Three-Dimensional Models of Soil-Transmitted Helminth Eggs from Light Microscopy Images
Abstract
1. Introduction
2. Materials and Methods
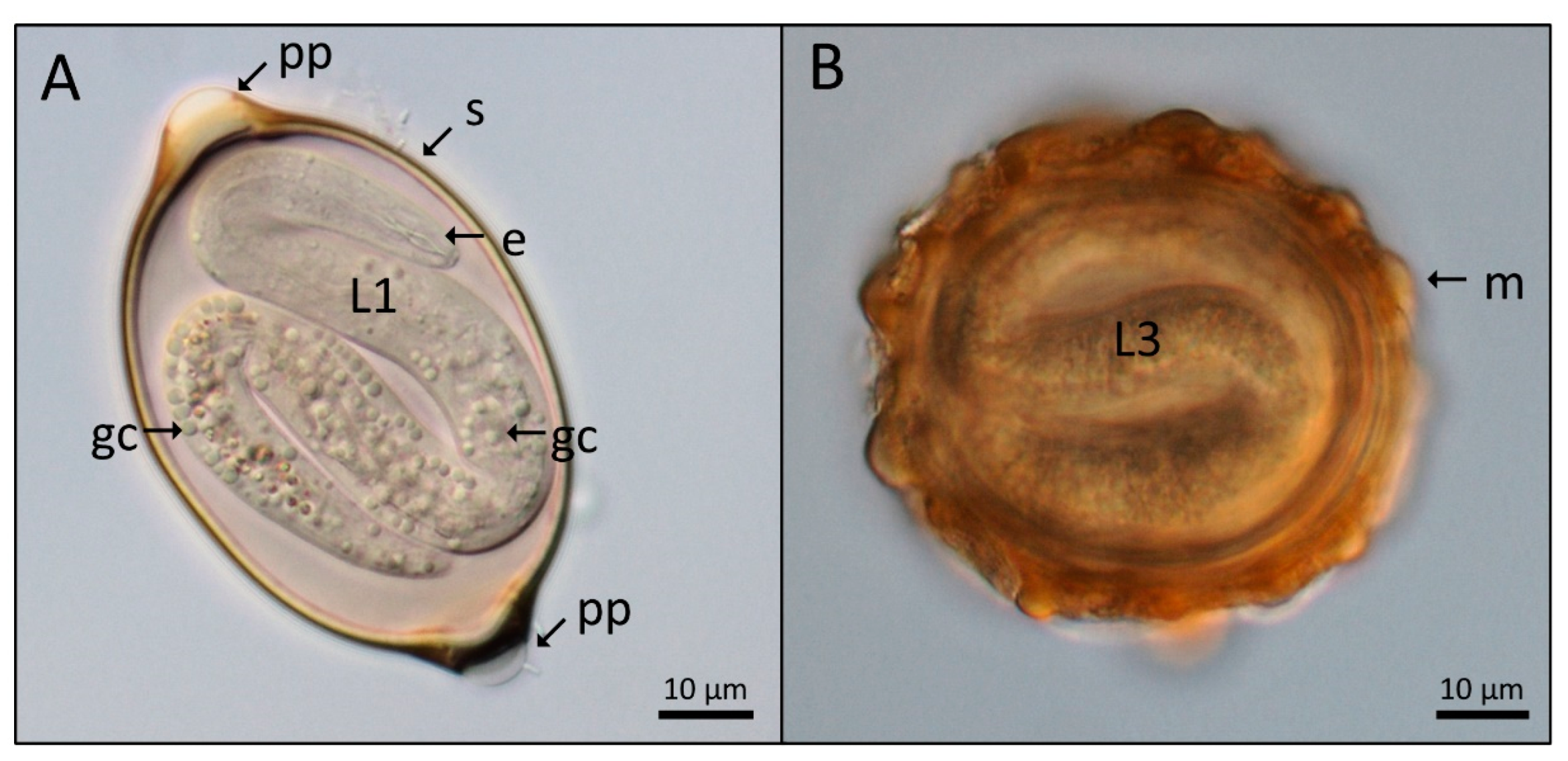
2.1. Light Microscopy of the Nematode Eggs
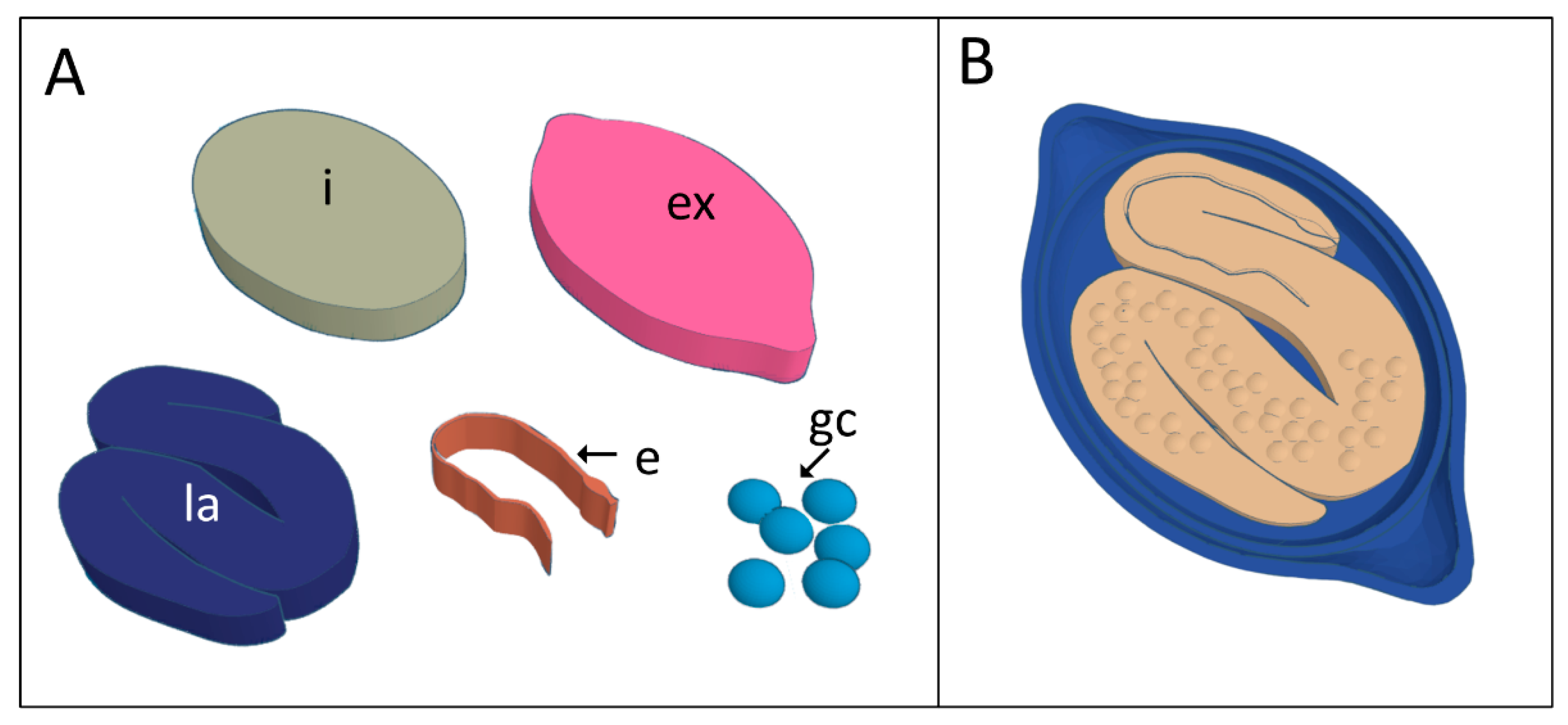
2.2. Three-Dimensional Virtual Modeling
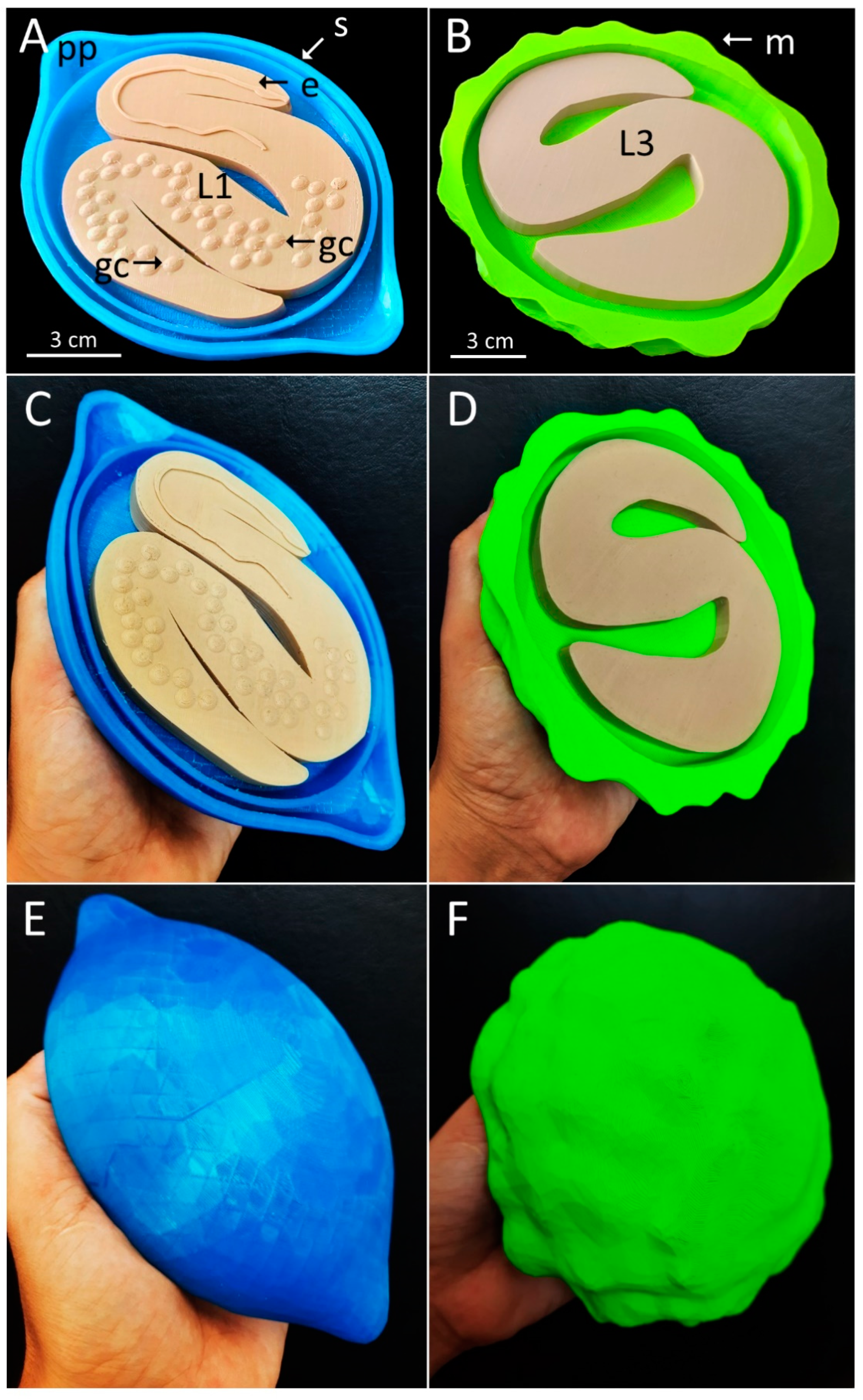
2.3. Three-Dimensional Printing
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, P.; Vats, O.; Kumar, D.; Singh, S. Coccidian intestinal parasites among immunocompetent children presenting with diarrhea: Are we missing them? Trop. Parasitol. 2017, 7, 37–40. [Google Scholar] [CrossRef] [PubMed]
- WHO World Health Organization. Geohelminthiasis. 10 January 2022. Available online: https://www.who.int/es/news-room/fact-sheets/detail/soil-transmitted-helminth-infections (accessed on 30 June 2022).
- WHO World Health Organization. Geographical distribution—Intestinal Worms. Available online: https://apps.who.int/neglected_diseases/ntddata/sth/sth.html (accessed on 30 June 2022).
- Chelkeba, L.; Mekonnen, Z.; Alemu, Y.; Emana, D. Epidemiology of intestinal parasitic infections in preschool and school-aged Ethiopian children: A systematic review and meta-analysis. BMC Public Health 2020, 20, 117. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; May, R.M. Herd immunity to helminth infection and implications for parasite control. Nature 1985, 315, 493–496. [Google Scholar] [CrossRef]
- Adegnika, A.A.; Lötsch, F.; Mba, R.M.O.; Ramharter, M. Update on Treatment and Resistance of Human Trichuriasis. Curr. Trop. Med. Rep. 2015, 2, 218–223. [Google Scholar] [CrossRef][Green Version]
- Katz, N. Inquérito Nacional de Prevalência da Esquistossomose Mansoni e Geo-Helmintoses. Instituto René Rachou. Fundação Oswaldo Cruz. 2018. Available online: https://www.cpqrr.fiocruz.br/pg/wp-content/uploads/2019/08/Inquerito.pdf (accessed on 30 June 2022).
- Liu, C.; Luo, R.; Yi, H.; Zhang, L.; Li, S.; Bai, Y.; Medina, A.; Rozelle, S.; Smith, S.; Wang, G.; et al. Soil-Transmitted Helminths in Southwestern China: A Cross-Sectional Study of Links to Cognitive Ability, Nutrition, and School Performance among Children. PLoS Negl. Trop. Dis. 2015, 9, e0003877. [Google Scholar] [CrossRef]
- Garrison, A.; Boivin, M.; Khoshnood, B.; Courtin, D.; Alao, J.; Mireku, M.; Ibikounle, M.; Massougbodji, A.; Cot, M.; Bodeau-Livinec, F. Soil-transmitted helminth infection in pregnancy and long-term child neurocognitive and behavioral development: A prospective mother-child cohort in Benin. PLoS Negl. Trop. Dis. 2021, 15, e0009260. [Google Scholar] [CrossRef]
- Araujo, A.; Reinhard, K.J.; Ferreira, L.F.; Gardner, S.L. Parasites as probes for prehistoric human migrations? Trends Parasitol. 2008, 24, 112–115. [Google Scholar] [CrossRef]
- Mitchell, P.D. The origins of human parasites: Exploring the evidence for endoparasitism throughout human evolution. Int. J. Paleopathol. 2013, 3, 191–198. [Google Scholar] [CrossRef]
- Lysek, H.; Malinsky, J.; Janisch, R. Ultrastructure of eggs of Ascaris lumbricoides Linnaeus, 1758 I. Egg-shells. Folia Parasitol. 1985, 32, 381–384. [Google Scholar]
- Yoshikawa, H.; Yamada, M.; Matsumoto, Y.; Yoshida, Y. Variations in egg size of Trichuris trichiura. Parasitol. Res. 1989, 75, 649–654. [Google Scholar] [CrossRef]
- Schmidt, G.D.; Roberts, L.S. Foundations of Parasitology, 8th ed.; McGraw-Hill: New York, NY, USA, 2009. [Google Scholar]
- Cardoso, D.C.; Cristiano, M.P.; Arent, C.O. Development of New Didactic Materials for Teaching Science and Biology: The Importance of the New Education Practices. OnLine J. Biol. Sci. 2009, 9, 1–5. [Google Scholar] [CrossRef][Green Version]
- Warburton, S. Second Life in higher education: Assessing the potential for and the barriers to deploying virtual worlds in learning and teaching. Br. J. Educ. Technol. 2009, 40, 414–426. [Google Scholar] [CrossRef]
- Suarez-Fontes, A.M.; Fontes, S.S.; Vannier-Santos, M.A. Enteroparasitosis in public schools in Bahia: Parasitology learning. Rev. Patol. Trop. 2017, 46, 185. [Google Scholar] [CrossRef]
- Bitter, G.; Corral, A. The pedagogical potential of augmented reality apps. Int. J. Eng. Sci. Invent. 2014, 3, 13–17. [Google Scholar]
- Addy, T.M.; Dube, D.; Pauze, B. How to Design a Classroom Activity that Integrates 3D Print Models with Active Learning. CourseSource 2018, 5. [Google Scholar] [CrossRef]
- Huang, T.-C.; Lin, C.-Y. From 3D modeling to 3D printing: Development of a differentiated spatial ability teaching model. Telemat. Inform. 2017, 34, 604–613. [Google Scholar] [CrossRef]
- Elrod, R.E. Classroom innovation through 3D printing. Libr. Hi Tech News 2016, 33, 5–7. [Google Scholar] [CrossRef]
- Ironside, P.M. Teaching Thinking and Reaching the Limits of Memorization: Enacting New Pedagogies. J. Nurs. Educ. 2005, 44, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Augusto, I.; Monteiro, D.; Girard-Dias, W.; Dos Santos, T.O.; Rosa Belmonte, S.L.; Pinto de Oliveira, J.; Mauad, H.; da Silva Pacheco, M.; Lenz, D.; Stefanon Bittencourt, A.; et al. Virtual Reconstruction and Three-Dimensional Printing of Blood Cells as a Tool in Cell Biology Education. PLoS ONE 2018, 11, e0161184. [Google Scholar] [CrossRef]
- Garcia, J.; Yang, Z.; Mongrain, R.; Leask, R.L.; Lachapelle, K. 3D printing materials and their use in medical education: A review of current technology and trends for the future. BMJ Simul. Technol. Enhanc. Learn. 2017, 4, 27–40. [Google Scholar] [CrossRef]
- Ye, Z.; Dun, A.; Jiang, H.; Nie, C.; Zhao, S.; Wang, T.; Zhai, J. The role of 3D printed models in the teaching of human anatomy: A systematic review and meta-analysis. BMC Med. Educ. 2020, 20, 335. [Google Scholar] [CrossRef]
- Giraud, S.; Brock, A.M.; Macé, M.J.-M.; Jouffrais, C. Map Learning with a 3D Printed Interactive Small-Scale Model: Improvement of Space and Text Memorization in Visually Impaired Students. Front. Psychol. 2017, 8, 930. [Google Scholar] [CrossRef] [PubMed]
- Valente, D.; Palama, A.; Gentaz, E. Exploring 3D miniatures with action simulations by finger gestures: Study of a new embodied design for blind and sighted children. PLoS ONE 2021, 16, e0245472. [Google Scholar] [CrossRef] [PubMed]
- Borba, V.; Enoki, M.; Lopes-Torres, E.J.; Machado-Silva, J.R.; Iñiguez, A.M. New data on eggshell structure of capillariid species: A SEM perspective. Parasitol. Res. 2021, 120, 963–970. [Google Scholar] [CrossRef]
- Nejsum, P.; Andersen, K.L.; Andersen, S.D.; Thamsborg, S.M.; Tejedor, A.M. Mebendazole treatment persistently alters the size profile and morphology of Trichuris trichiura eggs. Acta Trop. 2020, 204, 105347. [Google Scholar] [CrossRef]
- Divakara Shetty, S.; Shetty, N. Investigation of mechanical properties and applications of polylactic acids—A review. Mater. Res. Express 2019, 6, 112002. [Google Scholar]
- Yao, T.; Deng, Z.; Zhang, K.; Li, S. A method to predict the ultimate tensile strength of 3D printing polylactic acid (PLA) materials with different printing orientations. Compos. Part B Eng. 2019, 163, 393–402. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Wu, B.; Cui, C.; Guo, Y.; Yan, C. A critical review of fused deposition modeling 3D printing technology in manufacturing polylactic acid parts. Int. J. Adv. Manuf. Technol. 2019, 102, 2877–2889. [Google Scholar] [CrossRef]
- Zontek, T.L.; Ogle, B.R.; Jankovic, J.T.; Hollenbeck, S.M. An exposure assessment of desktop 3D printing. ACS Chem. Health Saf. 2017, 24, 15–25. [Google Scholar] [CrossRef]
- Storck, J.L.; Ehrmann, G.; Güth, U.; Uthoff, J.; Homburg, S.V.; Blachowicz, T.; Ehrmann, A. Investigation of Low-Cost FDM-Printed Polymers for Elevated-Temperature Applications. Polymers 2022, 14, 2826. [Google Scholar] [CrossRef]
- Vinyas, M.; Athul, S.; Harursampath, D.; Thoi, T.N. Experimental evaluation of the mechanical and thermal properties of 3D printed PLA and its composites. Mater. Res. Express 2019, 6, 115301. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, Y.E.; de Freitas, E.O.; de Oliveira, D.A.; Girard-Dias, W.; Crivano Machado, L.P.d.A.; Lopes-Torres, E.J. Three-Dimensional Models of Soil-Transmitted Helminth Eggs from Light Microscopy Images. Trop. Med. Infect. Dis. 2022, 7, 216. https://doi.org/10.3390/tropicalmed7090216
Dias YE, de Freitas EO, de Oliveira DA, Girard-Dias W, Crivano Machado LPdA, Lopes-Torres EJ. Three-Dimensional Models of Soil-Transmitted Helminth Eggs from Light Microscopy Images. Tropical Medicine and Infectious Disease. 2022; 7(9):216. https://doi.org/10.3390/tropicalmed7090216
Chicago/Turabian StyleDias, Yan Emygdio, Elisângela Oliveira de Freitas, Dayane Alvarinho de Oliveira, Wendell Girard-Dias, Lúcio Paulo do Amaral Crivano Machado, and Eduardo José Lopes-Torres. 2022. "Three-Dimensional Models of Soil-Transmitted Helminth Eggs from Light Microscopy Images" Tropical Medicine and Infectious Disease 7, no. 9: 216. https://doi.org/10.3390/tropicalmed7090216
APA StyleDias, Y. E., de Freitas, E. O., de Oliveira, D. A., Girard-Dias, W., Crivano Machado, L. P. d. A., & Lopes-Torres, E. J. (2022). Three-Dimensional Models of Soil-Transmitted Helminth Eggs from Light Microscopy Images. Tropical Medicine and Infectious Disease, 7(9), 216. https://doi.org/10.3390/tropicalmed7090216







