Evaluating Molecular Xenomonitoring as a Tool for Lymphatic Filariasis Surveillance in Samoa, 2018–2019
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Region
2.2. Selection of Primary Sampling Units (PSUs) and Households
2.3. Data Collection
2.3.1. Mosquito Survey
2.3.2. Human Seroprevalence Survey
2.4. Data Analysis
2.4.1. Mosquito Abundance
2.4.2. Prevalence of PCR-Positive Mosquitoes
Mosquito Infection Prevalence in Randomly vs. Purposively Selected PSUs in 2019
2.4.3. Human Antigen Prevalence
2.4.4. Comparison of Antigen Prevalence in PSUs with or without PCR-Positive Mosquito Pools
2.4.5. Comparison of Human Ag and Mosquito Infection Prevalence between Baseline (2018) and Follow-Up (2019)
3. Results
3.1. Mosquito Abundance
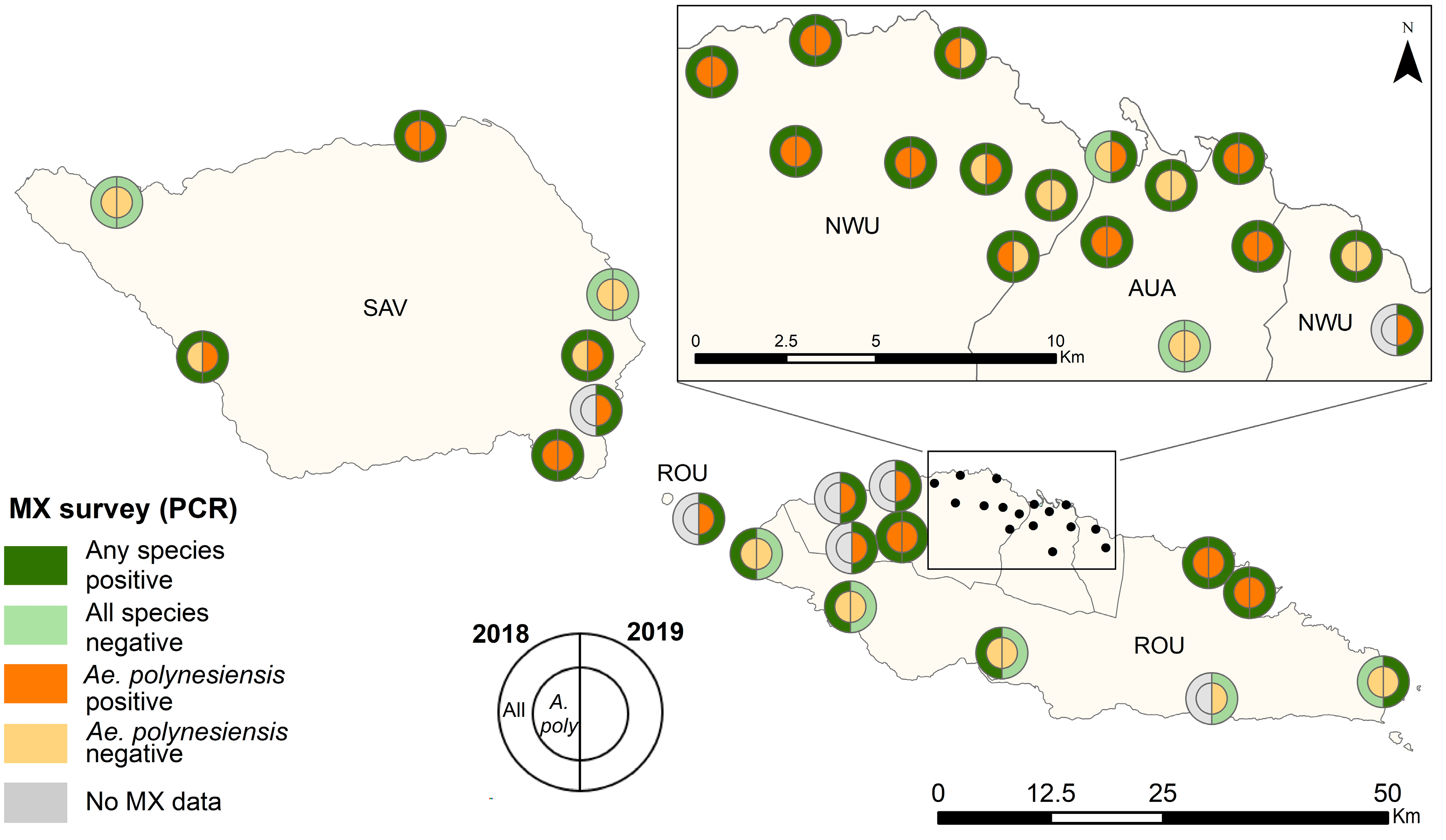
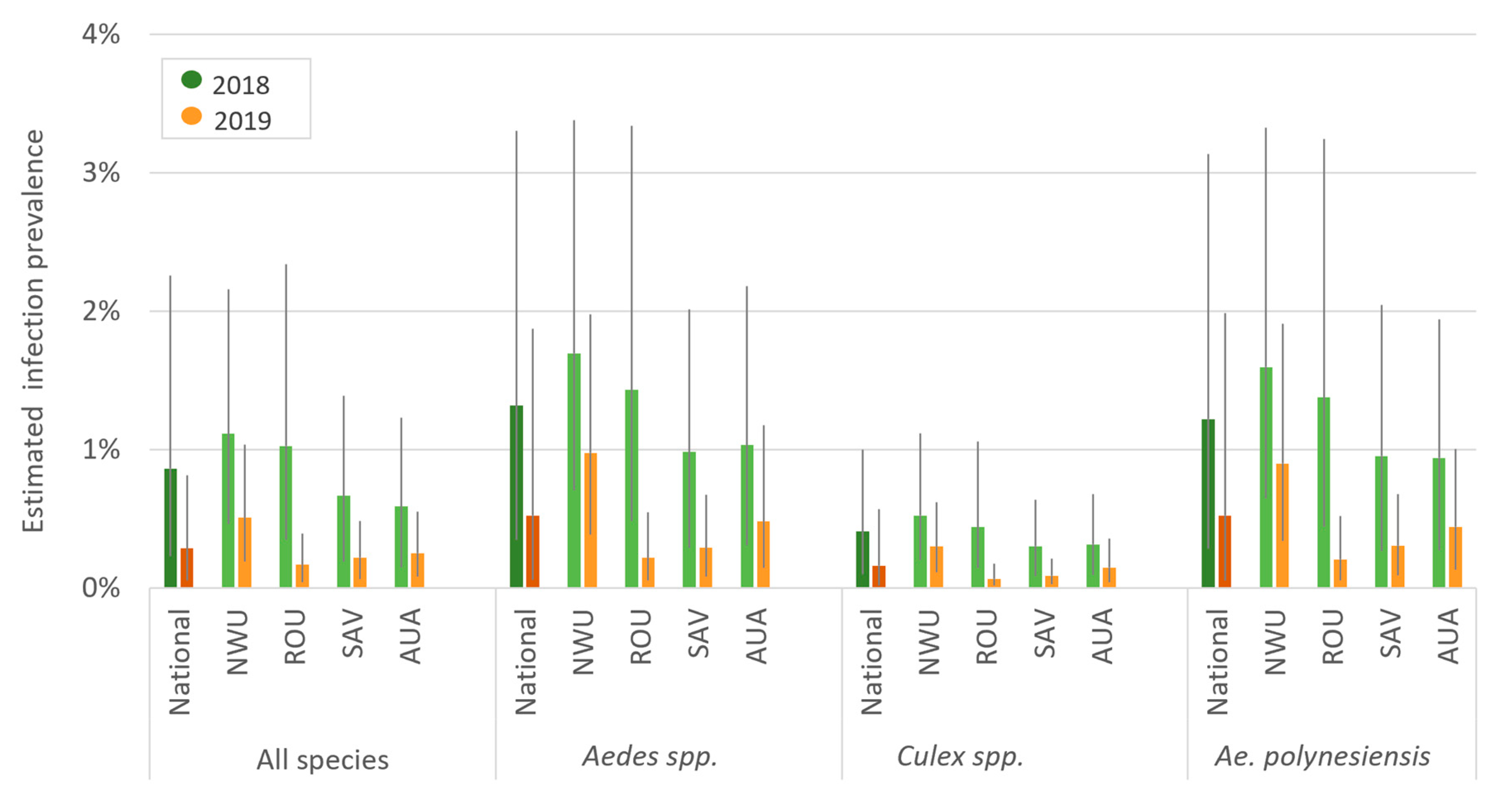
3.2. Precence and Prevalence of PCR-Positive Mosquitoes
Mosquito Infection Prevalence in Randomly vs. Purposively Selected PSUs in 2019
3.3. Human Antigen Prevalence
3.4. Comparison of Antigen Prevalence in PSUs with or without PCR-Positive Mosquito Pools
3.5. Comparison of Human Ag and Mosquito Infection Prevalence between Baseline (2018) and Follow-Up (2019) Surveys
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramaiah, K.D.; Ottesen, E.A. Progress and Impact of 13 Years of the Global Programme to Eliminate Lymphatic Filariasis on Reducing the Burden of Filarial Disease. PLoS Negl. Trop. Dis. 2014, 8, e3319. [Google Scholar] [CrossRef]
- Weil, G.J.; Curtis, K.C.; Fakoli, L.; Fischer, K.; Gankpala, L.; Lammie, P.J.; Majewski, A.C.; Pelletreau, S.; Won, K.Y.; Bolay, F.K.; et al. Laboratory and field evaluation of a new rapid test for detecting wuchereria bancrofti Antigen in human blood. Am. J. Trop. Med. Hyg. 2013, 89, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, E.M.; Stolk, W.A.; Laney, S.J.; Michael, E. The role of monitoring mosquito infection in the Global Programme to Eliminate Lymphatic Filariasis. Trends Parasitol. 2009, 25, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.L.; Won, K.Y.; Lammie, P.J.; Graves, P.M. Lymphatic Filariasis Elimination in American Samoa: Evaluation of Molecular Xenomonitoring as a Surveillance Tool in the Endgame. PLoS Negl. Trop. Dis. 2016, 10, e0005108. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.U.; Samarasekera, S.D.; Nagodavithana, K.C.; Goss, C.W.; Punchihewa, M.W.; Dassanayaka, T.D.M.; Ranasinghe, U.S.B.; Mendis, D.; Weil, G.J. Comprehensive Assessment of a Hotspot with Persistent Bancroftian Filariasis in Coastal Sri Lanka. Am. J. Trop. Med. Hyg. 2018, 99, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Jambulingam, P.; Krishnamoorthy, K.; Sivagnaname, N.; Sadanandane, C.; Vasuki, V.; Palaniswamy, C.; Vijayakumar, B.; Srividya, A.; Raju, H.K.K. Molecular xenomonitoring as a post—MDA surveillance tool for global programme to eliminate lymphatic filariasis: Field validation in an evaluation unit in India. PLoS Negl. Trop. Dis. 2020, 14, e0007862. [Google Scholar] [CrossRef] [PubMed]
- Schmaedick, M.A.; Koppel, A.L.; Pilotte, N.; Torres, M.; Williams, S.A.; Dobson, S.L.; Lammie, P.J.; Won, K.Y. Molecular Xenomonitoring Using Mosquitoes to Map Lymphatic Filariasis after Mass Drug Administration in American Samoa. PLoS Negl. Trop. Dis. 2014, 8, e3087. [Google Scholar] [CrossRef] [PubMed]
- Dorkenoo, M.A.; de Souza, D.K.; Apetogbo, Y.; Oboussoumi, K.; Yehadji, D.; Tchalim, M.; Etassoli, S.; Koudou, B.; Ketoh, G.K.; Sodahlon, Y.; et al. Molecular xenomonitoring for post-validation surveillance of lymphatic filariasis in Togo: No evidence for active transmission. Parasites Vectors 2018, 11, 52. [Google Scholar] [CrossRef]
- Byrd, E.; St. Amant, L.; Bromberg, L. Studies on filariasis in the Samoan area. U.S. Naval Med. Bull. 1945, 44, 1–20. [Google Scholar]
- Jachowski, J.L.A. Filariasis in American Samoa. V. Bionomics of the principal vector, Aedes polynesiensis Marks. Am. J. Hyg. 1954, 60, 186–203. [Google Scholar]
- Graves, P.M.; Joseph, H.; Coutts, S.P.; Mayfield, H.J.; Maiava, F.; Ah Leong-Lui, T.A.; Tupuimatagi Toelupe, P.; Toeaso Iosia, V.; Loau, S.; Pemita, P.; et al. Control and elimination of lymphatic filariasis in Oceania: Prevalence, geographical distribution, mass drug administration, and surveillance in Samoa, 1998–2017. Adv. Parasitol. 2021, 114, 27–73. [Google Scholar] [CrossRef]
- Willis, G.A.; Mayfield, H.J.; Kearns, T.; Naseri, T.; Thomsen, R.; Gass, K.; Sheridan, S.; Graves, P.M.; Lau, C.L. A community survey of coverage and adverse events following country-wide triple-drug mass drug administration for lymphatic filariasis elimination, Samoa 2018. PLoS Negl. Trop. Dis. 2020, 14, e0008854. [Google Scholar] [CrossRef]
- Lau, C.L.; Meder, K.; Mayfield, H.J.; Kearns, T.; McPherson, B.; Naseri, T.; Thomsen, R.; Hedtke, S.M.; Sheridan, S.; Gass, K.; et al. Lymphatic filariasis epidemiology in Samoa in 2018: Geographic clustering and higher antigen prevalence in older age groups. PLoS Negl. Trop. Dis. 2020, 14, e0008927. [Google Scholar] [CrossRef]
- King, C.L.; Weil, G.J.; Kazura, J.W. Single-Dose Triple-Drug Therapy for Wuchereria bancrofti—5-Year Follow-up. N. Engl. J. Med. 2020, 382, 1956–1957. [Google Scholar] [CrossRef] [PubMed]
- Samoa Bureau of Statistics. 2016 Census Brief No 2, Population Dynamics and Trends. 2018. Available online: https://www.sbs.gov.ws/digi/3-2016%20Census%20Brief%20No.2.pdf (accessed on 17 July 2022).
- Government of Samoa Meteorology Division. Climate of Samoa. Available online: http://www.samet.gov.ws/index.php/climate-of-samoa (accessed on 29 November 2019).
- Samarawickrema, W.A.; Kimura, E.; Spears, G.F.S.; Penaia, L.; Sone, F.; Paulson, G.S.; Cummings, R.F. Distribution of vectors, transmission indices and microfilaria rates of subperiodic Wuchereria bancrofti in relation to village ecotypes in Samoa. Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 129–135. [Google Scholar] [CrossRef]
- WHO. Samoa Rolls Out Triple Drug Therapy to Accelerate Elimination of Lymphatic Filariasis 2018. Available online: https://www.who.int/news/item/24-08-2018-samoa-rolls-out-triple-drug-therapy-to-accelerate-elimination-of-lymphatic-filariasis (accessed on 17 July 2022).
- Chambers, E.W.; McClintock, S.K.; Avery, M.F.; King, J.D.; Bradley, M.H.; Schmaedick, M.A.; Lammie, P.J.; Burkot, T.R. Xenomonitoring of Wuchereria bancrofti and Dirofilaria immitis infections in mosquitoes from American Samoa: Trapping considerations and a comparison of polymerase chain reaction assays with dissection. Am. J. Trop. Med. Hyg. 2009, 80, 774–781. [Google Scholar] [CrossRef]
- Hapairai, L.K.; Plichart, C.; Naseri, T.; Silva, U.; Tesimale, L.; Pemita, P.; Bossin, H.C.; Burkot, T.R.; Ritchie, S.A.; Graves, P.M.; et al. Evaluation of traps and lures for mosquito vectors and xenomonitoring of Wuchereria bancrofti infection in a high prevalence Samoan Village. Parasites Vectors 2015, 8, 287. [Google Scholar] [CrossRef]
- Ramalingam, S. An annotated checklist and keys to the mosquitoes of Samoa and Tonga. Mosq. Syst. 1976, 8, 298–318. [Google Scholar]
- Huang, G.H. The mosquitoes of Polynesia with a pictorial key to some species associated with filariasis and. Mosq. Syst. 1977, 9, 289–322. [Google Scholar]
- Rueda, L. Pictorial keys for the identification of mosquitoes (Diptera: Culicidae) associated with dengue virus transmission. Zootaxa 2004, 589, 1–60. [Google Scholar] [CrossRef]
- Zulch, M.F.; Pilotte, N.; Grant, J.R.; Minetti, C.; Reimer, L.J.; Williams, S.A. Selection and exploitation of prevalent, tandemly repeated genomic targets for improved real-time PCR-based detection of Wuchereria bancrofti and Plasmodium falciparum in mosquitoes. PLoS ONE 2020, 15, e0232325. [Google Scholar] [CrossRef]
- McLure, A.; O’Neill, B.; Mayfield, H.; Lau, C.; McPherson, B. PoolTestR: An R package for estimating prevalence and regression modelling for molecular xenomonitoring and other applications with pooled samples. Environ. Model. Softw. 2021, 145, 105158. [Google Scholar] [CrossRef]
- Vehtari, A.; Gelman, A.; Gabry, J. Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Stat. Comput. 2017, 27, 1413–1432. [Google Scholar] [CrossRef]





| Region | Ae. polynesiensis | Ae. (Finlaya) spp. | Aedes spp. (Other) | Culex spp. (All) | All Species |
|---|---|---|---|---|---|
| AUA | 467 | 242 | 179 | 848 | 1736 |
| NWU | 1140 | 60 | 803 | 1954 | 3957 |
| ROU | 313 | 178 | 296 | 847 | 1634 |
| SAV | 578 | 218 | 165 | 684 | 1645 |
| Total | 2498 | 698 | 1443 | 4333 | 8972 |
| Region | Ae. polynesiensis | Ae. aegypti | Ae. (Finlaya) spp. | Aedes spp. (Other) | Cx. quinquefasciatus | Culex spp. (Other) | Other | All Species |
|---|---|---|---|---|---|---|---|---|
| AUA | 1232 | 623 | 15 | 54 | 2965 | 0 | 2 | 4891 |
| NWU | 3443 | 1432 | 107 | 283 | 5823 | 0 | 9 | 11,097 |
| ROU | 2642 | 822 | 206 | 508 | 7179 | 1 | 3 | 11,361 |
| SAV | 3223 | 519 | 1049 | 291 | 1840 | 23 | 5 | 6950 |
| Total | 10,540 | 3396 | 1377 | 1136 | 17,807 | 24 | 19 | 34,299 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McPherson, B.; Mayfield, H.J.; McLure, A.; Gass, K.; Naseri, T.; Thomsen, R.; Williams, S.A.; Pilotte, N.; Kearns, T.; Graves, P.M.; et al. Evaluating Molecular Xenomonitoring as a Tool for Lymphatic Filariasis Surveillance in Samoa, 2018–2019. Trop. Med. Infect. Dis. 2022, 7, 203. https://doi.org/10.3390/tropicalmed7080203
McPherson B, Mayfield HJ, McLure A, Gass K, Naseri T, Thomsen R, Williams SA, Pilotte N, Kearns T, Graves PM, et al. Evaluating Molecular Xenomonitoring as a Tool for Lymphatic Filariasis Surveillance in Samoa, 2018–2019. Tropical Medicine and Infectious Disease. 2022; 7(8):203. https://doi.org/10.3390/tropicalmed7080203
Chicago/Turabian StyleMcPherson, Brady, Helen J. Mayfield, Angus McLure, Katherine Gass, Take Naseri, Robert Thomsen, Steven A. Williams, Nils Pilotte, Therese Kearns, Patricia M. Graves, and et al. 2022. "Evaluating Molecular Xenomonitoring as a Tool for Lymphatic Filariasis Surveillance in Samoa, 2018–2019" Tropical Medicine and Infectious Disease 7, no. 8: 203. https://doi.org/10.3390/tropicalmed7080203
APA StyleMcPherson, B., Mayfield, H. J., McLure, A., Gass, K., Naseri, T., Thomsen, R., Williams, S. A., Pilotte, N., Kearns, T., Graves, P. M., & Lau, C. L. (2022). Evaluating Molecular Xenomonitoring as a Tool for Lymphatic Filariasis Surveillance in Samoa, 2018–2019. Tropical Medicine and Infectious Disease, 7(8), 203. https://doi.org/10.3390/tropicalmed7080203








