BNT162b2 mRNA Vaccine against Covid-19 in Israel. N. Engl. J. Med. 2021, 385, 2140–2149. [Google Scholar] [CrossRef]
- Gargano, J.W.; Wallace, M.; Hadler, S.C.; Langley, G.; Su, J.R.; Oster, M.E.; Broder, K.R.; Gee, J.; Weintraub, E.; Shimabukuro, T.; et al. Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 977–982. [Google Scholar] [CrossRef]
- Caforio, A.L.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [PubMed]
- Su, J.R.; McNeil, M.M.; Welsh, K.J.; Marquez, P.L.; Ng, C.; Yan, M.; Cano, M.V. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990–2018. Vaccine 2021, 39, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, T.T.; Nguyen, M.; Martin, D.; DeStefano, F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine 2015, 33, 4398–4405. [Google Scholar] [CrossRef]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2019, 5, 811–818. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practice (ACIP). Coronavirus Disease 2019 (COVID-19) Vaccines. Available online: https://www.cdc.gov/vaccines/acip/meetings/slides-2021-10.html (accessed on 23 January 2022).
- Kuntz, J.; Crane, B.; Weinmann, S.; Naleway, A.L. Myocarditis and pericarditis are rare following live viral vaccinations in adults. Vaccine 2018, 36, 1524–1527. [Google Scholar] [CrossRef]
- García, J.B.; Ortega, P.P.; Fernández, J.A.B.; León, A.C.; Burgos, L.R.; Dorta, E.C. Acute myocarditis after administration of the BNT162b2 vaccine against COVID-19. Rev. Esp. Cardiol. 2021, 74, 812–814. [Google Scholar] [CrossRef]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis With COVID-19 mRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.J.; Dutta, S.; Charan, J.; Bhardwaj, P.; Tandon, A.; Yadav, D.; Islam, S.; Haque, M. Cardiovascular Adverse Events Reported from COVID-19 Vaccines: A Study Based on WHO Database. Int. J. Gen. Med. 2021, 14, 3909–3927. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines-a new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Adolescents in Another Importance Action in Flight Against Pandemic; US Food and Drug Administration: Silver Spring, MD, USA, 2021. [Google Scholar]
- Montgomery, J.; Ryan, M.; Engler, R.; Hoffman, D.; McClenathan, B.; Collins, L.; Loran, D.; Hrncir, D.; Herring, K.; Platzer, M.; et al. Myocarditis Following Immunization With mRNA COVID-19 Vaccines in Members of the US Military. JAMA Cardiol. 2021, 6, 1202–1206. [Google Scholar] [CrossRef]
- Mouch, S.A.; Roguin, A.; Hellou, E.; Ishai, A.; Shoshan, U.; Mahamid, L.; Zoabi, M.; Aisman, M.; Goldschmid, N.; Yanay, N.B. Myocarditis following COVID-19 mRNA vaccination. Vaccine. 2021, 39, 3790–3793. [Google Scholar] [CrossRef] [PubMed]
- Power, J.R.; Keyt, L.K.; Adler, E.D. Myocarditis following COVID-19 vaccination: Incidence, mechanisms, and clinical considerations. Expert Rev. Cardiovasc. Ther. 2022, 20, 241–251. [Google Scholar] [CrossRef]
- Hajjo, R.; Sabbah, D.A.; Bardaweel, S.K.; Tropsha, A. Shedding the Light on Post-Vaccine Myocarditis and Pericarditis in COVID-19 and Non-COVID-19 Vaccine Recipients. Vaccines 2021, 9, 1186. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247 Pt 1, 1465–1468. [Google Scholar] [CrossRef]
- Ahin, U.; Karikó, K.; Türeci, Ö. mRNA-based therapeutics—developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef] [PubMed]
- Vora, S.M.; Lieberman, J.; Wu, H. Inflammasome activation at the crux of severe COVID-19. Nat. Rev. Immunol. 2021, 21, 694–703. [Google Scholar] [CrossRef]
- Chilamahuri, R.; Agarwal, S. COVID-19: Characteristics and Therapeutics. Cells 2021, 10, 206. [Google Scholar] [CrossRef]
- Aikawa, T.; Takagi, H.; Ishikawa, K.; Kuno, T. Myocardial injury characterized by elevated cardiac troponin and in-hospital mortality of COVID-19: An insight from a meta-analysis. J. Med. Virol. 2021, 93, 51–55. [Google Scholar] [CrossRef]
- Maiese, A.; Frati, P.; Del Duca, F.; Santoro, P.; Manetti, A.C.; La Russa, R.; Di Paolo, M.; Turillazzi, E.; Fineschi, V. Myocardial Pathology in COVID-19-Associated Cardiac Injury: A Systematic Review. Diagnostics 2021, 11, 1647. [Google Scholar] [CrossRef]
- Tano, E.; San Martin, S.; Girgis, S.; Martinez-Fernandez, Y.; Sanchez Vegas, C. Perimyocarditis in adolescents after Pfizer-BioNTech COVID-19 vaccine. J. Pediatr. Infect. Dis. Soc. 2021, 10, 962–966. [Google Scholar] [CrossRef]
- Marshall, M.; Ferguson, I.D.; Lewis, P.; Jaggi, P.; Gagliardo, C.; Collins, J.S.; Shaughnessy, R.; Caron, R.; Fuss, C.; Corbin, K.J.E.; et al. Symptomatic acute myocarditis in 7 adolescents after Pfizer-BioNTech COVID-19 vaccination. Pediatrics 2021, 148, e2021052478. [Google Scholar] [CrossRef] [PubMed]
- Ashor, A.W.; Lara, J.; Mathers, J.C.; Siervo, M. Effect of vitamin C on endothelial function in health and disease: A systematic review and meta-analysis of randomized controlled trials. Athersclerosis 2014, 235, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Berretta, M.; Quagliariello, V.; Maurea, N.; Di Francia, R.; Sharifi, S.; Facchini, G.; Rinaldi, L.; Piezzo, M.; Manuela, C.; Nunnari, G.; et al. Multiple Effects of Ascorbic Acid against Chronic Diseases: Updated Evidence from Preclinical and Clinical Studies. Antioxidants 2020, 9, 1182. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, J.F.; Koshino, Y.; Bonnichsen, C.R.; Yu, Y.; Miller FAJr Pellikka, P.A.; Cooper, L.T., Jr. Villarraga HR. Speckle tracking echocardiography in acute myocarditis. Int. J. Cardiovasc. Imaging 2013, 29, 275–284. [Google Scholar] [CrossRef] [PubMed]

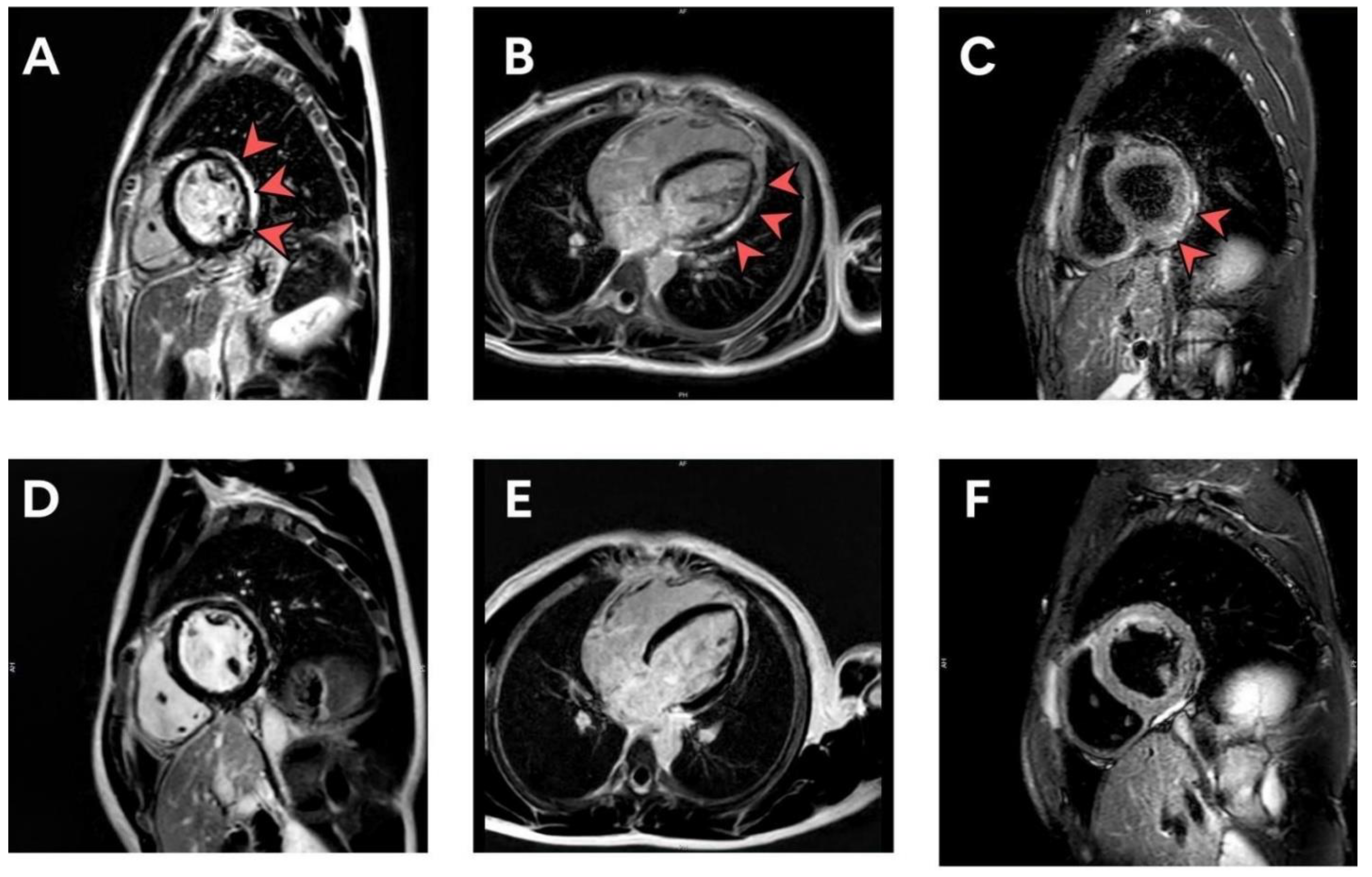
| Characteristic | Overall (n = 301) | 13–15 y (n = 207) | 16–18 y (n = 94) | p-Value |
|---|---|---|---|---|
| Age, y | 15 ± 1.6 | 14 ± 0.8 | 17 ± 0.7 | - |
| BMI (kg/m2) | 21 ± 5.0 | 20 ± 4.8 | 22 ± 5.2 | 0.017 |
| Male sex, n (%) | 202 (67.1) | 110 (53.1) | 92 (97.9) | <0.0001 * |
| Underlying disease n (%) | 44 (14.6) | 31 (15.0) | 13 (13.8) | 0.795 |
| Allergic rhinitis | 24 (8.0) | 17 (8.2) | 7 (7.4) | 0.813 |
| Asthma | 7 (2.3) | 5 (2.4) | 2 (2.1) | 0.869 |
| Thalassemia trait | 5 (1.7) | 3 (1.4) | 2 (2.1) | 0.688 |
| G6PD deficiency | 4 (1.3) | 3 (1.4) | 1 (1.1) | 0.782 |
| Attention deficit | 1 (0.3) | 1 (0.5) | 0 | 0.500 |
| Epilepsy | 1 (0.3) | 1 (0.5) | 0 | 0.500 |
| Migraine | 1 (0.3) | 1 (0.5) | 0 | 0.500 |
| Thyrotoxicosis | 1 (0.3) | 0 | 1 (1.1) | 0.500 |
| Symptoms, n (%) | ||||
| Fever | 50 (16.6) | 30 (14.5) | 20 (21.3) | 0.093 |
| Palpitation | 12 (4.0) | 10 (4.8) | 2 (2.1) | 0.268 |
| Chest pain | 8 (2.7) | 5 (2.4) | 3 (3.2) | 0.699 |
| Shortness of breath | 19 (6.3) | 16 (7.7) | 3 (3.2) | 0.134 |
| Headache | 35 (11.6) | 27 (13.0) | 8 (8.5) | 0.257 |
| Laboratory findings | ||||
| Troponin-T, ng/L | 5.6 ± 2.5 | 5.4 ± 2.5 | 5.9 ± 2.5 | 0.112 |
| CK-MB ng/mL | 1.4 ± 0.9 | 1.4 ± 0.9 | 1.5 ± 0.9 | 0.473 |
| Treatment and hospital course | ||||
| NSAIDS, n (%) | 3 (1.0) | 1 (0.5) | 2 (2.1) | 0.178 |
| Hospitalization, n (%) | 2 (0.7) | 0 | 2 (2.1) | 0.035 |
| ICU admission, n (%) | 1 (0.3) | 0 | 1 (1.1) | 0.138 |
| Variable | Value |
|---|---|
| Presenting symptoms and signs—Number/total number (%) Chest pain Chest discomfort Pericardial effusion Fever Headache Palpitation Dyspnea | 3/7 (42.86) 3/7 (42.86) 3/7 (42.86) 4/7 (57.14) 2/7 (28.57) 1/7 (14.29) 1/7 (14.29) |
| Vital signs on day of symptoms (Mean ± SD) | |
| Temperature—°C Blood pressure—mmHg Systolic Diastolic Heart rate—beats/min | 36.4 ± 0.4 114.9 ± 10.9 70.7 ± 7.8 92.71 ± 21.3 |
| Shock-Number/total number. (%) | 0/7 (0) |
| Electrocardiographic findings—Number/total number (%) Normal sinus rhythm Sinus rhythm with sinus arrhythmia Diffuse ST elevation with PR depression Sinus arrhythmia with PAC Sinus tachycardia Junctional escape rhythm | 1/7 (14.29) 2/7 (28.57) 1/7 (14.29) 1/7 (14.29) 1/7 (14.29) 1/7 (14.29) |
| Laboratory values Elevated troponin-T | 5/7 (71.43) |
| Clinical course Arrhythmia ICU admission Need for inotrope or vasopressor Death | 4/7 (57.14) 1/7 (14.29) 0/7 (0) 0/7 (0) |
| Treatment and hospital course Ibuprofen (NSAIDs) | 3/7 (42.86) |
| Demographic | Clinical Presentation | Echocardiography | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Age (y) | Sex | Classification | Peak CRP (mg/ L) | Peak ESR (mm/hr) | CK-MB Level (ng/mL) | Troponin-T (pg/mL) | LVEF% | Pericardial Effusion | |||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |||||||
| 1 | 16 | Male | Myopericarditis | 86.6 | 19 | 1.25 | 109.6 | 2.36 | 1.67 | 3.18 | 593 | 37.2 | 10.9 | 75.3 | 73.7 | 77.2 | 84.7 | Yes |
| 2 | 15 | Male | Pericarditis | 1.3 | 7 | 1.11 | 1.34 | 1.52 | 1.46 | 2.58 | 3.77 | 6.04 | 3.93 | 61.5 | 60.2 | 74.1 | 70.7 | Yes |
| 3 | 17 | Male | Pericarditis | 10.5 | 8 | 1.99 | 1.87 | 1.72 | 2.71 | 4.54 | 8.03 | 7.87 | 6.75 | 78.9 | 77.5 | 61.0 | 67.2 | Yes |
| 4 | 13 | Male | Subclinical myocarditis | 0.3 | - | 1.39 | 1.72 | 2.28 | - | 8.56 | 10.3 | 34.94 | - | 58.6 | 59.2 | 75.4 | - | No |
| 5 | 14 | Male | Subclinical myocarditis | 0.5 | - | 3.00 | 2.06 | 3.06 | - | 3.73 | 28.6 | 38.68 | - | 79.6 | 60.1 | 76.2 | - | No |
| 6 | 13 | Male | Subclinical myocarditis | 0.9 | - | 3.90 | 3.67 | 5.10 | - | 5.35 | 14.87 | 16.81 | - | 64.3 | 76.2 | 78.9 | - | No |
| 7 | 17 | Male | Subclinical myocarditis | 4.3 | - | 2.25 | 2.32 | 2.41 | - | 3.12 | 13.06 | 15.44 | - | 70.8 | 52.4 | 53.8 | - | No |
| Rhythm | Number (n = 301) |
|---|---|
| Normal sinus rhythm | 247 (82.06%) |
| Sinus rhythm with sinus arrhythmia | 22 (7.31%) |
| Sinus tachycardia | 20 (6.64%) |
| Sinus bradycardia | 4 (1.33%) |
| Premature atrial contraction (PAC) | 3 (1%) |
| Premature ventricular contraction (PVC) | 2 (0.66%) |
| Junctional escape rhythm | 1 (0.33%) |
| Ectopic atrial rhythm | 1 (0.33%) |
| Diffuse ST elevation with PR depression | 1 (0.33%) |
| Cardiac Function | Day0 | Day3 | Day7 | p-Value D0 vs. D3 | p-Value D0 vs. D7 | p-Value D3 vs. D7 |
|---|---|---|---|---|---|---|
| IVSD, mean ± SD | 1.18 ± 4.83 | 0.99 ± 1.51 | 0.93 ± 1.32 | 0.508 | 0.383 | 0.596 |
| LVIDd, mean ± SD | 4.43 ± 3.22 | 4.34 ± 3.02 | 4.81 ± 5.02 | 0.735 | 0.209 | 0.168 |
| LVPWd, mean ± SD | 0.96 ± 0.67 | 0.91 ± 0.81 | 1.28 ± 5.16 | 0.431 | 0.299 | 0.234 |
| LVIDs, mean ± SD | 2.64 ± 1.65 | 2.85 ± 2.82 | 2.60 ± 0.5 | 0.250 | 0.730 | 0.128 |
| LVEF, mean ± SD | 68.68 ± 9.27 | 68.21 ± 9.18 | 68.30 ± 8.56 | 0.490 | 0.585 | 0.878 |
| MV flow E-wave velocity mean ± SD | 99.32 ± 18.77 | 98.98 ± 20.47 | 99.93 ± 21.05 | 0.791 | 0.773 | 0.391 |
| MV flow A-wave velocity mean ± SD | 53.37 ± 16.10 | 52.39 ± 15.39 | 51.23 ± 16.18 | 0.432 | 0.046 | 0.217 |
| MV annulus E-wave velocity, mean ± SD | 2.11 ± 1.28 | 2.03 ± 0.63 | 2.06 ± 1.18 | 0.281 | 0.632 | 0.653 |
| e’, mean ± SD | 0.12 ± 0.05 | 0.13 ± 0.10 | 0.18 ± 0.55 | 0.227 | 0.097 | 0.065 |
| E/e´, mean ± SD | 8.33 ± 2.63 | 8.22 ± 2.03 | 9.29 ± 8.27 | 0.551 | 0.277 | 0.125 |
| a´, mean ± SD | 0.08 ± 0.06 | 0.08 ± 0.04 | 0.16 ± 0.79 | 0.393 | 0.201 | 0.091 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansanguan, S.; Charunwatthana, P.; Piyaphanee, W.; Dechkhajorn, W.; Poolcharoen, A.; Mansanguan, C. Cardiovascular Manifestation of the BNT162b2 mRNA COVID-19 Vaccine in Adolescents. Trop. Med. Infect. Dis. 2022, 7, 196. https://doi.org/10.3390/tropicalmed7080196
Mansanguan S, Charunwatthana P, Piyaphanee W, Dechkhajorn W, Poolcharoen A, Mansanguan C. Cardiovascular Manifestation of the BNT162b2 mRNA COVID-19 Vaccine in Adolescents. Tropical Medicine and Infectious Disease. 2022; 7(8):196. https://doi.org/10.3390/tropicalmed7080196
Chicago/Turabian StyleMansanguan, Suyanee, Prakaykaew Charunwatthana, Watcharapong Piyaphanee, Wilanee Dechkhajorn, Akkapon Poolcharoen, and Chayasin Mansanguan. 2022. "Cardiovascular Manifestation of the BNT162b2 mRNA COVID-19 Vaccine in Adolescents" Tropical Medicine and Infectious Disease 7, no. 8: 196. https://doi.org/10.3390/tropicalmed7080196
APA StyleMansanguan, S., Charunwatthana, P., Piyaphanee, W., Dechkhajorn, W., Poolcharoen, A., & Mansanguan, C. (2022). Cardiovascular Manifestation of the BNT162b2 mRNA COVID-19 Vaccine in Adolescents. Tropical Medicine and Infectious Disease, 7(8), 196. https://doi.org/10.3390/tropicalmed7080196






